Abstract
OBJECTIVE
No guidelines for A1C measurement exist for women with gestational diabetes mellitus (GDM). The aim of this study was to document the rate of A1C decline in women with GDM.
RESEARCH DESIGN AND METHODS
Women with GDM in the Santa Barbara County Endocrine Clinic are managed with a carbohydrate-restricted diet and self-monitored blood glucose before and 1-h postprandial. Insulin is started if the preprandial glucose concentration is ≥90 mg/dl and/or a 1-h postprandial glucose concentration is ≥120 mg/dl. Capillary A1C was tested weekly using the DCA2000+ analyzer.
RESULTS
Twenty-four women with GDM (aged 29.0 ± 7.3 years) with initial A1C ≥7.0% were recruited. Baseline A1C was 8.8 ± 1.8%. Mean A1C decline was 0.47% per week (range 0.10–1.15%); the maximum was 4.3% in 4 weeks.
CONCLUSIONS
This study documents rapid decline in A1C during pregnancy and the utility of weekly A1C to guide therapy.
A1C is routinely measured approximately every 3 months in individuals with diabetes to assess the mean glucose concentration. The erythrocyte life span is ∼120 days (1). Thus, the 3-month interval between tests of A1C reflects the mean blood glucose over the preceding weeks to months. There are no clear guidelines for the frequency of testing A1C during pregnancy (2). In pregnancies complicated by type 1 or type 2 diabetes, most studies report the relationship between the first trimester A1C and the risk of spontaneous abortion and/or congenital malformations (3,4). The goal for therapy in pregestational diabetes is to sustain the A1C at <6.0%, although this level of A1C assumes that the measurement is performed only once each trimester (5–9). In addition, A1C is not recommended routinely in women with gestational diabetes mellitus (GDM). The life span of the erythrocyte during pregnancy is shortened to ∼90 days, and thus the test measures the mean glucose over a shorter time interval than in the nonpregnant state (10). Hence, the rate of change of A1C in pregnancy reflects the glycemic control over the past few weeks. Therefore, the measurement of A1C more frequently during pregnancy may be used to guide therapeutic decisions in all pregnancies complicated by diabetes including GDM. The aim of this study was to document the rate of A1C decline during the first 4 weeks after the initiation of treatment in women with GDM.
RESEARCH DESIGN AND METHODS
This was an observational study of pregnant women attending the Santa Barbara County Health Care Services Obstetrics Clinic for care who have the diagnosis of GDM (diabetes first diagnosed during pregnancy [2]) and who were referred to the Prenatal Diabetes/Endocrine Clinic for management. Because of the high rates of diabetes in this largely Latino population and because many of the women only seek medical attention during pregnancy, women are screened for GDM very early in pregnancy. Many women found to have diabetes early in pregnancy undoubtedly have preexisting type 2 diabetes, which falls under the classification of GDM when first identified during pregnancy. Treatment consists of a carbohydrate-restricted meal plan (11) and fingerstick blood glucose monitoring before and 1-h after each meal (11). Insulin is initiated if the diet does not achieve premeal glucose concentrations <90 mg/dl and/or 1-h postprandial glucose concentrations <120 mg/dl within 1 week of the carbohydrate restriction prescription (12,13). For simplicity in the clinic and to impress upon the diabetic women the importance of tight glucose control, a point of care A1C by fingerstick is routinely obtained at every weekly visit and analyzed immediately using the DCA2000+ analyzer (14,15). The DCA A1C is a Clinical Laboratory Improvement Amendments (CLIA)-waived test with a coefficient of variation of 2.3–3.3% at a normal A1C concentration of 5.2 and of 2.8–3.7% at an elevated A1C concentration of 11.9%.
RESULTS
Twenty-four Latina women with the diagnosis of GDM whose initial A1C was ≥7.0% and followed during the first 1–4 weeks of treatment were included in the analysis. The mean ± SD age was 29.0 ± 7.3 years, A1C at enrollment was 8.80 ± 1.83, and the duration of follow-up for this study was 3.2 ± 1.0 weeks. Mean gestational age at diagnosis of GDM was 12.2 ± 7.4 weeks.
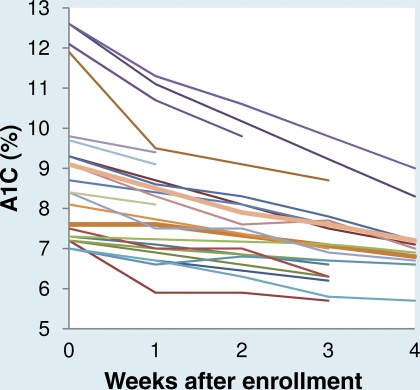
Figure 1 shows the A1C decline during the first 4 weeks of treatment (range 1.0–4.0 weeks) for all 24 women. The mean decline was 0.47 ± 0.30% per week, and the maximum decline over the 4 weeks was 4.3%. The decline was greatest among women with the highest A1C at baseline. Among the 20 women with an initial A1C <10.0%, the decline was 0.36 ± 0.15% per week. After the 1st month of decline, the A1C stabilized with a small drop of only 0.05% per week to the end of gestation (data not shown).
Figure 1.
A1C (%) at enrollment and weekly for up to 4 weeks of follow-up in 24 women with GDM.
CONCLUSIONS
This study documents that a rapid decline in A1C can be achieved during pregnancy when normoglycemia is vigorously instituted and achieved and thereafter sustained for 4 weeks. Home glucose monitoring on a regular basis is certainly the cornerstone of management of GDM and was initiated in the women enrolled in this study from the beginning. However, many high glucose values can be missed with the usual six or seven fingersticks per day. When A1C is measured at every weekly visit and the result is compared with the woman's previous value, then the rate of change of A1C (decline or rise) can be used to assess the glycemic control and guide therapeutic decisions. A randomized trial in a larger group of women will be needed to determine whether weekly A1C measurements will lead to an improvement in outcome.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
L.J., H.S., M.M., A.T., and D.J.P. designed the study, collected data, analyzed data, and wrote the manuscript.
Parts of this study were presented in poster form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Jovanovič L, Peterson CM: The clinical utility of glycosylated hemoglobin. Am J Med 1981;70:33l-337 [DOI] [PubMed] [Google Scholar]
- 2.Jovanovič L: Ed. Medical Management of Pregnancy Complicated by Diabetes. Alexandria, VA, American Diabetes Association, 1993, revised 1995, 2000, and 2009 [Google Scholar]
- 3.Mills JL, Simpson JL, Driscoll SG, Jovanovič-Peterson L, Van Allen M, Aarons JH, Metzger B, Bieber FR, Knopp RH, Holmes LB, Peterson CM, Withiam-Wilson M, Brown Z, Ober C, Harley E, Macpherson TA, Duckles AE, Mueller-Heubach EThe National Institutes of Child Health and Human Development-Diabetes In Early Pregnancy Study Incidence of spontaneous abortion among normal women and insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. N Engl J Med 1988;319:1617–1623 [DOI] [PubMed] [Google Scholar]
- 4.Mills JL, Knopp RH, Simpson JL, Jovanovič-Peterson L, Metzger BE, Holmes LB, Aarons JH, Brown Z, Reed GF, Bieber FR, Van Allen M, Holtzman I, Ober C, Peterson CM, Withiam MJ, Duckles AE, Mueller-Heubach E, Polk BFNational Institute of Child Health and Human Development Diabetes in Early Pregnancy Study Lack of relation of increased malformation rates in infants of diabetic mothers to glycemic control during organogenesis. N Engl J Med 1988;318:671–676 [DOI] [PubMed] [Google Scholar]
- 5.Sapienza AD, Francisco RP, Trindade TC, Zugaib M: Factors predicting the need for insulin therapy in patients with gestational diabetes mellitus. Diabetes Res Clin Pract 2010;88:81–86 [DOI] [PubMed] [Google Scholar]
- 6.Karcaaltincaba D, Yalvac S, Kandemir O, Altun S: Glycosylated hemoglobin level in the second trimester predicts birth weight and amniotic fluid volume in non-diabetic pregnancies with abnormal screening test. J Matern Fetal Neonatal Med 2010;23:1193–1199 [DOI] [PubMed] [Google Scholar]
- 7.Balaji V, Madhuri BS, Ashalatha S, Sheela S, Suresh S, Seshiah V: A1C in gestational diabetes mellitus in Asian Indian women. Diabetes Care 2007;30:1865–1867 [DOI] [PubMed] [Google Scholar]
- 8.Agarwal MM, Dhatt GS, Punnose J, Koster G: Gestational diabetes: a reappraisal of HBA1c as a screening test. Acta Obstet Gynecol Scand 2005;84:1159–1163 [DOI] [PubMed] [Google Scholar]
- 9.Agarwal MM, Dhatt GS, Punnose J, Koster G: Gestational diabetes: a reappraisal of HBA1c as a screening test. J Womens Health (Larchmt) 2008;17:1183–118718774897 [Google Scholar]
- 10.Lurie S, Mamet Y: Red blood cell survival and kinetics during pregnancy. Eur J Obstet Gynecol Reprod Biol 2000;93:185–192 [DOI] [PubMed] [Google Scholar]
- 11.Schrader HM, Jovanovič-Peterson L, Bevier WC, Peterson CM: Fasting plasma glucose and glycosylated plasma protein at 24 to 28 weeks of gestation predict macrosomia in the general obstetric population. Am J Perinatol 1995;12:247–251 [DOI] [PubMed] [Google Scholar]
- 12.Jovanovič-Peterson L, Bevier W, Peterson CM: The Santa Barbara County Health Care Services Program: birth weight change concomitant with screening for and treatment of glucose-intolerance of pregnancy: a potential cost-effective intervention. Am J Perinatol 1997;14:221–228 [DOI] [PubMed] [Google Scholar]
- 13.Bevier WC, Fischer R, Jovanovič L: Treatment of women with an abnormal glucose challenge test (but a normal oral glucose tolerance test) decreases the prevalence of macrosomia. Am J Perinatol 1999;16:269–275 [DOI] [PubMed] [Google Scholar]
- 14.Bode BW, Irvin BR, Pierce JA, Allen M, Clark AL: Advances in hemoglobin A1c point of care technology. J Diabetes Sci Technol 2007;1:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamborlane WV, Kollman C, Steffes MW, Ruedy KJ, Dongyuan X, Beck RW, Chase P, Fox LA, Wilson DM, Tsalikian EDiabetes Research in Children Network (DirecNet) Study Group Comparison of fingerstick hemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: results of a Diabetes Research in Children Network (DirecNet) Study. Pediatr Diabetes. 2005;6:13–16 [DOI] [PubMed] [Google Scholar]