Abstract
OBJECTIVE
To compare the effectiveness of a telephonic and a print intervention over 1 year to improve diabetes control in low-income urban adults.
RESEARCH DESIGN AND METHODS
A randomized trial in Spanish and English comparing a telephonic intervention implemented by health educators with a print intervention. Participants (N = 526) had an A1C ≥7.5% and were prescribed one or more oral agents. All were members of a union/employer jointly sponsored health benefit plan. Health coverage included medications. Primary outcomes were A1C and pharmacy claims data; secondary outcomes included self-report of two medication adherence measures and other self-care behaviors.
RESULTS
Participants were 62% black and 23% Hispanic; 77% were foreign born, and 42% had annual family incomes <$30 thousand. Baseline median A1C was 8.6% (interquartile range 8.0–10.0). Insulin was also prescribed for 24% of participants. The telephone group had mean ± SE decline in A1C of 0.23 ± 0.11% over 1 year compared with a rise of 0.13 ± 0.13% for the print group (P = 0.04). After adjusting for baseline A1C, sex, age, and insulin use, the difference in A1C was 0.40% (95% CI 0.10–0.70, P = 0.009). Change in medication adherence measured by claims data, but not by self-report measures, was significantly associated with change in A1C (P = 0.01). Improvement in medication adherence was associated (P = 0.005) with the telephonic intervention, but only among those not taking insulin. No diabetes self-care activities were significantly correlated with the change in A1C.
CONCLUSIONS
A 1-year tailored telephonic intervention implemented by health educators was successful in significantly, albeit modestly, improving diabetes control compared with a print intervention in a low-income, insured, minority population.
Improving glycemic control in type 2 diabetes significantly decreases the risk of serious chronic complications such as retinopathy, neuropathy, and nephropathy, as shown by large-scale clinical trials from the last 2 decades (1,2). These studies, along with smaller trials, set the stage for evidence-based medical management of diabetes (3). Although effective therapies for management have been developed, treatment goals are often not reached—especially in lower income and minority populations (4)—and many individuals find it challenging to perform routine self-management (5). Critical reviews of the scientific literature on interventions to improve glycemic control show promising results for improved processes of care, such as screening for complications and laboratory tests, as well as for behavioral interventions and self-management training (6,7).
Evidence is emerging for the use of telephonic interventions to improve diabetes self-care and health outcomes; studies include use of automated calls with nurse follow-up (8) or calls implemented by individuals with graduate degrees (9). Telephonic interventions may enhance self-care adherence (10) by offering the opportunity to customize information to individuals under real-world conditions. Nonetheless, the efficacy of telephonic interventions in all populations and settings has not been established, and improvements in health outcomes for patients remain challenging even with many new pharmaceutical agents becoming available and combinations of type 2 diabetes medications becoming a standard of care.
As an adjunct to diabetes self-management education and medical care, a telephonic intervention by health educators may provide the coaching and motivation needed for individuals to perform diabetes self-management activities over time, especially medication adherence. The Improving Diabetes Outcomes (I DO) study aimed to evaluate the incremental effect of a tailored telephone intervention, in English and Spanish, on the mean A1C levels and medication adherence beyond that achieved with the mailing of print self-management materials. The population is insured, lower-income, mostly minority individuals who had health care and medication benefits covered in full by their labor union/employer plan. However, the study protocol allowed only telephonic and print contact with participants so that individuals who might not have agreed to participate in more conventional in-person studies could take part. The main study outcomes were changes in A1C and medication adherence. The study also sought to determine what demographic and behavioral factors might mediate the effect of the interventions. We now report the main results of this translational randomized controlled behavioral trial, including self-care data to more fully explain the results.
RESEARCH DESIGN AND METHODS
The I DO study is a randomized controlled behavioral intervention study comparing the effectiveness of a telephonic intervention with a print (active control) intervention. It was developed at the Einstein Diabetes Research and Training Center in collaboration with a union/employer jointly sponsored health benefit plan (1199SEIU Benefit and Pension Funds). As previously described (11), eligible participants were adult (≥30 years of age) members of the health care worker union Fund based in New York City. These Fund members include current full-time health workers or their spouses. The majority of members are service and clerical workers in nursing homes or hospitals, and others work as home health attendants. The Fund provides full coverage of prescription medications, medical visits, hospitalizations, and laboratory tests. Eligible participants had to read and speak English or Spanish, with no evidence of cognitive impairment. Eligibility also included the prescription of at least one oral glucose-lowering agent (OGLA) in the year prior to enrollment. The eligible A1C was ≥7.5%, which is above the usual management goal of <7% (3), but would provide a margin for lowering the A1C in a telephonic intervention with no in-person contact without raising safety concerns.
The study protocol aimed to evaluate interventions among individuals who might face challenges in completing in-person diabetes self-management education programs. It did not include any face-to-face interactions. Oral informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization were obtained by telephone with approval of the institutional review board of the Albert Einstein College of Medicine.
There was a two-step recruitment process. The Fund database was used to identify members who might be eligible, and they were telephoned by study staff. If a person seemed eligible and completed a screening questionnaire, oral informed consent was documented. The second step was the mailing and completion of an A1C capillary blood test kit. Individuals with lab results of A1C ≥7.5% were enrolled and randomized using a computerized randomization scheme to either the telephone or the print intervention group.
Interventions
All telephone participants could receive up to 10 calls at 4- to 6-week intervals from their health educator over the 1-year intervention. Health educators were trained and supervised by a certified diabetes educator nurse. Calls were tailored to the participant-reported needs but focused primarily on diabetes medication adherence and secondarily on lifestyle changes through healthy eating and physical activity. Problem solving (12), goal setting (13), communication skills, and preplanning for medical visits were important elements in the intervention. The protocol was based on improving empowerment and self-efficacy (14) using social-ecological approaches (15). Health educators used a manual to guide the telephone call content, but participants were encouraged to choose topics for each call. See the online appendix supplementary Table A1 (available at http://care.diabetesjournals.org/cgi/content/full/dc10-1005/DC1) for an example of a call log that both guided and documented implementation of the intervention. All participants received selected high-quality self-management materials by mail after randomization. Only telephone participants were prompted by health educators to use these materials.
Measures
The primary outcome was change in A1C, measured only at baseline and postintervention using mail-in kits with “filter paper” methodology (also called “dry-dot”) from a laboratory vendor, Home Healthcare Laboratory of America (“Lab-in-an-Envelope”) (16). This A1C test processed with a Roche analyzer had been approved by the National Glycosylation Standardization Program (17). Participants were asked to call the health educator to guide them through the blood sampling while using a spring-loaded lancet to draw blood from their fingertips and fill in one to three circles (1.2 cm diameter) on a special filter paper card. This card was then mailed directly to the laboratory in a prepaid envelope for analysis. A1C values from the filter paper method have been reported to correspond to those obtained by conventional venous whole-blood samples (18,19). If insufficient blood was obtained for a valid result, another test kit was sent to participants.
Medication adherence measures
Pharmacy claims (i.e., administrative) data from the Fund, including each OGLA prescription filled, its class, the date, number of pills dispensed, and number of pills per day, were used to calculate a medication possession ratio (MPR) for each participant. This type of measure of medication adherence has been used in many studies (20,21). For each class of OGLA taken by a participant within the previous year, the number of pill-days available from each filled prescription was calculated. For each participant, MPRs (number of days' supply of pills dispensed in 1 year/365) for the 1 year prior to randomization (baseline) and 1 year post randomization (follow-up) were calculated (range 0–1) for each OGLA class, and then an average of the class MPRs was used to denote separately the participant's pre- and postintervention MPR. The methods and rationale for this approach have been previously described (11). A recording of insulin use during the study year was categorized “ever” or “never” on the basis of prescription orders for any insulin product.
Other diabetes self-management behaviors were collected by telephone at baseline and end of study. The four-item Morisky Self-Reported Medication-Taking Scale (22) was administered, and scores ≤2 were considered poor adherence to diabetes medications. The Summary of Diabetes Self Care Activities (SDSCA) (23) scale was also administered, including a single medication adherence item: How many days in the most recent week were diabetes pills taken as prescribed? This was treated as a nonparametric continuous variable (0–7 days) and categorized as adherent (7 days) or not. Other SDSCA survey items addressed healthy eating and physical activity and were analyzed similarly. Hours of TV watching per day were recorded in categories (0, 1, 2, 3, 4, >4 h) and dichotomized as <2 or ≥2 h per day. Self-reported demographics including sex, age, race/ethnicity, work status, marital status, income, education, and birthplace were collected, as were other characteristics including self-reported height and weight for calculating BMI, years since diabetes diagnosis, and insulin use in the previous year.
Statistical analysis
The study outcomes, change in A1C (ΔA1C) and change in MPR (ΔMPR), were calculated as follow-up minus baseline values (negative values represent a decline) and were assessed for normality assumptions. ΔMPR was also dichotomized as ≥20 percentage points (e.g., going from 60 to 80%) because very small changes were not expected to have a meaningful impact on A1C. Changes in SDSCA during follow-up were also calculated both as continuous variables (days) and categorized as improved, worsened, or remained the same. Tests of bivariate associations with study arm were performed similarly to the comparison of baseline characteristics. Analyses of ΔA1C and ΔMPR outcomes were always adjusted for baseline levels. Adjustments for potential confounders or mediators were performed using linear regression models for continuous outcomes and binary logistic models for dichotomous outcomes. To test potential mediation, baseline MPR and ΔMPR ≥20% were added to the model predicting ΔA1C. The number of educator calls received by participants in the telephone group was used as a proxy for intensity of the intervention. Among those in the telephone group, the number of calls completed during the intervention (range 0–10) was categorized as 0–5, 6–8, and 9–10, and these were entered into regression models as dummy variables with print group allocation as reference. A test for trend of the association of these call categories with ΔA1C was also performed. Baseline values of the outcome variables were available as an inclusion criterion prior to randomization, but not all participants provided follow-up data. Outcome analyses were performed for those with complete data with sensitivity analyses using two alternate imputation methods to simulate intention-to-treat analyses. Imputation for missing outcome data were carried out with STATA (version 11) multiple imputation procedure based on a Bayesian paradigm pooling 100 repeated imputations taking into account baseline A1C, age, sex, insulin use, and baseline MPR. An alternate imputation used baseline A1C values for missing follow-up that in this study was the same as a last observation carried forward (LOCF) approach (24). Those with missing outcome data were compared by study arm to assess assumptions of missing at random. Residuals-based regression diagnostics were performed to check linear regression model assumptions, and first-order interactions of covariates with study arm were tested with interaction product terms while simultaneously adjusting for main effects terms. Hosmer-Lemeshow test for goodness-of-fit was performed for binary logistic models and first-order interactions were assessed.
RESULTS
The study flow diagram is in online appendix as supplementary Figure A1; it shows the database recruitment pool of 8,083 adults with diabetes taking OGLAs. Of the 4,548 individuals assessed for eligibility, 4,021 were excluded (ineligible 55%, refused 45%), and 527 individuals were randomized, with intention-to-treat analysis of 526 cases. Description of baseline characteristics by group and total is found in Table 1. Participants were mainly minority in terms of race/ethnicity, and were lower-income, middle-aged, and foreign born. The median baseline A1C was 8.6% (interquartile range 8.0–10).
Table 1.
Participant characteristics at baseline
| Telephone group | Print group | Total | |
|---|---|---|---|
| n | 262 | 264 | 526 |
| Female (%) | 68.3 | 65.9 | 67.1 |
| Race/ethnicity (%) | |||
| Black | 61.5 | 61.7 | 61.6 |
| Hispanic | 24.8 | 20.5 | 22.6 |
| White | 5.7 | 6.1 | 5.9 |
| Other | 8.0 | 11.7 | 9.9 |
| Age (years) | 55.7 ± 7.4 | 55.4 ± 7.2 | 55.5 ± 7.3 |
| Married (%) | 59.2 | 63.6 | 61.4 |
| Foreign born* (%) | 75.2 | 78.4 | 76.8 |
| Spanish preferred (%) | 18.7 | 12.9 | 15.8 |
| Duration of diabetes (years) | 8.8 ± 6.8 | 9.5 ± 6.4 | 9.2 ± 6.6 |
| Duration of diabetes (%) | |||
| <6 years | 37.0 | 30.7 | 33.8 |
| 6–10 years | 33.2 | 34.8 | 34.0 |
| >10 years | 29.8 | 34.5 | 32.1 |
| Employed full time (%) | 73.3 | 74.6 | 74.0 |
| Household income (%) | |||
| <$20,000 | 17.2 | 14.4 | 15.8 |
| $20–29,000 | 26.7 | 26.5 | 26.6 |
| $30–39,000 | 29.0 | 29.2 | 29.1 |
| $40–49,000 | 10.7 | 9.1 | 9.9 |
| ≥$50,000 | 16.4 | 20.8 | 18.6 |
| Education (%) | |||
| ≤8th grade | 16.4 | 16.7 | 16.5 |
| 9–11th grade | 11.8 | 10.2 | 11.0 |
| HS or GED | 36.3 | 28.8 | 32.5 |
| Some college | 22.1 | 26.1 | 24.1 |
| ≥College | 13.4 | 18.2 | 15.8 |
| Self-reported insulin use (%) | 21.0 | 25.0 | 23.0 |
| Insulin Rx in last year (%) | 23.3 | 24.6 | 24.0 |
| >2 diabetes pill classes (%) | 68.7 | 68.2 | 68.4 |
| BMI (kg/m2) | 31.8 ± 6.2 | 30.7 ± 6.0 | 31.2 ± 6.1 |
| A1C (%) | 8.6 (8.0–9.6) | 8.7 (8.0–10.2) | 8.6 (8.0–10.0) |
| Morisky scale ≤2 (%) | 35.1 | 38.6 | 36.9 |
| Report taking diabetes pills <7 days per week (%) | 27.9 | 25.4 | 26.6 |
Data are means ± SD or median (interquartile range).
*Foreign born does not include those born in Puerto Rico. GED, high school equivalency; HS, high school; Rx, prescription.
Primary outcomes
Among the 444 participants (84.4%) with follow-up A1C, the 228 in the telephone group exhibited a mean ± SE decline in A1C of 0.23 ± 0.11% over the study year compared with a rise of 0.13 ± 0.13% for the 216 in the print group (P = 0.04). After adjusting for baseline A1C, sex, age, and insulin use, the difference in ΔA1C between telephone and print groups was 0.40% (95% CI 0.10–0.70, P = 0.009). There was no strong evidence for mediation of the ΔA1C by ΔMPR. With regard to the ΔA1C outcome, no statistically significant first order interactions with intervention group were observed.
When ΔMPR was assessed as an outcome variable, whether as a continuous variable or as ≥20% improvement, statistically significant (P = 0.04 and 0.01, respectively) interactions of intervention with insulin use (n = 141, 26.8%) during the 12-month study period were observed. ΔMPR as a continuous variable was not significantly associated with the telephone intervention either among those taking (P = 0.23) or not taking (P = 0.39) insulin, whereas ΔMPR ≥20% was significantly associated (P = 0.005) with the telephone intervention after adjusting for baseline MPR, age, and sex among those not taking insulin, but not among those taking insulin (P = 0.28). Among those not taking insulin, there was a significant (P = 0.001) linear trend with ΔMPR ≥20% for the numbers of intervention calls received. Significant associations with intervention calls compared with print were only observed for those receiving at least six telephone calls (Table 2).
Table 2.
Adjusted odds ratios for change in MPR ≥20% stratified by insulin use during study
| No insulin use (n = 385) |
Insulin use (n = 141) |
|||
|---|---|---|---|---|
| OR (95% CI)* | P | OR (95% CI)* | P | |
| Reference | Reference | |||
| 0–5 Calls† | 1.0 (0.4–2.8) | 0.98 | 0.3 (0.0–2.8) | 0.29 |
| 6–8 Calls | 1.9 (1.0–3.5) | 0.04 | 0.6 (0.2–2.1) | 0.41 |
| 9–10 Calls | 2.6 (1.4–4.6) | 0.002 | 0.4 (0.3–2.2) | 0.61 |
| Call linear trend | 0.001 | 0.88 | ||
| Baseline MPR | 0.04 (0.02–1.3) | <0.001 | 0.01 (0.001–0.10) | <0.001 |
| Age (years) | 1.0 (1.0–1.0) | 0.81 | 0.9 (0.9–1.0) | 0.03 |
| Male | 0.9 (0.6–1.6) | 0.81 | 1.0 (0.4–2.6) | >0.99 |
| Telephone | 2.0 (1.2–3.2) | 0.005 | 0.6 (0.3–1.5) | 0.28 |
*Odds ratio (OR) (95% CI) estimated with binary logistic regression models.
†Call categories for the telephone intervention with print as reference. Linear trend is across the categories. Telephone gives the overall odds ratio (irrespective of number of calls) with print as reference, estimated in separate adjusted models.
Secondary outcomes
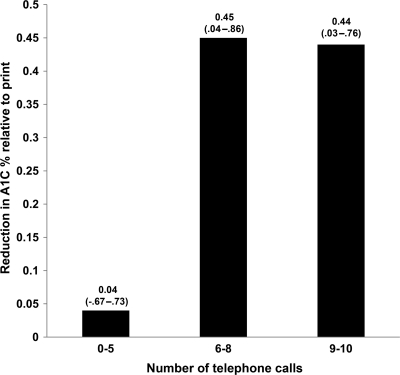
Attempts were made to complete 10 phone calls over 12 months to telephone participants (mean ± SD number of completed calls was 7.9 ± 2.1). Fewer phone calls resulted from participants being unreachable or refusing the telephone call. Only 3% (n = 7) of participants had no phone calls even after much staff effort. Mean length of each call was 14.1 ± 4.6 min. Having at least six completed phone calls was associated with significant improvement in A1C (Fig. 1).
Figure 1.
Decline in A1C, expressed as median (interquartile range), per category of telephone intervention intensity (number of calls) compared with print group (no calls), estimated in a multiple linear regression model adjusting for baseline A1C, age, sex, insulin use, and improvement in MPR ≥20%.
Table 2 highlights the differences between those who took insulin and at least one OGLA and those who took only an OGLA. The telephone intervention was not associated with a change in medication adherence (≥20% MPR) if the regimen included insulin. Despite the lack of a statistically significant association of ΔMPR with the intervention among the minority taking insulin and an OGLA, for the group as a whole the multivariable model provided evidence that the improvement of MPR was a mediator of the intervention association with improved glycemic control.
From the SDSCA survey, two items showed significant improvement associated with the telephone intervention: number of days per week following a healthy eating plan and number of days with ≥30 min of exercise. The other items, as well as hours of TV watched per day, showed a direction toward improvement associated with the telephone intervention, but not significantly so. However, none of the changes in SDSCA or TV watching were significantly correlated with ΔA1C. Although ΔMPR derived from pharmacy claims data were significantly (P = 0.01) associated with ΔA1C in the adjusted model, changes in the two self-report medication adherence measures (number of days taking medication as prescribed item from the SDSCA and the Morisky score) were not significantly associated with ΔA1C.
Missing values and analysis with imputation
Of the 526 randomized participants, follow-up A1C values were not available for 15.6% (18.2% telephone, 13.0% print, P = 0.10). Of the 82 with missing values, 5 (2 deaths and 3 withdrawals) also had missing values for the follow-up MPR. For all the baseline characteristics in Table 1, there were no statistically significant associations with those missing a follow-up A1C among the print group; there was a single significant association in the telephone group, with those missing an A1C being (mean ± SE) 3.3 ± 1.3 years younger than those not missing an A1C. Median baseline A1C was 0.6% higher for those missing in the print group (P = 0.07), and in the telephone group the difference in median was 0.2% (P = 0.54). Using the multiple imputation approach, being in the telephone group compared with print group was significantly associated with greater decline in A1C, whether adjusting only for baseline A1C or also adjusting for age, sex, insulin use, and ΔMPR (both P = 0.03). These significant associations were also seen (both P = 0.01) when the LOCF imputation approach was used.
CONCLUSIONS
A tailored telephonic behavioral intervention implemented by health educators under the supervision of a certified diabetes educator nurse was successful in significantly, albeit modestly, improving A1C compared with a print intervention. Greater intensity of the intervention (≥6 calls) was associated with greater improvement in A1C.
A possible explanation for the reported differences in intervention effectiveness for medication adherence related to insulin use (Table 2) may be that being prescribed insulin in combination with an OGLA is a regimen complexity that reduces adherence to the OGLAs. An alternative explanation may be that nonclinical health educators, though supervised by a nurse certified diabetes educator, may not have been as effective in medication adherence counseling for participants also on various insulin regimens as they were with those on OGLAs alone.
Only a few self-care activity changes on the SDSCA were significantly associated with the intervention. It is possible, however, that there was an overall cumulative effect on glycemic control of small improvements in multiple self-care activities, even if they were individually too small to show significant associations with the intervention.
Limitations
The dry-dot methodology for the A1C measure completed by the participant and mailed to the laboratory had its own limitations, which were imposed by the nature of the protocol to not require subject visits to a lab or research center. This A1C methodology may contribute to greater measurement variability. In this randomized trial, it would not be expected to introduce a differential bias; and if a nondifferential bias were introduced for the change in A1C, it would be more likely toward the null. Not all patients completed the end of protocol survey or final A1C assessment. Although the final A1C was unobtainable for 15.6% of participants, this may be expected because we had no in-person contact with them. However, this did not impact the ΔMPR outcomes that were available administratively for all but five participants. Further, we used two alternate methods of imputation for an intention-to-treat analysis and both were consistent with results for those with complete data. A modest number of participants in the intervention group had fewer than six calls. The observation that only those with ≥6 calls over 1 year had statistically significant though modest improvements in glycemic control adds to our confidence that the mechanism of the calls, and not type 1 error, was responsible for the difference between the telephone and print group outcomes. However, those accepting more calls may be more amenable to change, which could possibly confound these results.
Strengths
This study explored the comparative effectiveness of two interventions in a lower-income, urban population that was racially and ethnically diverse; the majority were lower-income immigrants working in support of health-care systems. They were homogeneous, however, in that they did not have economic barriers to securing medications or medical visits because of their union/employer-sponsored health benefits. The sample was drawn from those with evidence of difficulty managing their diabetes; they were individuals who often, because of life circumstances, are unlikely to volunteer for a study requiring them to visit a research center. Therefore, a strength of this study is that we may have avoided selection bias.
Evidence supports diabetes self-management education having greater success in health outcomes when it is maintained over a longer period of time (25). A telephone intervention may be a convenient and feasible intervention to support those who have difficulty accessing diabetes self-management education. This intervention could be more successful in improving A1C if embedded in either provider or payer models, especially if synergistic with other targeted quality improvement initiatives. In the context of current related literature (5–9), this study provides a successful model of an intervention delivering self-management support at lower cost than studies using licensed health professionals or more intensive interventions, such as in-person or those having greater frequency of contact. This study extends previous research because it focused on a population with known health disparities. Health educators trained and supervised by a certified diabetes educator may promote and maintain self-management skills and provide crucial support needed by individuals managing their diabetes.
Acknowledgments
This study was supported by NIH grants R18 DK62038 and DK020541.
No potential conflicts of interest relevant to this article were reported.
E.A.W. and H.W.C. contributed to every aspect of this article. C.S., M.S.-K., and R.U. contributed to study design, discussion, research data, and editing of the manuscript. E.B. contributed to the research data, discussion, and review and editing of the manuscript.
Parts of this study were presented in oral form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
We gratefully acknowledge the data management contributions of Fionnuala King of the 1199SEIU Benefit and Pension Funds; our talented health educators, including Giovanna DiFrancesca, Kathleen McCabe, Gisela Mojica, Jennifer Case, Tara DeWitt, Gabriel Ferreira, Marlene Taveras, Samara Lipsky, Dr. Hollie Jones, and Hector Cariello; data management support from Maria Kalten and Jennifer Lukin, all staff from the Albert Einstein College of Medicine; and especially, the participants in New York City who volunteered for our study.
Footnotes
Clinical trial reg. no. NCT00179374, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying editorial, p. 240.
References
- 1.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 2.ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes–2010. Diabetes Care 2010;33:S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS: Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med 2009;122:443–453 [DOI] [PubMed] [Google Scholar]
- 5.Norris SL, Engelgau MM, Narayan KM: Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001;24:561–587 [DOI] [PubMed] [Google Scholar]
- 6.Vermeire E, Wens J, Van Royen P, Biot Y, Hearnshaw H, Lindenmeyer A: Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;2:CD003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark M: Diabetes self-management education: a review of published studies. Primary Care Diabetes 2008;2:113–120 [DOI] [PubMed] [Google Scholar]
- 8.Piette JD, Weinberger M, Kraemer FB, McPhee SJ: Impact of automated calls with nurse follow-up on diabetes treatment outcomes in a Department of Veterans Affairs Health Care System: a randomized controlled trial. Diabetes Care 2001;24:202–208 [DOI] [PubMed] [Google Scholar]
- 9.Wolever RQ, Dreusicke M, Fikkan J, Hawkins TV, Yeung S, Wakefield J, Duda L, Flowers P, Cook C, Skinner E: Integrative health coaching for patients with type 2 diabetes: a randomized clinical trial. Diabetes Educ 2010;36:629–639 [DOI] [PubMed] [Google Scholar]
- 10.Walker EA, Schechter CB, Caban A, Basch CE: Telephone intervention to promote diabetic retinopathy screening among the urban poor. Am J Prev Med 2008;34:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen HW, Shmukler C, Ullman R, Rivera CM, Walker EA: Measurements of medication adherence in diabetic patients with poorly controlled HbA(1c). Diabet Med 2010;27:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill-Briggs F: Problem solving in diabetes self-management: a model of chronic illness self-management behavior. Ann Behav Med 2003;25:182–193 [DOI] [PubMed] [Google Scholar]
- 13.Bodenheimer T, Handley MA: Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns 2009;76:174–180 [DOI] [PubMed] [Google Scholar]
- 14.Bandura A: Self-Efficacy: The Exercise of Control. New York, W.H. Freeman and Company, 1997 [Google Scholar]
- 15.Stokols D: Translating social ecological theory into guidelines for community health promotion. Am J Health Promot 1996;10:282–298 [DOI] [PubMed] [Google Scholar]
- 16.Lab in an Envelope [Internet]. Home Healthcare Laboratories of America. Availabe from http://www.hhla.com/products_services_lab_in_an_envelope.html Accessed 5 May 2010
- 17.National Glycosylation Standardization Program. [Accessed 5 May 2010]. Available from http://www.ngsp.org.
- 18.Jeppsson JO, Jerntorp P, Almër LO, Persson R, Ekberg G, Sundkvist G: Capillary blood on filter paper for determination of HbA1c by ion exchange chromatography. Diabetes Care 1996;19:142–145 [DOI] [PubMed] [Google Scholar]
- 19.Gay EC, Cruickshanks KJ, Chase HP, Klingensmith G, Hamman RF: Accuracy of a filter paper method for measuring glycosylated hemoglobin. Diabetes Care 1992;15:108–110 [DOI] [PubMed] [Google Scholar]
- 20.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE: Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care 2004;27:2800–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC: An empirical basis for standardizing adherence measures derived from administrative claims data among diabetic patients. Medical Care 2008;46:1125–1133 [DOI] [PubMed] [Google Scholar]
- 22.Morisky DE, Green LW, Levine DM: Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74 [DOI] [PubMed] [Google Scholar]
- 23.Toobert DJ, Hampson SE, Glasgow RE: The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 24.StataCorp Multiple-Imputation Reference Manual. Release 11 College Station, Texas, Stata Press, 2009 [Google Scholar]
- 25.Funnell MM, Brown TL, Childs BP, Haas LB, Hosey GM, Jensen B, Maryniuk M, Peyrot M, Piette JD, Reader D, Siminerio LM, Weinger K, Weiss MA: National standards for diabetes self-management education. Diabetes Care 2007;30:1630–1637 [DOI] [PubMed] [Google Scholar]