Abstract
OBJECTIVE
Some obese individuals have normal insulin sensitivity. It is controversial whether this phenotype is associated with increased all-cause mortality risk.
RESEARCH DESIGN AND METHODS
Fifteen-year all-cause mortality data were obtained through the Regional Health Registry for 2,011 of 2,074 Caucasian middle-aged individuals of the Cremona Study, a population study on the prevalence of diabetes in Italy. Individuals were divided in four categories according to BMI (nonobese: <30 kg/m2; obese: ≥30 kg/m2) and estimated insulin resistance (insulin sensitive: homeostasis model assessment of insulin resistance <2.5; insulin resistant ≥2.5).
RESULTS
Obese insulin-sensitive subjects represented 11% (95% CI 8.1–14.5) of the obese population. This phenotype had similar BMI but lower waist circumference, blood pressure, fasting glucose, triglycerides, and fibrinogen and higher HDL cholesterol than obese insulin-resistant subjects. In the 15-year follow-up, 495 deaths (cardiovascular disease [CVD]: n = 221; cancer: n = 180) occurred. All-cause mortality adjusted for age and sex was higher in the obese insulin-resistant subjects (hazard ratio 1.40 [95% CI 1.08–1.81], P = 0.01) but not in the obese insulin-sensitive subjects (0.99 [0.46–2.11], P = 0.97) when compared with nonobese insulin-sensitive subjects. Also, mortality for CVD and cancer was higher in the obese insulin-resistant subjects but not in the obese insulin-sensitive subjects when compared with nonobese insulin-sensitive subjects.
CONCLUSIONS
In contrast to obese insulin-resistant subjects, metabolically healthy obese individuals are less common than previously thought and do not show increased all-cause, cancer, and CVD mortality risks in a 15-year follow-up study.
Metabolically healthy obese (MHO) individuals are considered as a subset of obese subjects without metabolic abnormalities (such as insulin resistance, proatherogenic lipoprotein profile, proinflammatory state, or hypertension) and a model for better understanding the pathogenesis of insulin resistance (1–3). The prevalence of the MHO phenotype in the general population, the reasons for not developing metabolic alterations, and the less aggressive therapeutic approach with respect to obese individuals with metabolic abnormalities are currently debated (4,5). In the Framingham Offspring Study, Meigs et al. (6) found that MHO individuals do not have increased risk of incident diabetes and cardiovascular disease (CVD). Conversely, in the Third National Health and Nutrition Examination Survey (NHANES III), Kuk et al. (7) reported increased all-cause mortality associated with the MHO phenotype. Finally, in a Scandinavian study (8), middle-aged overweight/obese subjects without metabolic syndrome also had an increased risk of CVD when compared with normal-weight individuals without metabolic syndrome. The present study shows the prevalence of the MHO phenotype, its metabolic features, and 15-year all-cause, CVD, and cancer mortality rates in the Caucasian population of the Cremona Study (9,10).
RESEARCH DESIGN AND METHODS
Study cohort and follow-up
The Cremona Study is a population survey carried out in 1990–1991 in the health district of Cremona (Lombardia, Italy) to determine the prevalence of diabetes according to the oral glucose tolerance test (OGTT) and World Health Organization criteria (8,9). A total of 2,074 individuals were enrolled. Past medical history, anthropometric measures, and clinical data of subjects were collected by trained interviewers using standardized procedures. A venous blood sample was collected after a 12-h overnight fast, and thereafter a 75-g oral glucose monohydrate was given. An additional blood sample was collected 2 h later. Heart rate and blood pressure were recorded twice, at the beginning and at the end of the visit, in the sitting position, and after at least 10 min rest using a full automatic noninvasive sphyngomanometer. The lowest figure was considered. Further details concerning the study protocol were previously described (8,9). Vital status and time of death were acquired from the Regional Health Registry (updated to 31 December 2005), and causes of death were classified using the ICD-9 (death codes for CVD are from 401 to 448 and cancer from 140.0 to 208.9). Median follow-up was 180 months, and median follow-up of those still alive at 182 months (98% of those who were still alive had a minimum follow-up period of 174 months). Data for 2,011 of 2,074 individuals were available.
Definition of study groups
Study subjects were divided in four categories based on BMI (nonobese: <30 kg/m2; obese: ≥30 kg/m2) and estimated insulin resistance (insulin sensitive: homeostasis model assessment of insulin resistance [HOMA-IR] <2.5; insulin resistant ≥2.5). The cutoff of 2.5 for HOMA-IR was chosen to compare our data with those recently published by Kuk et al. (7). Therefore, the four categories were 1) the nonobese subjects with normal insulin sensitivity, 2) the obese but insulin-sensitive subjects, 3) the nonobese but insulin-resistant subjects, and 4) the obese and insulin-resistant subjects. The features of these subgroups are summarized in Table 1.
Table 1.
Baseline anthropometric, clinical, and laboratory features of study groups
| Nonobese insulin-sensitive subjects | Obese insulin-sensitive subjects | Nonobese insulin-resistant subjects | Obese insulin-resistant subjects | |
|---|---|---|---|---|
| Anthropometric parameters | ||||
| n (female/male) | 708 (392/316) | 43 (31/12) | 923 (512/411) | 337 (191/146) |
| Age (years) | 55 ± 11*† | 55 ± 9 | 59 ± 11‡§ | 59 ± 10‡§ |
| BMI (kg/m2) | 23.8 ± 2.8*†§ | 32.5 ± 4.3†‡ | 25.8 ± 2.3*‡§ | 33.3 ± 3.4†‡ |
| Waist circumference (cm) | 82 ± 9*†§ | 94 ± 4*†‡ | 89 ± 10*‡§ | 104 ± 11†‡§ |
| Actual smoking | 201 (28%)¶ | 8 (19%) | 177 (19%) | 64 (19%) |
| Alcohol intake (g/day) | 44 ± 59 | 39 ± 45 | 42 ± 59 | 39 ± 54 |
| Systolic blood pressure (mmHg) | 139 ± 20*† | 143 ± 23* | 147 ± 21*‡ | 154 ± 20†‡§ |
| Diastolic blood pressure (mmHg) | 77 ± 11*† | 79 ± 13* | 81 ± 12*‡ | 85 ± 12†‡§ |
| Heart rate (beats/min) | 73 ± 11*† | 72 ± 10 | 76 ± 13‡ | 77 ± 11‡ |
| Biochemical lab parameters | ||||
| Glucose (mmol/l) | 4.83 ± 0.50*† | 4.83 ± 0.33*† | 5.44 ± 1.05†‡§ | 6.00 ± 1.67†‡§ |
| Cholesterol (mmol/l) | 5.92 ± 1.09† | 6.20 ± 1.19 | 6.20 ± 1.14‡ | 6.10 ± 1.14 |
| HDL cholesterol (mmol/l) | 1.52 ± 0.36*† | 1.50 ± 0.34*† | 1.29 ± 0.36 *‡§ | 1.21 ± 0.34†‡§ |
| LDL cholesterol (mmol/l) | 3.90 ± 1.03*† | 4.13 ± 1.00 | 4.19 ± 1.03‡ | 4.11 ± 1.06‡ |
| Triglycerides (mmol/l) | 1.15 ± 0.63*† | 1.26 ± 0.58*† | 1.56 ± 1.04*‡ | 1.72 ± 0.94†‡§ |
| Alanine aminotransferase (units/l) | 21 ± 14*† | 23 ± 12* | 27 ± 22*‡ | 31 ± 26†‡§ |
| Aspartate aminotransferase (units/l) | 26 ± 12* | 25 ± 8 | 28 ± 13 | 30 ± 19‡ |
| γGT (units/l) | 31 ± 38*† | 33 ± 41 | 42 ± 58‡ | 50 ± 82‡ |
| ALP (units/l) | 169 ± 64*† | 159 ± 52* | 180 ± 66‡ | 187 ± 76‡§ |
| Fibrinogen (mg/dl) | 271 ± 66*† | 274 ± 48*† | 286 ± 74*‡ | 302 ± 76‡§ |
| Hormones | ||||
| Insulin (pmol/l) | 50 ± 13*† | 56 ± 13*† | 112 ± 70*‡§ | 154 ± 89†‡§ |
| Insulin sensitivity, metabolic syndrome, diabetes status | ||||
| HOMA-IR | 1.80 ± 0.45*† | 2.00 ± 0.43*† | 4.65 ± 3.70*‡§ | 7.18 ± 5.57†‡§ |
| Metabolic syndrome | 37 (5%) | 3 (7%) | 218 (24%)‖ | 139 (41%)‖ |
| Diabetes | 16 (2%) | 0 (0%) | 98 (11%)‖ | 74 (28%)‖ |
Data are means ± SD or n (%), unless otherwise indicated. One-way ANOVA and Tukey post hoc for continuous variables.
*P < 0.05 vs. obese insulin-resistant subjects;
†P < 0.05 vs. nonobese insulin-resistant subjects;
‡P < 0.05 vs. nonobese insulin-sensitive subjects;
§P < 0.05 vs. obese insulin-sensitive subjects. χ2 for categorical variables
¶P < 0.05 vs. all;
‖P < 0.05 vs. nonobese and obese insulin-sensitive subjects.
Definition of diabetes, impaired glucose tolerance, and metabolic syndrome
Diabetes was defined according to the use of oral hypoglycemic agents or insulin and according to the World Health Organization diagnostic criteria for the OGTT (basal plasma glucose >7.8 mmol/l or >11.1 mmol/l after a 2-h oral glucose load). Patients with manifest diabetes did not undergo the OGTT. Impaired glucose tolerance was defined as basal plasma glucose <7.8 mmol/l and plasma glucose >7.8 but <11 mmol/l after a 2-h oral glucose load. Metabolic syndrome was defined accordingly to the definition of the National Cholesterol Education Program Adult Treatment Program III.
Analytical determinations
Blood, serum, and plasma measurements were done as previously described (8,9).
Calculations
BMI was calculated as weight in kilograms divided by the square of height in meters and alcohol consumption as grams of alcohol (glass of wine = 20 g, glass of aperitif = 30 g, and glass of liquor = 80 g). HOMA-IR was calculated as previously described (11), and LDL cholesterol was calculated using the Friedwald formula.
Statistical analysis
Data are presented as means ± SD, unless otherwise indicated. Serum insulin, triglycerides, fibrinogen, and glucose had a skewed distribution; therefore, log-transformed values were used in the analysis. ANOVA and Tukey post hoc analysis were used for comparison between groups. Differences in proportion between groups were tested by the χ2 test. The associations of each investigated risk factor with all-cause, CVD, and cancer mortality were estimated by the Cox proportional hazard model, with adjustments for age and sex. Multivariate Cox regression analysis was performed to adjust the comparisons of mortality among the different subgroups for possible confounding factors. Hazard ratios (HRs) and 95% CIs are presented. Proportions' 95% CIs were calculated using the normal approximation or the exact method. Kaplan and Meier curves for all-cause mortality were plotted for the four groups, as previously described. A P value <0.05 indicated statistical significance. Analyses were performed using SAS software (version 9.1).
RESULTS
Prevalence of the obese insulin-sensitive phenotype
Of 2,011 subjects, 708 were nonobese and insulin sensitive, 923 nonobese and insulin resistant, and 337 obese and insulin resistant. There were a total of 43 obese insulin-sensitive individuals, representing 11.0% (95% CI 8.1–14.5) of the obese population and 2.1% (1.6–2.9) of the entire population.
Anthropometric and metabolic features of obese insulin-sensitive individuals
The features of the four groups are summarized in Table 1. Sex distribution did not differ among all groups, whereas cigarette smoking was more frequent in nonobese insulin-sensitive subjects than all other groups. Systolic and diastolic blood pressure, heart rate, plasma glucose, insulin, total cholesterol, HDL cholesterol, triglycerides, transaminases, γ-glutamyltransferase (γGT), alkaline phosphatase (ALP), and fibrinogen did not differ between the two insulin-sensitive groups.
Individuals in the insulin-sensitive groups were younger, had lower heart rates, had higher plasma HDL cholesterol, and had lower fibrinogen and triglycerides, as well as had a lower prevalence of diabetes and metabolic syndrome than insulin-resistant groups. Waist circumference was higher in obese insulin-sensitive than nonobese insulin-resistant subjects but were lower than in obese insulin-resistant subjects. Systolic and diastolic blood pressure, plasma transaminases, γGT, and ALP were higher in the two groups of insulin-resistant subjects than in nonobese insulin-sensitive subjects.
Mortality in the cohort
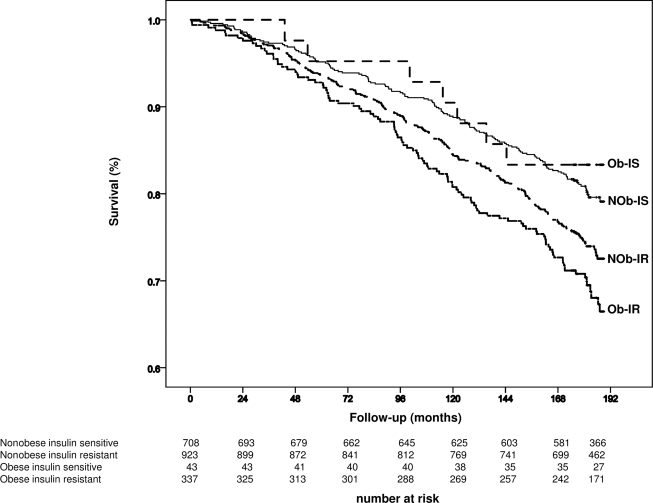
During the 15-year observation period, 495 deaths occurred. A total of 221 deaths were CVD related and 180 were cancer related. Age and sex were associated with higher all-cause mortality (age: HR 1.11 [95% CI 1.10–1.12], P < 0.0001; female sex: 0.42 [0.35–0.50], P < 0.0001), mortality for CVD (age: 1.15 [1.13–1.17], P < 0.0001; female sex: 0.40 [0.31–0.53], P < 0.0001), and mortality for cancer (age: 1.07 [1.06–1.09], P < 0.0001; female sex: 0.39 [0.29–0.52], P < 0.0001). All-cause mortality was higher in the obese insulin-resistant subjects (31%) in comparison with the reference group of nonobese insulin-sensitive subjects (20%) (age- and sex-adjusted HR 1.4, P = 0.01) (Table 2) (Fig. 1) but not in the obese insulin-sensitive subjects (12%) (0.99, P = 0.97) (Table 2) (Fig. 1) and in the nonobese insulin-resistant subjects (26%) (1.11, P = 0.35) (Table 2) (Fig. 1).
Table 2.
Cox proportional hazard model adjusting for age and sex
| n events/n | HR (95% CI) | P | |
|---|---|---|---|
| All-cause mortality | |||
| Nonobese insulin-sensitive subjects | 141/708 | — | — |
| Obese insulin-sensitive subjects | 7/43 | 0.99 (0.46–2.11) | 0.97 |
| Nonobese insulin-resistant subjects | 241/923 | 1.11 (0.90–1.36) | 0.35 |
| Obese insulin-resistant subjects | 106/337 | 1.40 (1.08–1.81) | 0.01 |
| CVD mortality | |||
| Nonobese insulin-sensitive subjects | 58/708 | — | — |
| Obese insulin-sensitive subjects | 2/43 | 0.73 (0.18–3.00) | 0.66 |
| Nonobese insulin-resistant subjects | 112/923 | 1.19 (0.86–1.64) | 0.29 |
| Obese insulin-resistant subjects | 49/337 | 1.61 (1.10–2.36) | 0.015 |
| Cancer mortality | |||
| Nonobese insulin-sensitive subjects | 51/708 | — | — |
| Obese insulin-sensitive subjects | 3/43 | 1.04 (0.32–3.30) | 0.95 |
| Nonobese insulin-resistant subjects | 85/923 | 1.09 (0.78–1.52) | 0.64 |
| Obese insulin-resistant subjects | 41/337 | 1.52 (1.02–2.26) | 0.04 |
Figure 1.
Survival by Kaplan-Meier estimates of all-cause mortality. Follow-up period was 15 years (180 months). Subjects were divided according to BMI (nonobese: <30 kg/m2; obese: ≥30 kg/m2) and estimated insulin resistance (insulin sensitive: HOMA-IR <2.5; insulin resistant: ≥2.5). At the bottom are the detailed figures of the number at risk for each subgroup of individuals. NOb-IR, nonobese insulin-resistant subjects; NOb-IS, nonobese insulin-sensitive subjects (the reference); Ob-IR, obese insulin-resistant subjects; Ob-IS, obese insulin-sensitive subjects.
Also, mortality for CVD (15%, P = 0.015) and cancer (12%, P = 0.04) (Table 2) was higher in the obese insulin-resistant subjects but not in the obese insulin-sensitive subjects (CVD related: 5%, P = 0.66, and cancer related: 7%, P = 0.95) and nonobese insulin-resistant subjects (CVD related: 12%, P = 0.29, and cancer related: 9%, P = 0.64) when compared with nonobese insulin-sensitive subjects (CVD related: 8% and cancer related: 7%).
Because the prevalence of cigarette smoking and baseline plasma LDL cholesterol were different among the groups, we performed the analysis adjusting also for these two factors. All-cause mortality remained higher in the obese insulin-resistant subjects (HR 1.66 [95% CI 1.12–2.46], P = 0.011) but not in obese insulin-sensitive subjects (0.79 [0.19–3.28], P = 0.75) and in nonobese insulin-resistant subjects (1.22 [0.88–1.70], P = 0.23) when compared with nonobese and insulin-sensitive subjects.
The analysis was also repeated after the exclusion of diabetic patients. When compared with nonobese insulin-sensitive (reference group), all-cause mortality tended to be higher in obese insulin-resistant subjects (HR 1.29 [95% CI 0.96–1.73], P = 0.087) but was again not different in obese insulin-sensitive subjects (1.01 [0.47–2.17], P = 0.97) and nonobese insulin-resistant subjects (1.00 [0.80–1.25], P = 0.98). Similarly, mortality for CVD and cancer tended to be higher in obese insulin-resistant subjects (CVD: HR 1.40 [95% CI 0.94–2.11], P = 0.071; cancer: 1.46 [0.94–2.27], P = 0.097) but was not different in obese insulin-sensitive subjects (CVD: 0.76 [0.197–3.11], P = 0.71; cancer: 1.05 [0.33–3.56], P = 0.94) and nonobese insulin-resistant subjects (CVD: [1.09 [0.77–1.54], P = 0.65; cancer: 1.01 [0.93–2.27], P = 0.95) than nonobese insulin-sensitive subjects.
Finally, instead of the preselected HOMA-IR of 2.5, we repeated the analysis using cutoff values (top tertile and top quartile) for HOMA-IR obtained from the present study. Even in this case, the results did not change (see the online appendix, available at http://care.diabetesjournals.org/cgi/content/full/dc10-0665/DC1).
CONCLUSIONS
The 15-year follow-up of the Cremona Study demonstrates that obese insulin-sensitive individuals, also known as MHO individuals 1) have a prevalence of 11% in the obese population and 2% in the entire population; 2) have less features of the metabolic syndrome, when compared with obese insulin-resistant individuals; and 3) do not have increased all-cause, CVD, and cancer mortality, when compared with nonobese insulin-sensitive (reference group) subjects.
Major findings and comparison with the literature
The prevalence of the obese insulin-sensitive phenotype (11%) in our obese cohort was lower than reported by Iacobellis et al. (3) (27.5%) in a cohort of 681 obese individuals living in Rome and the surrounding areas. The discrepancy may be related to the different regional habits of the Italian cohorts but most likely to the different definition of MHO. Iacobellis et al. based their definition mainly on the metabolic syndrome; meanwhile, our definition was centered on HOMA-IR, a surrogate index of insulin resistance, in order to compare our results with those recently published by Kuk and Ardern (7), who analyzed the NHANES III survey in U.S. using HOMA-IR <2.5 as the cutoff. Interestingly, they reported a prevalence of metabolically healthy subjects of 6%. Our finding is in line with this report (7); therefore, we think that the frequency of this phenotype is lower than previously thought.
The present study has also clearly shown that the obese insulin-sensitive phenotype carries less features of the metabolic syndrome. These subjects were characterized by lower waist circumference, blood pressure, circulating triglycerides, transaminases, γGT (as a surrogate markers of fatty liver), and fibrinogen (as a surrogate marker of low-grade inflammation), when compared with the obese insulin-resistant subjects, in spite of similar BMIs. Not surprisingly, they had a lower prevalence of the metabolic syndrome (7% in comparison to the observed 41% in the obese insulin-resistant subjects) and of diabetes (0 vs. 28% of the obese insulin-resistant subjects). We think, therefore, that the deleterious metabolic features associated with obesity are largely related to the presence of insulin resistance rather than obesity, per se.
The third aim was to establish the prognosis of the MHO subjects. The present study has also shown that all-cause mortality is significantly higher in obese insulin-resistant subjects but not obese insulin-sensitive subjects, when compared with nonobese insulin-sensitive individuals (considered as reference group). These findings were confirmed when the analysis was adjusted for LDL cholesterol and cigarette smoking (risk factors not related to metabolic syndrome). However, our findings are in contrast with recent data from a U.S. population that suggest increased all-cause mortality in MHO subjects (defined according to the same BMI and HOMA-IR criteria we use here) (7). The potential explanations for this discrepancy are number of events, reference HR, and different ethnicity. Even though our population was smaller, the number of events was higher (495 vs. 292, or 25 vs. 5%). This was likely because of the longer observational period (15 vs. 8.7 years). It is important to point out that a 10- to 15-year follow-up may be the least to see the effects of metabolic risk factors on mortality (8,12). Regarding reference HRs, we studied nonobese insulin-sensitive individuals, which also includes overweight individuals with BMIs ranging between 25 and 29.9 kg/m2, whereas Kuk et al. (7) studied normal-weight insulin-sensitive subjects with BMI <25 kg/m2.
Our finding is also in contrast with another report by Arnlov et al. (8) in a Scandinavian population in which overweight and obese individuals without the metabolic syndrome showed a higher mortality when compared with normal-weight and insulin-sensitive individuals. We believe that the reason for this discrepancy could be attributed to sex differences because our study included both male and female subjects, whereas only male subjects were included in the Scandinavian study. This is worth mentioning because male sex was a significant risk factor for all-cause mortality in our study.
We used all-cause mortality as primary outcome (because this variable is less affected by errors in reporting), whereas CVD and cancer mortality were considered secondary outcomes. Mortality for CVD and cancer, as for all-cause mortality, were also higher in obese insulin-resistant individuals but not in the MHO subjects.
Strengths and limitations
The following are major strengths of the present study: 1) this was a population-based study including both male and female subjects, 2) there was careful and homogeneous acquisition of the anthropometric parameter of interest, 3) there was a robust end point (all-cause mortality) whose ascertainment was based on the Regional Health Registry, and 4) there was a long follow-up period (15 years).
The following are the limitations of this study: 1) there was a small sample size of the group of obese insulin-sensitive subjects (n = 43), and their low prevalence in the cohort (2%) could represent a problem because of the consequent small number of events even if it was similar to previously reported data (8); 2) there was a lack of collection of intermediate data points about the parameters of interest during the 15-year observation period; 3) the glucose clamp technique is the gold standard for the assessment of insulin sensitivity and HOMA is inferior; nevertheless, it was suggested that HOMA appeared to be specifically suited to large-scale epidemiologic studies in which only fasting glucose and insulin concentrations were available (13); and 4) there was a lack of collection of the dietary habits and habitual physical activity, known to have a well recognized impact on insulin sensitivity.
Pathogenic remarks
It is currently unclear why these MHO subjects may be protected. It was reported that a lower amount of visceral fat content may contribute to the favorable metabolic profile (1,6). Fitting this view, the waist circumference was lower in MHO subjects than in the obese insulin-resistant subjects; on the other hand, it was higher in comparison to the nonobese and insulin-sensitive group (Table 1), in spite of a similar all-cause mortality. We speculate that visceral fat and insulin resistance may, in combination, explain the difference and the trends observed between groups in our study, and, in addition, an undetectable effect of ectopic fat accumulation in the skeletal muscle (14) and the liver (15) should be considered. In particular, the potential, but yet-to-be-demonstrated, role of the liver (see the profile of surrogate markers of fatty liver) in mediating the increased CVD mortality may be hypothesized based on the proinflammatory and proatherosclerotic profile of individuals with nonalcoholic fatty liver disease but also based on some initial epidemiological data (16).
All-cause mortality in obese insulin-resistant subjects but not in MHO subjects is higher when compared with nonobese insulin-sensitive subjects. The effect of obesity on the increasing risk is strongly related with insulin resistance, and we therefore agree with Bonora et al. (17) and McLaughlin et al. (18) that it is important to not limit our risk evaluation to the identification of obesity alone but to put more effort into identifying those at higher risk, insulin-resistant obese individuals.
Acknowledgments
This work was supported by grants from the Italian Minister of Health (030.5/RF96.305 and 030.5/RF98.49), Ministero dell' Università e della Ricerca Scientifića e Tecnologica Cofin (9806409093), and the Italian National Research Council (Consiglo Nazionale delle Ricerche 97.00485.CT04). The financial support of the European Foundation for the Study of Diabetes is also gratefully acknowledged.
No potential conflicts of interest relevant to this article were reported.
G.C. researched data, contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript. G.L. researched data and reviewed/edited the manuscript. L.P. contributed to the discussion and reviewed/edited the manuscript. M.P.G. researched data and reviewed/edited the manuscript. F.R. researched data and reviewed/edited the manuscript. M.V. researched data and reviewed/edited the manuscript. S.M. researched data and reviewed/edited the manuscript. P.C. researched data and reviewed/edited the manuscript. E.B. contributed to the discussion and reviewed/edited the manuscript. L.L. contributed to the discussion and reviewed/edited the manuscript. G.R. contributed to the discussion and reviewed/edited the manuscript. G.P. contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript.
This work was presented in abstract form at the American Diabetes Association's 70th Scientific Sessions, 25–29 June 2010, Orlando, Florida.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, Poehlman ET: What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab 2001;86:1020–1025 [DOI] [PubMed] [Google Scholar]
- 2.Karelis AD, Faraj M, Bastard J-P, St-Pierre DH, Brochu M, Prud'homme D, Rabasa-Lhoret R: The metabolically healthy but obese individual presents a favourable inflammation profile. J Clin Endocrinol Metab 2005;90:4145–4150 [DOI] [PubMed] [Google Scholar]
- 3.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F: Prevalence of uncomplicated obesity in an Italian obese population. Obes Res 2005;13:116–1122 [DOI] [PubMed] [Google Scholar]
- 4.Perseghin G: Is a nutritional therapeutic approach unsuitable for metabolically healthy but obese women? Diabetologia 2008;51:1567–1569 [DOI] [PubMed] [Google Scholar]
- 5.Karelis AD: Metabolically healthy but obese individuals. Lancet 2008;372:1281–1283 [DOI] [PubMed] [Google Scholar]
- 6.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB: Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006;91:2906–2912 [DOI] [PubMed] [Google Scholar]
- 7.Kuk JL, Ardern CI: Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 2009;32:2297–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnlov J, Ingelsson E, Sundstrom J, Lind L: Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 2010;121:230–236 [DOI] [PubMed] [Google Scholar]
- 9.Garancini MP, Calori G, Manara E, Izzo A, Ebbli E, Galli L, Boari L, Gallus G: An Italian population-based study of the prevalence of diabetes: some methodological aspects. Diabetes Metab 1993;19:116–120 [PubMed] [Google Scholar]
- 10.Garancini MP, Calori G, Ruotolo G, Manara E, Izzo A, Ebbli E, Bozzetti AM, Boari L, Lazzari P, Gallus G: Prevalence of NIDDM and impaired glucose tolerance in Italy: a OGTT-based population study. Diabetologia 1995;38:306–313 [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1995;28:412–419 [DOI] [PubMed] [Google Scholar]
- 12.Sundstrom J, Riserus U, Byberg L, Zethelius B, Lithell H, Lind L: Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ 2006;332:878–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M: Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 2000;23:57–63 [DOI] [PubMed] [Google Scholar]
- 14.Perseghin G, Scifo P, Danna M, Piceni Sereni L, Maffi P, Battezzati A, De Cobelli F, Secchi A, Del Maschio A, Luzi L: Normal insulin sensitivity and IMCL content in overweight humans are associated with higher fasting lipid oxidation. Am J Physiol Endocrinol Metab 2002;283:E556–E564 [DOI] [PubMed] [Google Scholar]
- 15.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring H-U: Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008;168:1609–1616 [DOI] [PubMed] [Google Scholar]
- 16.Perseghin G: Viewpoints on the way to a consensus session: where does insulin resistance start? The liver. Diabetes Care 2009;32(Suppl. 2):S164–S167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M: Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: the Bruneck Study. Diabetes Care 2007;30:318–324 [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin T, Abbasi F, Lamendola C, Reaven G: Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med 2007;167:642–648 [DOI] [PubMed] [Google Scholar]