Abstract
OBJECTIVE
To evaluate whether asymptomatic bacteriuria (ASB) is more common in patients with diabetes than among control subjects. In addition, we wanted to clarify the clinical significance of ASB in patients with diabetes.
RESEARCH DESIGN AND METHODS
We conducted a systematic review and meta-analysis of published data since 1966. Twenty-two studies fulfilled the inclusion criteria of the meta-analysis.
RESULTS
ASB was present in 439 of 3,579 (12.2%) patients with diabetes and in 121 of 2,702 (4.5%) healthy control subjects. ASB was more common both in patients with type 1 diabetes (odds ratio 3.0 [95% CI 1.1–8.0]) and type 2 diabetes (3.2 [2.0–5.2]) than in control subjects. The point prevalence of ASB was higher in both women (14.2 vs. 5.1%; 2.6 [1.6–4.1]) and men (2.3 vs. 0.8%; 3.7 [1.3–10.2]) as well as in children and adolescents (12.9 vs. 2.7%; 5.4 [2.7–11.0]) with diabetes than in healthy control subjects. Albuminuria was more common in patients with diabetes and ASB than those without ASB (2.9 [1.7–4.8]). History of urinary tract infections was associated with ASB (1.6 [1.1–2.3]).
CONCLUSIONS
We were able to show that the prevalence of ASB is higher in all patients with diabetes compared with control subjects. We also found that diabetic subjects with ASB more often had albuminuria and symptomatic urinary tract infections.
As the prevalence of both type 1 diabetes and type 2 diabetes increases world wide, factors associated with diabetes and its complications become more important (1,2). Asymptomatic bacteriuria (ASB) refers to the presence of bacteria in bladder urine in an asymptomatic individual. Usually, samples are collected indirectly by clean-voided midstream urine, and growth of the same uropathogen (≥105 cfu/ml) in two consecutive specimens is considered to be a significant indication of the presence of bacteria in bladder urine (3). ASB is found in 2–5% of healthy adult women, is quite unusual in healthy men, and has been claimed to be three to four times more common in women with diabetes than in healthy women (3). A prevalence as high as 30% in diabetic women has been reported (4).
ASB is considered clinically significant and worth treating during pregnancy because treatment effectively reduces the risk of pyelonephritis and preterm delivery (5,6). Although ASB has been found to associate with increased risk of hospitalization for urosepsis in a prospective observational study among women with diabetes (7), the treatment of ASB in one randomized controlled trial did not reduce the risk of symptomatic urinary tract infection (8). Associations between ASB, metabolic control of diabetes, and impaired renal function have been brought up repeatedly (9–15). To evaluate whether ASB is truly more common in patients with diabetes than among control subjects and to clarify the clinical significance of ASB in diabetic subjects we did a systematic literature search and performed a meta-analysis of the published data.
RESEARCH DESIGN AND METHODS
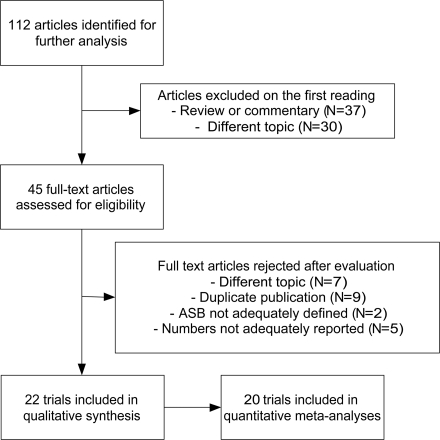
We performed a literature search in PubMed for the years 1966–2007 using the following MeSH terms: “asymptomatic bacteriuria” and “diabetes” in order to find all the articles that considered epidemiology, risk factors, and prognosis of ASB in patients with diabetes. Altogether, 112 hits were found. Reviews, commentary articles, and editorials were excluded. On the basis of the title and abstract, 45 articles were found to be original-research articles on the selected topic. All members of the study group read these 45 articles. Studies where ASB was defined as growth of one or two bacteria species for ≥105 cfu/ml urine in one or more samples taken from asymptomatic patients were included. After excluding 24 articles in which study design, presentation, or reporting was not adequate, 21 articles were finally accepted and analyzed (Fig. 1). Of the non-English articles, only abstracts in English were reviewed.
Figure 1.
Flowchart of the literature search.
We focused on the point prevalence of ASB in diabetic patients and control subjects and the associations of ASB and specific risk and prognostic factors among people with diabetes. Analyses were performed using the Comprehensive Meta-Analysis Program, version 1.0.25. Heterogeneity was assessed and quantified by calculating I2 (inconsistency) values. Without the heterogeneity (test for inconsistency not significant), pooled estimates of odds ratios (ORs) or effect sizes and 95% CIs for the estimates were derived using a fixed-effects model; otherwise, a random-effects model was used (16). The possibility of publication bias was assessed with funnel plots (not shown). The analyses were performed separately for women and men and for patients with type 1 diabetes and type 2 diabetes, whenever possible. The quality of the articles was assessed by all members of the study group, using a scale from 1 to 5, and the summary scoring was then decided after a discussion on the flaws and biases of the study. Because using one figure indicative for the quality of included studies has been shown to be problematic or even misleading, the numbers were not included in the final analyses (17).
RESULTS
Twenty-two studies fulfilled the inclusion criteria of the meta-analysis (Table 1). The design was cross-sectional in 16 and follow-up in 5 studies, whereas 10 studies comprised only women. The mean quality score of the studies included in the analyses was 2.6 (range 1–4). The only randomized intervention trial was evaluated separately (8).
Table 1.
Characteristics of the included studies
| Reference | Study design | Number of patients (diabetic subjects/control subjects) | Mean age (years) (diabetic subjects/control subjects) | Patient group and Source (diabetic subjects/control subjects) | Type of diabetes | Outcomes | Language | Quality score (1–5) |
|---|---|---|---|---|---|---|---|---|
| Ishay et al. 2005 (19) | Cross-sectional, controlled | 411/160 | 59.6/53.3 | Only women from a diabetes outpatient clinics | Type 2 diabetes | Prevalence, duration, urinary protein, creatinine, A1C | English | 4 |
| Bonadio et al. 2004 (9) | Cross-sectional, controlled | 228/146 | 57.7/59.0 | Only women from metabolic/cardiology outpatient clinics | Type 1 and type 2 diabetes | Prevalence, duration, A1C, GFR | English | 3 |
| Makuyana et al. 2002 (25) | Cross-sectional, controlled | 123/53 | 51.0/46.0 | Only black race from diabetes outpatient clinics/outpatient clinics | Type 1 and type 2 diabetes | Prevalence | English | 2 |
| Geerlings et al. 2000 (10) | Cross-sectional, controlled | 636/153 | Not available/47.8 | Only women from diabetes outpatient clinics/eye and trauma outpatient clinics | Type 1 and type 2 diabetes | Prevalence, duration, urinary protein, A1C, UTI anamnesis | English | 3 |
| Kelestimur et al. 1990 (26) | Cross-sectional, controlled | 110/100 | Not available | Hospital patients | Type 1 and type 2 diabetes | Prevalence | Turkish | 1 |
| Schmitt et al. 1986 (27) | Cross-sectional, controlled | 752/200 | 55.0/54.0 | Outpatient clinics/outpatient clinics | Type 2 diabetes | Prevalence | English | 4 |
| Abu-Bakare et al. 1986 (28) | Cross-sectional, controlled | 190/190 | Not available | Only black race from diabetes outpatient clinics/outpatient clinics | Type 1 and type 2 diabetes | Prevalence | English | 4 |
| Rozsai et al. 2006 (18) | Cross-sectional, controlled | 133/178 | 15.6/14.1 | Children and adolescents from diabetes outpatient clinics/medical students | Type 1 diabetes | Prevalence | English | 4 |
| Mendoza et al. 2002 (29) | Cross-sectional, controlled | 50/50 | Not available | Only women from Diabetes outpatient clinic/outpatient clinic | Type 2 diabetes | Prevalence | Spanish | 1 |
| Vigg et al. 1977 (30) | Cross-sectional, controlled | 87/93 | 18–60/18–60 (range) | Diabetes outpatient clinics/outpatient clinics | Type 1 and type 2 diabetes | Prevalence | English | 1 |
| Joffe et al. 1974 (31) | Cross-sectional, controlled | 100/36 | 57.0/72.0 | Diabetes outpatient clinics/outpatient clinics | Type 1 and type 2 diabetes | Prevalence | English | 1 |
| Rozsai et al. 2003 (12) | Cross-sectional, controlled | 178/194 | 15.1/14.4 | Children and adolescents | Type 1 diabetes | Prevalence | English | 3 |
| Boroumand et al. 2006 (20) | Cross-sectional | 202 | 56.0 | Only women from diabetes outpatient clinics/outpatient clinics | Type 2 diabetes | Urinary protein | English | 1 |
| Zhanel et al. 1995 (11) | Cross-sectional | 1,072 | >16 | Only women from diabetes outpatient clinics/outpatient clinics | Type 1 and type 2 diabetes | Creatinine, A1C, UTI anamnesis | English | 1 |
| Boyko et al. 2005 (32) | Controlled follow-up (2 years) | 218/799 | Not available | Postmenopausal women from an epidemiological cohort study | Type 1 and type 2 diabetes | Prevalence | English | 2 |
| Sotiropoulos et al. 2005 (13) | Controlled follow-up (12 months) | 363/350 | 61.3/63.0 | Only women from diabetes outpatient clinics/outpatient clinics | Type 2 diabetes | Prevalence, duration, A1C | English | 3 |
| Ribera-Montes et al. 2006 (21) | Follow-up (12 months) | 457 | 68.3 | Diabetes outpatient clinics/health center | Type 2 diabetes | UTI during follow-up | Spanish | 3 |
| Karunajeewa et al. 2005 (7) | Follow-up (2.9 years) | 496 | Not available | Diabetes outpatient clinics/outpatient clinics | Type 1 and type 2 diabetes | Creatinine, UTI during follow-up | English | 3 |
| Geerlings et al. 2001 (14) | Follow-up (18 months) | 378 | 59.4 | Only women from diabetes outpatient clinics/health center | Type 1 and type 2 diabetes | UTI during follow-up | English | 3 |
| Semetkowska-Jurk 1995 (22) | Follow-up (14 years) | 49 | Not available | Diabetes outpatient clinics/outpatient clinics | Type 1 and type 2 diabetes | UTI during follow-up | English | 3 |
| Meiland et al. 2,006 (15) | Follow-up (6 years) | 348 | 51.1 | Only women from diabetes outpatient clinics/outpatient clinics | Type 1 and type 2 diabetes | GFR, hypertension | English | 4 |
| Harding et al. 2002 (8) | Intervention | 105 | Antibiotics 57.0/placebo 53.7 | Only women from diabetes outpatient clinics/outpatient clinics | Type 1 and type 2 diabetes | UTI | English | 5 |
Of the non-English articles, only abstracts in English were reviewed.
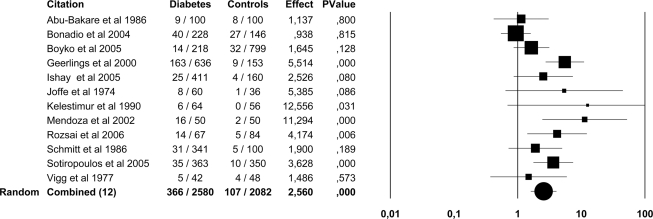
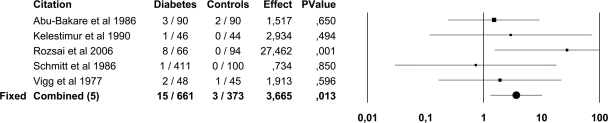
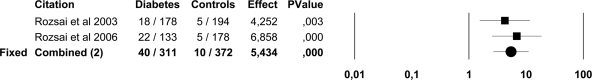
In the pooled data, ASB was present in 439 of 3,579 (12.2%) patients with diabetes and in 121 of 2,702 (4.5%) healthy control subjects. ASB was more common in both patients with type 1 diabetes (OR 3.0 [95% CI 1.1–8.0]) and type 2 diabetes (3.2 [2.0–5.2]) than in control subjects. The point prevalence of ASB was higher in both women (14.2 vs. 5.1%; 2.6 [1.6–4.1]) and men (2.3 vs. 0.8%; 3.7 [1.3–10.2]) with diabetes than in healthy control subjects (Figs. 2 and 3). There were only two trials (12,18) that included children and adolescents and comprised 683 subjects and was published by the same study group. In these surveys, ASB was more common in children and adolescents with diabetes (12.9%) than in healthy control subjects (2.7%; 5.4 [2.7–11.0]) (Fig. 4).
Figure 2.
Forest plot of 12 studies on the prevalence of ASB in women with diabetes and healthy control subjects. Because of the heterogeneity of the studies (I2 63%, P < 0.001), the results of the random-effects model are presented.
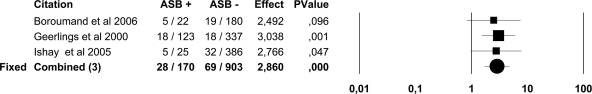
Figure 3.
Forest plot of five studies on the prevalence of ASB in men with diabetes and healthy control subjects. Because the heterogeneity test was not significant (I2 25.6%, P = 0.24) the results of the fixed-effects model are presented.
Figure 4.
Forest plot of two studies on the prevalence of ASB in children and adolescents with diabetes and healthy control subjects. Because the heterogeneity test was not significant (I2, *P = 0.51) the results of the fixed-effects model are presented.
The effect of the duration of diabetes on the point prevalence of ASB was reported in four studies (9,10,13,19) all comprising only women. The mean duration of diabetes was longer in patients with ASB than in those without ASB (pooled difference 0.17 years [95% CI 0.03–0.31]; P = 0.01). The mean A1C, as a measurement of glycemic control in diabetes, did not differ in diabetic subjects with ASB compared with those without ASB (pooled difference 0.21 [−0.07 to 0.50]; P = 0.14).
The mean creatinine level did not differ in diabetic subjects with or without ASB in three cross-sectional surveys (pooled difference 0.21 μmol/l [95% CI −0.3 to 0.8]; P = 0.36) (7,11,19). Association of proteinuria and ASB was studied in three trials (10,19,20). Proteinuria, defined as ≥30 mg/24 h in two of the studies and as presence of macroalbuminuria in one study, was more common in patients with diabetes and ASB than those without ASB (OR 2.9 [95% CI 1.7–4.8]; P < 0.0001) (Fig. 5).
Figure 5.
Forest plot of three studies on albuminuria in patients with diabetes with and without ASB. Because the heterogeneity test was not significant (I2 0%, P = 0.96) the results of the fixed-effects model are presented.
Renal function was measured with glomerulus filtration rate (GFR) in two studies, both of which included only women with diabetes. In the cross-sectional survey, there was no difference in GFR values between diabetic subjects with and without ASB, but in a 6-year follow-up study the GFR values decreased more in patients with diabetes and ASB than in those without ASB (14 vs. 9%, P = 0.03) (9,15). In multivariate analyses adjusted for age, length of follow-up, duration of diabetes, and microalbuminuria at baseline, the difference was no longer statistically significant (15). Hypertension was more common in women with diabetes and ASB (54%) than without ASB (37%), but this difference was not statistically significant when adjusting for confounding variables in logistic modeling (15).
In two cross-sectional surveys (10,11) in which the history of having had a urinary tract infection (UTI) ever in the past was compared in diabetic subjects with and without ASB, positive UTI anamnesis was associated with ASB (OR 1.6 [95% CI 1.1–2.3]). In follow-up studies that included both women and men, symptomatic UTIs tended to be more common in diabetic subjects with ASB than in those without ASB (2.8 [0.8–9.8]) (7,14,21,22).
CONCLUSIONS
In this meta-analysis of observational studies, we were able to show that the prevalence of ASB was three times higher in all patients with diabetes compared with control subjects. We also found that diabetic subjects with ASB more often had albuminuria and symptomatic UTIs than those without ASB. Only one randomized controlled trial on the effect of active treatment of ASB on occurrence of symptomatic UTIs has been performed (8).
Whether glucosuria, as such, could increase the rate of ASB is unclear. Even though adding glucose to urine enhances the growth of bacteria in vitro, the association has not been verified in vivo (23). In this meta-analysis, A1C was slightly higher in diabetic subjects with ASB than in those without ASB, but the difference was neither statistically nor clinically significant. Thus, it seems unlikely that ASB would be just a consequence of a poor metabolic control of diabetes.
Urinary albumin is an important marker of diabetic nephropathy. We found that albuminuria was more common in diabetic subjects with than without ASB. The presence of bacteriuria, as such, does not seem to interfere with urinary albumin measurements. Kramer et al. (24) measured urine albumin concentrations in the same 81 diabetic individuals during ASB and with sterile urine, and no statistically significant differences were found.
Systematic reviews and meta-analyses of observational studies are very sensitive to biases atrributed to confounding factors. Meta-analyses of observational studies are good in developing new hypotheses that then have to be tested in intervention studies. In our meta-analysis, we were able to verify the higher incidence of ASB in diabetic compared with control subjects. Associations between ASB and important clinical outcomes, such as occurrence of symptomatic UTIs and complications of diabetes, have been evaluated in several surveys (10,11,13–15), but the conclusion has been that screening of ASB in diabetes is not beneficial. Lack of association has been interpreted as an evidence for equality (6). In this case, ASB does not cause any clinical consequences, and most of the research findings would show this. However, by chance alone, there would also be findings showing both negative and positive associations with ASB and clinical end points. Yet there are reports of no association and reports showing positive associations between ASB and clinical outcomes but no real contradictory reports. This was seen also in our meta-analysis, in which because a small number of studies and patients were included, only the association between albuminuria and ASB reached statistical significance. The lack of contradictory reports may well be because of publication bias, but we suggest that the associations of ASB and clinical outcomes should be further tested in prospective trials to better define the questions raised in this meta-analysis.
ASB is not a stable phenomenon but fluctuates over time even without any interventions. The pathophysiology of UTIs is unclear, but it is probable that the biologic reasons for asymptomatic and symptomatic urinary infections are similar. In the randomized controlled trial, routine screening and treatment of ASB in diabetic women did not change the occurrence of symptomatic UTsI or hospitalization because of UTIs (8). Harding et al.'s (8) trial is a landmark study in this field, but only women were included, mostly with type 2 diabetes. It is important to repeat these results and also include men and adolescents in the material. Altogether, the only way to thoroughly clarify the significance of ASB in patients with diabetes is to perform high-quality prospective studies on screening and treating ASB, with UTIs, metabolic control, and occurrence of long-term complications of diabetes as outcomes.
The limitations of this meta-analysis arise mainly from the difficulties in obtaining detailed information from the articles included. We were not able to perform all analyses separately for the age-groups, sexes, or diabetes types. Also, the methodological quality of the majority of the studies included in this meta-analysis was poor. Almost all studies were performed among elderly women with type 2 diabetes, and whenever there were men, adolescents, or young adults included, the data for the different patient groups were not possible to separate. Yet this meta-analysis supports previous observations, verifies the incidence of ASB in the more seldom–investigated patient groups, and found significant association between albuminuria and ASB in patients with diabetes.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
M.R. participated in designing and planning the study, made the literature searches, read and reviewed the articles, made the analyses, and wrote the first version of the manuscript. P.Ta. participated in designing and planning the study, read and reviewed the articles, and edited the manuscript. P.To. participated in designing and planning the study, read and reviewed the articles, and edited the manuscript. T.P. participated in designing and planning the study, helped with the analyses, and edited the manuscript. M.U. participated in designing and planning the study, read and reviewed the articles, and edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Harjutsalo V, Sjoberg L, Tuomilehto J: Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 3.Raz R: Asymptomatic bacteriuria: clinical significance and management. Int J Antimicrob Agents 2003;22(Suppl. 2):45–47 [DOI] [PubMed] [Google Scholar]
- 4.Balasoiu D, Van Kessel KC, van Kats-Renaud HJ, Collet TJ, Hoepelman AI: Granulocyte function in women with diabetes and asymptomatic bacteriuria. Diabetes Care 1997;20:392–395 [DOI] [PubMed] [Google Scholar]
- 5.Smaill F: Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev CD000490, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Lin K, Fajardo K: Screening for asymptomatic bacteriuria in adults: evidence for the U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med 2008;149:W20–W24 [DOI] [PubMed] [Google Scholar]
- 7.Karunajeewa H, McGechie D, Stuccio G, Stingemore N, Davis WA, Davis TM: Asymptomatic bacteriuria as a predictor of subsequent hospitalisation with urinary tract infection in diabetic adults: the Fremantle Diabetes Study. Diabetologia 2005;48:1288–1291 [DOI] [PubMed] [Google Scholar]
- 8.Harding GK, Zhanel GG, Nicolle LE, Cheang M: Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med 2002;347:1576–1583 [DOI] [PubMed] [Google Scholar]
- 9.Bonadio M, Boldrini E, Forotti G, Matteucci E, Vigna A, Mori S, Giampietro O: Asymptomatic bacteriuria in women with diabetes: influence of metabolic control. Clin Infect Dis 2004;38:e41–e45 [DOI] [PubMed] [Google Scholar]
- 10.Geerlings SE, Stolk RP, Camps MJ, Netten PM, Hoekstra JB, Bouter KP, Bravenboer B, Collet JT, Jansz AR, Hoepelman AI: Asymptomatic bacteriuria may be considered a complication in women with diabetes: Diabetes Mellitus Women Asymptomatic Bacteriuria Utrecht Study Group. Diabetes Care 2000;23:744–749 [DOI] [PubMed] [Google Scholar]
- 11.Zhanel GG, Nicolle LE, Harding GK: Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus: the Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis 1995;21:316–322 [DOI] [PubMed] [Google Scholar]
- 12.Rozsai B, Lanyi E, Soltesz G: Asymptomatic bacteriuria and leukocyturia in type 1 diabetic children and young adults. Diabetes Care 2003;26:2209–2210 [DOI] [PubMed] [Google Scholar]
- 13.Sotiropoulos A, Skourtis S, Merkouris P, Peppas T, Apostolou O, Kontela E, Skliros E, Pappas S: Incidence and outcome of asymptomatic bacteriuria in females with type 2 diabetes mellitus over a 1-year follow-up period and association with risk factors. Diabet Med 2005;22:1625–1626 [DOI] [PubMed] [Google Scholar]
- 14.Geerlings SE, Stolk RP, Camps MJ, Netten PM, Collet JT, Schneeberger PM, Hoepelman AI: Consequences of asymptomatic bacteriuria in women with diabetes mellitus. Arch Intern Med 2001;161:1421–1427 [DOI] [PubMed] [Google Scholar]
- 15.Meiland R, Geerlings SE, Stolk RP, Netten PM, Schneeberger PM, Hoepelman AI: Asymptomatic bacteriuria in women with diabetes mellitus: effect on renal function after 6 years of follow-up. Arch Intern Med 2006;166:2222–2227 [DOI] [PubMed] [Google Scholar]
- 16.Littell JH, Corcoran J, Pillai V: Systematic Reviews and Meta-Analysis. New York, Oxford University Press, 2008 [Google Scholar]
- 17.Juni P, Witschi A, Bloch R, Egger M: The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054–1060 [DOI] [PubMed] [Google Scholar]
- 18.Rozsai B, Lanyi E, Berki T, Soltesz G: Urinary cytokine response to asymptomatic bacteriuria in type 1 diabetic children and young adults. Pediatr Diabetes 2006;7:153–158 [DOI] [PubMed] [Google Scholar]
- 19.Ishay A, Lavi I, Luboshitzky R: Prevalence and risk factors for asymptomatic bacteriuria in women with type 2 diabetes mellitus. Diabet Med 2006;23:185–188 [DOI] [PubMed] [Google Scholar]
- 20.Boroumand MA, Sam L, Abbasi SH, Salarifar M, Kassaian E, Forghani S: Asymptomatic bacteriuria in type 2 Iranian diabetic women: a cross sectional study. BMC Womens Health 2006;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribera MC, Pascual R, Orozco D, Perez BC, Pedrera V, Gil V: Incidence and risk factors associated with urinary tract infection in diabetic patients with and without asymptomatic bacteriuria. Eur J Clin Microbiol Infect Dis 2006;25:389–393 [DOI] [PubMed] [Google Scholar]
- 22.Semetkowska-Jurkiewicz E, Horoszek-Maziarz S, Galinski J, Manitius A, Krupa-Wojciechowska B: The clinical course of untreated asymptomatic bacteriuria in diabetic patients: 14-year follow-up. Mater Med Pol 1995;27:91–95 [PubMed] [Google Scholar]
- 23.Geerlings SE, Meiland R, Hoepelman AI: Pathogenesis of bacteriuria in women with diabetes mellitus. Int J Antimicrob Agents 2002;19:539–545 [DOI] [PubMed] [Google Scholar]
- 24.Kramer CK, Camargo J, Ricardo ED, Almeida FK, Canani LH, Gross JL, Azevedo MJ: Does bacteriuria interfere with albuminuria measurements of patients with diabetes? Nephrol Dial Transplant 2009;24:1193–1196 [DOI] [PubMed] [Google Scholar]
- 25.Makuyana D, Mhlabi D, Chipfupa M, Munyombwe T, Gwanzura L: Asymptomatic bacteriuria among outpatients with diabetes mellitus in an urban black population. Cent Afr J Med 2002;48:78–82 [DOI] [PubMed] [Google Scholar]
- 26.Kelestimur F, Unal A, Pasaoglu H, Basar E, Kilic H, Doganay M: [Asymptomatic bacteriuria in patients with diabetes mellitus]. Mikrobiyol Bul 1990;24:126–132[Article in Turkish] [PubMed] [Google Scholar]
- 27.Schmitt JK, Fawcett CJ, Gullickson G: Asymptomatic bacteriuria and hemoglobin A1. Diabetes Care 1986;9:518–520 [DOI] [PubMed] [Google Scholar]
- 28.Abu-Bakare A, Oyaide SM: Asymptomatic bacteriuria in Nigerian diabetics. J Trop Med Hyg 1986;89:29–32 [PubMed] [Google Scholar]
- 29.Mendoza T, García de los Ríos M, Lafourcade M, Soto C, Durruty P, Alvo M: [Asymptomatic bacteriuria in type 2 diabetics women]. Rev Med Chil 2002;130:1001–1007[Article in Spanish] [PubMed] [Google Scholar]
- 30.Vigg B, Rai V: Asymptomatic bacteriuria in diabetics. J Assoc Physicians India 1977;25:57–61 [PubMed] [Google Scholar]
- 31.Joffe BI, Seftel HC, Distiller LA: Asymptomatic bacteriuria in diabetes mellitus. S Afr Med J 1974;48:1306–1308 [PubMed] [Google Scholar]
- 32.Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B: Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol 2005;161:557–564 [DOI] [PubMed] [Google Scholar]