Abstract
OBJECTIVE
Intramyocellular acetylcarnitine (IMAC) is involved in exercise-related fuel metabolism. It is not known whether levels of systemic glucose influence IMAC levels in type 1 diabetes.
RESEARCH DESIGN AND METHODS
Seven male individuals with type 1 diabetes performed 120 min of aerobic exercise at 55–60% of Vo2max randomly on two occasions (glucose clamped to 5 or 11 mmol/l, identical insulinemia). Before and after exercise, IMAC was detected by 1H magnetic resonance spectroscopy in musculus vastus intermedius.
RESULTS
Postexercise levels of IMAC were significantly higher than pre-exercise values in euglycemia (4.30 ± 0.54 arbitrary units [a.u.], P < 0.001) and in hyperglycemia (2.44 ± 0.53 a.u., P = 0.01) and differed significantly according to glycemia (P < 0.01). The increase in exercise-related levels of IMAC was significantly higher in euglycemia (3.97 ± 0.45 a.u.) than in hyperglycemia (1.71 ± 0.50 a.u.; P < 0.01).
CONCLUSIONS
The increase in IMAC associated with moderate aerobic exercise in individuals with type 1 diabetes was significantly higher in euglycemia than in hyperglycemia.
Intramyocellular acetylcarnitine (IMAC) is involved in the regulation of fat and carbohydrate oxidation in skeletal muscle during moderate aerobic exercise (1). Although carnitine metabolism appears comparable in patients with type 1 diabetes and healthy control subjects (2), it is not known whether IMAC is related to variations in fuel metabolism observed during exercise under differing glycemic levels in type 1 diabetes (3). Given the controversial results from previous studies linking IMAC to increased β-oxidation (4) but also to high glycolytic flux (1), the aim of the present study was to assess exercise-related concentrations of IMAC noninvasively by 1H magnetic resonance spectroscopy (1H MRS) (5,6) in patients with type 1 diabetes in euglycemia and hyperglycemia.
RESEARCH DESIGN AND METHODS
This was a randomized single-blind crossover study (3). Seven physically active individuals with type 1 diabetes without relevant diabetes-associated complications were investigated (mean ± SEM age 33.5 ± 2.4 years, diabetes duration 20.1 ± 3.6 years, A1C 6.7 ± 0.2%, BMI 24.3 ± 0.4 kg/m2, and Vo2max 50.3 ± 4.5 ml · min−1 · kg−1). All were treated with continuous subcutaneous insulin infusion (insulin dose 0.61 ± 0.05 units · kg−1 · day−1). Participants performed 120 min of exercise at 55–60% of Vo2max on two occasions in random order with glucose clamped at 5.4 ± 0.5 mmol/l (euglycemia) or 11.0 ± 0.3 mmol/l (hyperglycemia). Insulin infusion (7 mU · m−2 · min−1, range 5–8.2) was equal during both conditions (3). Muscle spectra were obtained with short echo time 1H MRS from musculus vastus intermedius 81 ± 2 and 83 ± 2 min after exercise in euglycemia and hyperglycemia, respectively (NS). Acquisition parameters were clinical 1.5-T scanner (GE Medical Systems, Waukesha WI), localized volume of 11 × 12 × 18 mm3, point-resolved spectroscopy localization, repetition time 3 s, echo time 20 ms, two spectra of 128 acquisitions, and water presaturation. Spectra were analyzed using TDFDFit (7) with a fit strategy optimized for the acetylcarnitine-peak. Acetylcarnitine content is expressed in absolute arbitrary units (a.u.) relative to the water signal intensity. Parameters are expressed as means ± SEM (median and range where appropriate). All analyses were performed with Stata (version 10.0; StataCorp, College Station, TX).
RESULTS
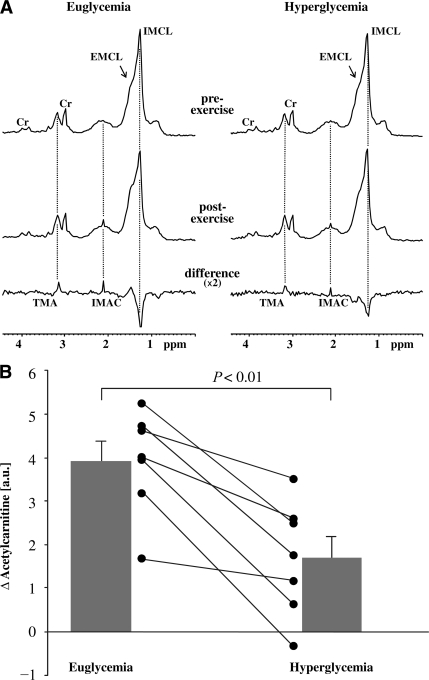
Figure 1A displays the summed spectra before and after exercise and corresponding differences with the acetyl peak of IMAC at 2.13 ppm. Pre-exercise levels of IMAC did not differ significantly according to the glycemic levels (0.33 ± 0.21 and 0.73 ± 0.33 a.u. for euglycemia and hyperglycemia, respectively; NS). Postexercise levels of IMAC were significantly higher than pre-exercise values in euglycemia (4.30 ± 0.54 a.u.; P < 0.001) and in hyperglycemia (2.44 ± 0.53 a.u.; P = 0.01). In addition, postexercise levels differed significantly according to glycemia (P < 0.01). As shown in Fig. 1B, this translated into a significantly higher increase of exercise-related levels of IMAC in euglycemia (3.97 ± 0.45 a.u.) compared with that in hyperglycemia (1.71 ± 0.50 a.u.; P < 0.01).
Figure 1.
A: 1H magnetic resonance spectra were obtained from musculus vastus intermedius before and after 120 min of moderate-intensity exercise in euglycemia or hyperglycemia. Summed spectra are portrayed for pre- and postexercise. The difference spectra show positive peaks for the acetyl group of IMAC at 2.13 ppm and its trimethylammonium (TMA) group at 3.2 ppm, as well as negative contribution from intramyocellular lipids (IMCL) (reduced lipid levels after exercise). The trimethylammonium resonance is not used in the present work because, as reported earlier (5), its difference peak does not readily scale in intensity with the acetylcarnitine peak, probably related to differences in molecular properties. Cr, methyl and methylene peaks of creatine/phosphocreatine. Note that in the summed spectra, differentiation between extramyocellular lipids (EMCL) and intramyocellular lipids is difficult, whereas all analyses were done in individual spectra. B: Exercise-related differences in IMAC levels in euglycemia and hyperglycemia. Black lines reflect individual results; bars reflect mean ± SEM. Acetylcarnitine content is expressed in absolute arbitrary units relative to the water signal obtained from separate reference scans. P represents the comparison of differences in euglycemia vs. hyperglycemia.
CONCLUSIONS
In the present study, levels of IMAC were assessed noninvasively in skeletal muscle of individuals with type 1 diabetes in euglycemia and hyperglycemia before and after moderate aerobic exercise. The principal finding was a significantly higher increase in IMAC in euglycemia than in hyperglycemia.
The effect of euglycemia and hyperglycemia on exercise-related substrate oxidation has also been assessed in healthy individuals (8), however, without determination of IMAC. Still, previous reports do not suggest genuinely different levels of acylated carnitine in patients with type 1 diabetes compared with nondiabetic control subjects (2). Conversely, a recent study in patients with type 1 diabetes investigated levels of IMAC during exercise in euglycemia but with differing insulin levels (9). The authors reported a similar increase in IMAC in euglycemia and hyperinsulinemia, implying that the flux through the pyruvate dehydrogenase complex (PDC) is independent of insulin concentrations (9). Taken together, these results indicate that in individuals with type 1 diabetes, levels of IMAC are influenced by the availability of energy substrates, in particular systemic glucose, whereas levels of insulin seem to have less influence. Of note, IMAC has been shown to increase after a few minutes of high-intensity exercise (10). This result has been hypothesized to be due to the production of acetyl-CoA exceeding its utilization by the Krebs cycle, the acetyl groups consequently being transferred to carnitine by carnitine acetyltransferase, which results in increases in IMAC as well as in free CoA (1). Although IMAC may thus reflect the imbalance of the fluxes through the PDC and the Krebs cycle in high-intensity exercise, its role during exercise at lower intensity or at rest is less clear. Interestingly, previous studies in healthy individuals (4) have suggested an association of IMAC with increased β-oxidation. Compatible with these findings, the present study revealed a higher contribution of net lipid oxidation to overall substrate oxidation in euglycemia than in hyperglycemia together with a tendency toward increased consumption of intramyocellular lipids in euglycemia (data published in ref. 3). Whether this association is due to a direct effect of substrate fluxes (e.g., increased glucose flux in hyperglycemia compared with euglycemia) on fuel metabolism or whether indirect regulation of key enzymes (PDC and carnitine palmitoyltransferase 1) were involved remains speculative because noninvasive determination of IMAC precluded further analysis of intramyocellular metabolic pathways in the present study.
The strength of the present analysis is its strictly controlled design with stable and identical insulin levels, systemic glucose being the sole variable that has been modified. The use of MRS allowed for a repetitive, noninvasive measurement of IMAC, although technical differences limit the direct comparability of IMAC levels in the present study with results from muscle biopsies. We acknowledge that our results are limited by the small sample size. However, all individuals in this study showed similar dynamics of IMAC according to glycemia. Because of the study design, magnetic resonance spectra could not be obtained directly after completion of the exercise. However, previous studies have shown that postexercise levels of IMAC are remarkably stable for similar time periods (6). The present study was performed in male individuals with type 1 diabetes exclusively, and the complex study design along with the limited resources did not allow us to include a control group of healthy individuals. Statements referring to a more general population thus clearly remain restrictive.
In summary, in individuals with well-controlled type 1 diabetes, an exercise-related increase in IMAC was detected in both glycemic conditions, but the effect was significantly higher in euglycemia than in hyperglycemia. These findings point to a role of IMAC as a marker of exercise-related fuel metabolism in skeletal muscle.
Acknowledgments
This study was supported by unrestricted grants from the Oetliker Foundation for Physiology; the Swiss Diabetes Foundation; Novo Nordisk, Switzerland; and the Swiss National Science Foundation (grants 3233B0-115212 and 320000-109522/1 to C.S., grant 320000-109522/1 to E.C., and grant PBBEP3-125600 to S.J.).
No other potential conflicts of interest relevant to this article were reported.
A.B. researched data and wrote the manuscript. R.K. and C.S. designed the study, researched data, and wrote the manuscript. S.J. researched data and performed statistical analysis. M.I. and J.-M.N. researched data. E.C. and C.B. researched data and contributed to the manuscript.
We are grateful to the study participants and for the assistance of Sabin Allemann, Institute for Social and Preventive Medicine Bern, Bern, Switzerland.
Footnotes
Clinical trial reg. no. NCT00325559, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Stephens FB, Constantin-Teodosiu D, Greenhaff PL: New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol 2007;581:431–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamoulakis D, Galanakis E, Dionyssopoulou E, Evangeliou A, Sbyrakis S: Carnitine deficiency in children and adolescents with type 1 diabetes. J Diabetes Complications 2004;18:271–274 [DOI] [PubMed] [Google Scholar]
- 3.Jenni S, Oetliker C, Allemann S, Ith M, Tappy L, Wuerth S, Egger A, Boesch C, Schneiter P, Diem P, Christ E, Stettler C: Fuel metabolism during exercise in euglycaemia and hyperglycaemia in patients with type 1 diabetes mellitus—a prospective single-blinded randomised crossover trial. Diabetologia 2008;51:1457–1465 [DOI] [PubMed] [Google Scholar]
- 4.Tsintzas K, Chokkalingam K, Jewell K, Norton L, Macdonald IA, Constantin-Teodosiu D: Elevated free fatty acids attenuate the insulin-induced suppression of PDK4 gene expression in human skeletal muscle: potential role of intramuscular long-chain acyl-coenzyme A. J Clin Endocrinol Metab 2007;92:3967–3972 [DOI] [PubMed] [Google Scholar]
- 5.Kreis R, Jung B, Rotman S, Slotboom J, Boesch C: Non-invasive observation of acetyl-group buffering by 1H-MR spectroscopy in exercising human muscle. NMR Biomed 1999;12:471–476 [DOI] [PubMed] [Google Scholar]
- 6.White LJ, Robergs RA, Sibbitt WL, Jr, Gasparovic CM, Petropoulos H, Brooks WM: Accumulation of acetyl groups following cycling: a 1H-MR spectroscopy study. Int J Sports Med 2006;27:100–104 [DOI] [PubMed] [Google Scholar]
- 7.Slotboom J, Boesch C, Kreis R: Versatile frequency domain fitting using time domain models and prior knowledge. Magn Reson Med 1998;39:899–911 [DOI] [PubMed] [Google Scholar]
- 8.Coyle EF, Hamilton MT, Alonso JG, Montain SJ, Ivy JL: Carbohydrate metabolism during intense exercise when hyperglycemic. J Appl Physiol 1991;70:834–840 [DOI] [PubMed] [Google Scholar]
- 9.Chokkalingam K, Tsintzas K, Norton L, Jewell K, Macdonald IA, Mansell PI: Exercise under hyperinsulinaemic conditions increases whole-body glucose disposal without affecting muscle glycogen utilisation in type 1 diabetes. Diabetologia 2007;50:414–421 [DOI] [PubMed] [Google Scholar]
- 10.Harris RC, Foster CV, Hultman E: Acetylcarnitine formation during intense muscular contraction in humans. J Appl Physiol 1987;63:440–442 [DOI] [PubMed] [Google Scholar]