Abstract
OBJECTIVE
Lower birth weight has been associated with a greater risk of metabolic diseases. The aim of this study was examine whether physical activity and aerobic fitness may modify associations between birth weigh and metabolic risk.
RESEARCH DESIGN AND METHODS
The European Youth Heart Study is a population-based study of 9 and 15 year olds (n = 1,254). Birth weight was maternally reported. Skin fold measures were used to calculate body fat and fat mass index (FMI = fat mass [kilograms]/height2). Insulin was measured using fasting blood samples. Physical activity was measured using a hip-worn accelerometer (MTI Actigraph) for >600 min/day for ≥3 days and is expressed as “average activity” (counts per minute) and time spent in above moderate intensity activity (>2000 cpm). Aerobic fitness was assessed using a maximal cycle ergometry test (watts per kilogram fat-free mass).
RESULTS
Higher birth weight was associated with higher FMI (β = 0.49 [95% CI 0.21–0.80]; P = 0.001) and greater waist circumference (0.90 [0.32–1.47]; P < 0.001), adjusted for sex, age-group, sexual maturity, height, and socioeconomic status. Lower birth weight was associated with higher fasting insulin only after further adjustment for adolescent waist circumference and height (−0.059 [−0.107 to −0.011]; P = 0.016). There was no evidence for any modification of the associations after adjustment for physical activity or aerobic fitness.
CONCLUSIONS
The present study did not find any evidence that physical activity or aerobic fitness can moderate the associations among higher birth weight and increased fat mass and greater waist circumference or between lower birth weight and insulin resistance in healthy children and adolescents.
Lower birth weight, as a marker of restricted fetal growth, has been consistently associated with greater risk of metabolic diseases in adult life such as cardiovascular disease (1) and diabetes (2). Evidence also suggests that these associations are detectable in childhood, with lower birth weight being associated with insulin resistance (3).
The associations between birth weight and later adiposity are more complex, and it can be difficult to disentangle the influence of birth weight from that of postnatal growth (4). Higher birth weight is associated with increased BMI in childhood (5,6), and this will reflect greater fat-free mass (FFM) as well as fat mass. Studies with more detailed measures of body composition in children from developed countries suggested that higher birth weight is associated with greater overall fat mass and greater FFM, when adjusted for current height (7). The magnitude of the association seems to be stronger between birth weight and FFM than for fat mass (8), whereas other studies suggested that lower birth weight may be associated with increased percent fat (9) and greater central obesity when adjusted for total fat mass (10), suggesting greater metabolic risk with low birth weight.
Studies using objectively measured physical activity have suggested that higher levels of physical activity and aerobic fitness are associated with a favorable metabolic risk profile (11) and lower levels of adiposity in healthy children and adolescents (12). It has also been suggested that physical activity (13) and aerobic fitness (14) may modify the associations between low birth weight and metabolic risk and that higher levels of physical activity and improved fitness may be a useful strategy to reduce the metabolic risks associated with compromised intrauterine growth. However, these previous studies assessed physical activity levels using self-reported methods in adults.
The aim of this present study was to examine whether objectively measured physical activity and aerobic fitness may modify associations between birth weight and metabolic risk in a population-based cohort of healthy children and adolescents. Given that increased metabolic risk and excess adiposity may already be present at an early age, it is important to understand whether higher levels of physical activity and aerobic fitness may provide benefits not only for the metabolic risks associated with low birth weight, but also for the increased risk of excess adiposity associated with higher birth weights.
RESEARCH DESIGN AND METHODS
The European Youth Heart Study (EYHS) is a population-based mixed longitudinal cohort study comprising two age-groups of 9-year-old children and 15-year-old adolescents from four European countries: Denmark, Portugal, Estonia, and Norway. The aim of the EYHS was to investigate the personal, environmental, and lifestyle influences on cardiovascular and metabolic disease risk factors. The study aims, population, selection criteria, and methods have been described previously (15). In brief, study participants were randomly selected on the basis of school-level groups, with at least 20 schools within each area.
The present study is based on those children and adolescents with data on maternally reported birth weight and fasting blood samples from Denmark, Portugal, and Estonia (n = 1,254) as fasting blood samples were not collected in the Norwegian cohort. A small number of children (n = 9), who were classified as very low birth weight (<1.5 kg) were excluded from the analyses because very low birth weight may be may be associated with other health issues and can be indicative of premature birth, as information on gestational age was not available. Data collected via parental self-report were available for socioeconomic status (SES), which was categorized according to the mean for parental income and educational level.
Written informed consent was obtained from a parent or guardian, and the study procedures were explained verbally to all children. Ethics approval for the study was obtained from the local research ethics committees in each study region.
Anthropometry and sexual maturity
Anthropometric measurements were collected while subjects were wearing light clothes. Trained observers evaluated sexual maturity according to Tanner stages of breast development in girls and pubic hair in boys (16), with children categorized as being prepubertal (Tanner stage 1), midpubertal (Tanner stage 2), or pubertal (Tanner stages 3, 4, and 5). Waist circumference was measured at the midpoint between the iliac crest and the lowest rib, using a metal anthropometric tape, with the mean of two measures being used for the analysis. Weight was measured with a standard calibrated beam balance to the nearest 0.1 kg. Height was measured using a stadiometer (Harpenden; Baty International, Burgess Hill, West Sussex, U.K.) to the nearest 0.5 cm. BMI was calculated as weight in kilograms divided by the square of height in meters.
Skinfold measures were taken with Harpenden skinfold calipers according to standardized methods. Body fat percentage or fat mass was estimated using seven different child-specific equations (17) and was expressed relative to height: fat mass index [FMI] = fat mass [kilograms]/height [meters]2. The results from each equation were pooled to provide a mean FMI for each participant.
Fasting insulin
Because the study population is generally composed of healthy children and adolescents, we used fasting insulin as a marker of insulin resistance. Overnight fasting blood samples were taken in the morning from the antecubital vein. Samples were divided into aliquots, separated within 30 min, and stored at −80°C until transport to World Health Organization–certified laboratories for analyses. All biochemical analysis was performed by one of two World Health Organization–certified laboratories. Insulin was analyzed using an enzyme immunoassay (microtiter plate format; Dako Diagnostics, Ely, U.K.) in the Bristol laboratory (Danish and Estonian samples) and by two-site immunometric assays with either 125I or alkaline phosphatase labels in the Cambridge laboratory (Portuguese samples). To provide a further marker of insulin resistance, the homeostasis model assessment (HOMA) index was calculated using the homeostasis model of insulin resistance: HOMA-IR = (fasting glucose × fasting insulin)/22.
Physical activity
Physical activity was objectively measured using a hip-worn accelerometer (MTI Actigraph; Manufacturing Technology, Fort Walton Beach, FL) for 4 days. We derived two measures of physical activity: Average activity was defined as total activity counts divided by monitor wear time and expressed as counts per minute per day. Time (minutes per day) spent in moderate and vigorous intensity physical activity (MVPA) was defined as all minutes above a threshold of 2,000 counts as described previously (18). We excluded all time blocks with ≥10 consecutive zero counts, assuming that the monitor was not worn, and all children with <600 min/day for <3 days were excluded. All physical activity variables were also adjusted for total monitor wear time, to adjust for how long the monitor was worn.
Aerobic fitness
Aerobic fitness was assessed using a progressive cycle ergometer test (19) with workloads increased every 3 min until the child was unable to continue even after verbal encouragement. Heart rate data were collected every 5 s throughout the duration of the test (Polar Sport Tester, Polar Oy, Finland). A heart rate ≥185 bpm at the end of the test was used as the criterion for achieving a maximal test, and aerobic fitness was expressed relative to FFM as watts per kilogram FFM.
Statistical analyses
All data were analyzed in continuous form, and nonnormally distributed variables (fasting insulin and HOMA-IR) were log transformed. Multiple linear regression analyses were performed to assess the associations between birth weight and outcome variables (FMI, waist, fasting insulin, and HOMA-IR). Our initial model was adjusted for sex, age-group, study location, sexual maturation, and SES. Further adjustment for adolescent height was made when waist circumference was the outcome of interest to investigate whether associations were independent of later height. Further adjustments for height and waist were also made when insulin was the outcome of interest to investigate whether these associations were independent of central adiposity. Models were assessed for age-group by sex interaction, but because inclusion of this interaction term did not materially change the effect size for any of the models, they were excluded from the final models. We also tested for nonlinear associations with birth weight by entering birth weight as a quadratic term.
These models were then repeated, adjusting for total physical activity (counts per minute) or minutes of MVPA to investigate whether these variables moderated the association between birth weight and FMI, waist, and insulin. Finally, the model was adjusted for aerobic fitness (watts per kilogram FFM). We also examined whether physical activity or fitness modified the associations between birth weight and the outcomes of interest by introducing an interaction term of birth weight × average activity, birth weight × MVPA, and birth weight × fitness in the models.
All analysis was performed using SPSS (version 14). The significance level was P < 0.05.
RESULTS
Descriptive statistics, including means ± SD for the study population are displayed in supplementary Table 1 (available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-1178/DC1). There were significant differences between sex and age-groups for most physical characteristics, so all models were adjusted for sex and age-group, as well as study location, SES, and sexual maturation.
Birth weight and FMI
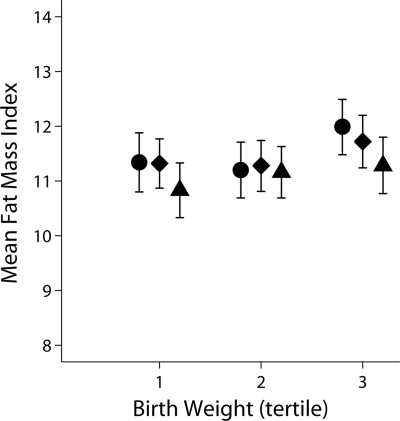
Higher birth weight was associated with higher FMI, with a 1-kg increase in birth weight associated with 0.49 kg fat mass/m2 increase in FMI (β = 0.49 [0.21–0.80]; P = 0.001), adjusted for sex, age-group, sexual maturity, and SES. Further adjustment for physical activity, as either average activity or minutes of MVPA per day, had little influence on the association between birth weight and FMI and the coefficients were materially unchanged (Table 1). Figure 1 shows FMI stratified by tertiles of birth weight and MVPA and indicates lower FMI by higher tertiles of MVPA, particularly in the third tertile of birth weight, although the confidence intervals overlap and do not reach statistical significance. Further adjustment for aerobic fitness also had little influence on the association between birth weight and FMI. A formal test for interaction showed no evidence that the association between birth weight and FMI was modified by physical activity levels or aerobic fitness. There was also no evidence for a sex × age-group interaction for FMI.
Table 1.
Associations between birth weight and FMI, waist circumference, and fasting insulin
| Model 1 | P value | Model 2 | P value | Model 3 | P value | Model 4 | P value | |
|---|---|---|---|---|---|---|---|---|
| FMI | 0.49 (0.21 to 0.80) | 0.001 | 0.49 (0.21 to 0.78) | 0.001 | 0.49 (0.21 to 0.78) | 0.001 | 0.52 (0.24 to 0.80) | <0.001 |
| Waist (cm)* | 0.90 (0.32 to 1.47) | 0.002 | 0.90 (0.32 to 1.47) | 0.002 | 0.89 (0.32 to 1.47) | 0.002 | 0.94 (0.37 to 1.50) | 0.001 |
| Insulin (log) | −0.005 (−0.055 to 0.045) | 0.844 | −0.007 (−0.057 to 0.043) | 0.788 | −0.008 (−0.058 to 0.042) | 0.745 | −0.005 (−0.053 to 0.044) | 0.856 |
| Insulin (log)† | −0.059 (−0.107 to −0.011) | 0.016 | −0.061 (−0.109 to −0.013) | 0.012 | −0.062 (−0.110 to −0.015) | 0.010 | −0.058 (−0.105 to −0.010) | 0.017 |
| HOMA-IR (log)† | −0.056 (−0.107 to −0.006) | 0.029 | −0.059 (−0.109 to −0.008) | 0.024 | −0.059 (−0.109 to −0.009) | 0.021 | −0.054 (−0.104 to −0.004) | 0.034 |
Data are β coefficients (95% CI). n = 1,254. Model 1: adjusted for sex, age-group, country, maturity, and SES. Model 2: adjusted for sex, age-group, country, maturity, SES, monitor worn time, and MVPA (minutes per day). Model 3: adjusted for sex, age-group, country, maturity, SES, monitor worn time, and average physical activity (counts per minute). Model 4: adjusted for sex, age-group, country, maturity, SES, monitor worn time, total physical activity (counts per minute), and aerobic fitness (watts per kilogram FFM).
*Additionally adjusted for adolescent height.
†Additionally adjusted for adolescent height and waist circumference.
Figure 1.
Associations between birth weight and FMI, stratified by tertiles of time spent in MVPA (minutes per day). Data (adjusted means, 95% CI) are adjusted for sex, age-group, country, sexual maturation, SES, and monitor wear time. ●, low MVPA; ♦, medium MVPA; ▴, high MVPA.
Similar results were observed if FMI was substituted with percent body fat as the outcome variable, with birth weight being positively associated with percent body fat, independently of sex, age-group, country, sexual maturation, and maternal BMI, with little change in the magnitude of association if further adjusted for physical activity or fitness (data not shown).
Birth weight and waist circumference
Higher birth weight was associated with a greater waist circumference in childhood, with a 1-kg increase in birth weight being associated with a 0.90-cm increase in waist circumference (β = 0.90 [0.32–1.47]; P = 0.002), adjusted for sex, age-group, sexual maturity, height, and SES. Again further adjustment for physical activity, either for average activity or time spent in MVPA, made little change in the birth weight and waist circumference association and the magnitude of the association was largely unaffected. Further adjustment to the model for aerobic fitness produced a very slight increase in the association between birth weight and waist circumference (0.94 [0.37–1.50]; P = 0.001) (Table 1). A formal test for interaction showed no evidence that the association between birth weight and waist circumference was modified by physical activity or aerobic fitness.
Birth weight and insulin resistance
Birth weight was not associated with fasting insulin (β = −0.005 [−0.055 to 0.045]; P = 0.844), which again was materially unchanged after further adjustment for either total physical activity or time spent in MVPA (Table 1). However, when adolescent waist circumference and height were introduced to the model, lower birth weight was associated with higher fasting insulin (−0.059 [−0.107 to −0.011]; P = 0.016), and further adjustment for physical activity or fitness had little influence on the birth weight fasting insulin association and the β coefficients remained almost unchanged (Table 1). Substituting fasting insulin for HOMA-IR did not change the observed effect size (−0.056 [−0.107 to −0.006]; P = 0.029), and further adjustment for physical activity or aerobic fitness did not modify this association.
Similar results were observed when waist circumference was substituted by current body weight in the model, in that there was a significant association between birth weight and fasting insulin (β = −0.058 [95% CI −0.106 to −0.010]; P = 0.018), after adjustment for sex, age-group, sexual maturity, SES, and current body weight (kilograms). However, if waist circumference was substituted by current FMI, the association between birth weight and fasting insulin was attenuated and no longer significant (−0.032 [−0.080 to 0.015]; P = 0.184). There was also no evidence of statistical interaction in the association between birth weight and fasting insulin by level of physical activity or aerobic fitness.
CONCLUSIONS
Current levels of physical activity and aerobic fitness did not moderate or modify the associations between birth weight and FMI, waist circumference, or fasting insulin. Our findings suggest that higher birth weight was associated with increased FMI and greater waist circumference, whereas lower birth weight was associated with greater metabolic risk in terms of higher fasting insulin but only after further adjustment for a measure of current adiposity (i.e., waist circumference or weight).
Our results are in contrast with two previous studies reporting that higher physical activity levels or aerobic fitness may moderate the associations between size at birth and metabolic risk. Laaksonen et al. (14) found the associations for ponderal index (ponderal index = birth length [meters]/birth weight [kilograms3]) were attenuated in adult men reporting >25 min/week of vigorous leisure time activity and in those classified in the “fit” above the 40th centile for Vo2 max (≥28.6 ml/kg/min). Eriksson et al. (13) observed an interaction between reported physical activity levels and glucose intolerance in men, with both higher frequency and intensity of physical activity being associated with lower glucose intolerance, especially in those with birth weight <3 kg. There are, however, a number of potential reasons for the differences between the current study and these previous reports.
The two previous studies investigating the influence of physical activity on the association between birth weight and metabolic risk both used self-reported methods to assess physical activity levels, whereas we used an objective measure of physical activity by accelerometry. Any self-report measure will be subject to issues of recall bias, but it should also be noted that questionnaires may capture different aspects of physical activity compared with accelerometry. Self-reported physical activity usually only considers regular, distinct activities, such as specific types of exercise and leisure time activity, whereas accelerometers measure body movement throughout the measurement period. Self-report methods are unlikely to fully capture incidental activity, which may be hard to recall, whereas accelerometers are limited to detecting certain movements. For example in this study the hip-worn monitor may not capture cycling or activities for which the monitor was removed, such as swimming. Indeed, these two measures are only weakly correlated, typically at ∼0.3 in adults (20). Furthermore, the questionnaires used in the previous studies captured information on physical activity during the preceding 12 months, whereas we, for practical reasons, only measured physical activity for 3–4 days. However, we included both weekdays and weekend days and a 4-day measurement period has been suggested to be sufficient when physical activity is assessed by accelerometry in youth (21). Although adiposity tends to reflect long-term energy balance, fasting insulin levels can be influenced by very recent physical activity levels (22).
The lack of a modifying effect of physical activity or aerobic fitness in the present study compared with previous observations may also be explained by differences in the study populations. We examined the influence of physical activity on the associations between birth weight and metabolic risk in healthy children and adolescents, whereas the two previous studies were performed in middle-aged men (14) and older adults aged 65–75 years, respectively (13). It is possible that the beneficial effect of physical activity or aerobic fitness on the association between birth weight and metabolic risk may not be apparent until later in life when the age-related increase in metabolic risk is more pronounced (2).
The finding that birth weight was positively associated with FMI is consistent with previous findings in adolescents (23). However, FMI includes an adjustment for current body size and therefore does not preclude the possibility that postnatal growth may influence the association between birth weight and FMI. A detailed measure of body fat distribution was not available; however, previous studies have also observed positive associations between birth weight and waist circumference in younger children (8).
The observed association between low birth weight and insulin resistance was only observed after adjustment for current waist circumference or weight, but this association was attenuated if adjusted for any measure of general adiposity (e.g., FMI or fat mass). This finding suggests that current adiposity more strongly influences insulin levels than current body size. However, it is hard to disentangle the pathways involved between birth weight and later insulin resistance, when adjusting for a measure of body size, because measures such as current weight will be influenced by both fat mass and FFM. A previous study investigating the associations between birth weight and insulin resistance in the EYHS study population showed an association between lower birth weight and HOMA score, which was augmented after further adjustment for current body size (BMI or height) (3). The statistically weaker association observed in the present study, which included a subset of the EYHS population with complete physical activity data, may be explained by reduced statistical power. It is also plausible that when an association between birth weight and a metabolic outcome (e.g., insulin resistance) is only detected after adjustment for current body size, it may be indicative of an association between change in size between birth and the later measurement, such as rapid weight gain (4). However, we did not have an intermediate measure of body size or composition in earlier childhood to be able to investigate this further. Further studies with repeated measures of body composition would be particularly valuable for investigating associations among birth weight, infant and childhood weight gain, and later metabolic risk.
Although we did not detect any influence of physical activity or aerobic fitness on the birth weight metabolic risk association, there is a wide range of evidence to suggest that higher levels of physical activity may be beneficial for reducing insulin resistance and metabolic risk (12) as well as adiposity (11). Given that the influence of physical activity and aerobic fitness on the association between birth weight and metabolic health in the two previous studies was in older adults, it may be difficult to detect the influence in young healthy populations. Further research in older populations or those at higher metabolic risk, using objectively measured physical activity and aerobic fitness data, would be particularly useful to investigate whether increasing physical activity levels or improved aerobic fitness may reduce insulin resistance in those born small or improve body composition, particularly in those born at the higher end of the birth weight spectrum who may be at increased risk of later obesity.
The following limitations should be considered when interpreting the results from the present study. First, we used skinfold, height, and weight equations to derive FMI, which may not be as accurate as other methods, such as dual-energy X-ray absorptiometry, deuterium dilution, or plethysmography. However, we estimated FMI as an individual aggregate using seven different equations to increase the accuracy of this method (17). Further, this method is more feasible to use in large population-based studies, provides a more comprehensive measure of adiposity than BMI, and is comparable to more detailed measures of body composition (24). Although birth weight was maternally reported and not objectively measured in this study, maternally reported birth weight correlates highly with measured birth weight (25). Unfortunately, data on gestational age were not available, so residual confounding by gestational age may persist, although very low-birth-weight infants (<1.5 kg) were excluded. Lastly, it should be noted that potential confounding variables included in the models (maternal BMI and SES) were self-reported and may be subject to recall bias. Strengths of this study include a large population-based sample of children and adolescents from three differing countries combined with an objective measure of physical activity, a maximal aerobic fitness test, and fasting blood samples.
In summary, the results from the present study did not show that physical activity or aerobic fitness can moderate or modify the associations between low birth weight and insulin resistance or between higher birth weight and increased fat mass and greater waist circumference in healthy children and adolescents. However, higher levels of physical activity are beneficial for both body composition and metabolic risk throughout the life course and the moderating effect of physical activity on the birth weight and metabolic risk association may only become apparent later in adult life or within populations at greater metabolic risk.
Acknowledgments
This study was supported by the following: the Danish Heart Foundation; the Danish Medical Research Council Health Foundation; the Danish Council for Sports Research; the Foundation in Memory of Asta Florida Bolding Renée Andersen; the Faculty of Health Sciences, University of Southern Denmark; the Estonian Science Foundation (grants 3277 and 5209); the Norwegian Council of Cardiovascular Diseases; the Eckbo Legacy; and the European Social Fund.
No potential conflicts of interest relevant to this article were reported.
C.L.R. conceived the hypothesis for the study, analyzed data, interpreted data, wrote the manuscript, and reviewed/edited the manuscript. S.B. conceived the hypothesis for the study, interpreted data, and reviewed/edited the manuscript. S.A.A., L.B.S., and L.B.A. collected data, organized the field work, and reviewed/edited the manuscript. U.E. conceived the hypothesis for the study, interpreted data, wrote the manuscript, and reviewed/edited the manuscript.
We thank all those involved with the European Youth Heart Study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Barker DJ: Mothers, Babies and Health in Later Life. Edinburgh, Churchill Livingstone, 1998 [Google Scholar]
- 2.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S, de Rooij SR, Dyck RF, Eriksson JG, Falkner B, Fall C, Forsén T, Grill V, Gudnason V, Hulman S, Hyppönen E, Jeffreys M, Lawlor DA, Leon DA, Minami J, Mishra G, Osmond C, Power C, Rich-Edwards JW, Roseboom TJ, Sachdev HS, Syddall H, Thorsdottir I, Vanhala M, Wadsworth M, Yarbrough DE: Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008;300:2886–2897 [DOI] [PubMed] [Google Scholar]
- 3.Lawlor DA, Riddoch CJ, Page AS, Anderssen SA, Froberg K, Harro M, Stansbie D, Smith GD: The association of birthweight and contemporary size with insulin resistance among children from Estonia and Denmark: findings from the European Youth Heart Study. Diabet Med 2005;22:921–930 [DOI] [PubMed] [Google Scholar]
- 4.Lucas A, Fewtrell MS, Cole TJ: Fetal origins of adult disease-the hypothesis revisited. BMJ 1999;319:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers I: The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord 2003;27:755–777 [DOI] [PubMed] [Google Scholar]
- 6.Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, Steer C, Sherriff A: Early life risk factors for obesity in childhood: cohort study. BMJ 2005;330:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers IS, Ness AR, Steer CD, Wells JC, Emmett PM, Reilly JR, Tobias J, Smith GD: Associations of size at birth and dual-energy X-ray absorptiometry measures of lean and fat mass at 9 to 10 y of age. Am J Clin Nutr 2006;84:739–747 [DOI] [PubMed] [Google Scholar]
- 8.Chomtho S, Wells JC, Williams JE, Lucas A, Fewtrell MS: Associations between birth weight and later body composition: evidence from the 4-component model. Am J Clin Nutr 2008;88:1040–1048 [DOI] [PubMed] [Google Scholar]
- 9.Elia M, Betts P, Jackson DM, Mulligan J: Fetal programming of body dimensions and percentage body fat measured in prepubertal children with a 4-component model of body composition, dual-energy X-ray absorptiometry, deuterium dilution, densitometry, and skinfold thicknesses. Am J Clin Nutr 2007;86:618–624 [DOI] [PubMed] [Google Scholar]
- 10.Dolan MS, Sorkin JD, Hoffman DJ: Birth weight is inversely associated with central adipose tissue in healthy children and adolescents. Obesity 2007;15:1600–1608 [DOI] [PubMed] [Google Scholar]
- 11.Steele RM, Brage S, Corder K, Wareham NJ, Ekelund U: Physical activity, cardiorespiratory fitness, and the metabolic syndrome in youth. J Appl Physiol 2008;105:342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekelund U, Sardinha LB, Anderssen SA, Harro M, Franks PW, Brage S, Cooper AR, Andersen LB, Riddoch C, Froberg K: Associations between objectively assessed physical activity and indicators of body fatness in 9- to 10-year-old European children: a population-based study from 4 distinct regions in Europe (the European Youth Heart Study). Am J Clin Nutr 2004;80:584–590 [DOI] [PubMed] [Google Scholar]
- 13.Eriksson JG, Ylihärsilä H, Forsén T, Osmond C, Barker DJ: Exercise protects against glucose intolerance in individuals with a small body size at birth. Prev Med 2004;39:164–167 [DOI] [PubMed] [Google Scholar]
- 14.Laaksonen DE, Lakka HM, Lynch J, Lakka TA, Niskanen L, Rauramaa R, Salonen JT, Kauhanen J: Cardiorespiratory fitness and vigorous leisure-time physical activity modify the association of small size at birth with the metabolic syndrome. Diabetes Care 2003;26:2156–2164 [DOI] [PubMed] [Google Scholar]
- 15.Riddoch C, Edwards D, Page A, Froberg K, Anderssen SA, Wedderkopp N, Brage S, Cooper A, Sardinha LB, Harro M, Heggebø LK, van Mechelen W, Boreham C, Ekelund U, Andersen LBEuropean Youth Heart Study Team The European Youth Heart Study—cardiovascular disease risk factors in children: rational, aims, study design, and validation of methods. J Phys Act Health 2005;2:115–129 [Google Scholar]
- 16.Tanner JM: Growth at Adolescence. Oxford, Blackwell, 1962 [Google Scholar]
- 17.Wells JC, Williams JE, Haroun D, Fewtrell MS, Colantuoni A, Siervo M: Aggregate predictions improve accuracy when calculating metabolic variables used to guide treatment. Am J Clin Nutr 2009;89:491–499 [DOI] [PubMed] [Google Scholar]
- 18.Ekelund U, Anderssen SA, Froberg K, Sardinha LB, Andersen LB, Brage SEuropean Youth Heart Study Group Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: the European youth heart study. Diabetologia 2007;50:1832–1840 [DOI] [PubMed] [Google Scholar]
- 19.Kolle E, Steene-Johannessen J, Andersen LB, Anderssen SA: Objectively assessed physical activity and aerobic fitness in a population-based sample of Norwegian 9- and 15-year-olds. Scand J Med Sci Sports 2009;14:14. [DOI] [PubMed] [Google Scholar]
- 20.Cust AE, Smith BJ, Chau J, van der Ploeg HP, Friedenreich CM, Armstrong BK, Bauman A: Validity and repeatability of the EPIC physical activity questionnaire: a validation study using accelerometers as an objective measure. Int J Behav Nutr Phys Act 2008;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trost SG, McIver KL, Pate RR: Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc 2005;37:S531–S43 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi Y, Nagasaka S, Takahashi N, Kusaka I, Ishibashi S, Numao S, Lee DJ, Taki Y, Ogata H, Tokuyama K, Tanaka K: A single bout of exercise at higher intensity enhances glucose effectiveness in sedentary men. J Clin Endocrinol Metab 2005;90:4035–4040 [DOI] [PubMed] [Google Scholar]
- 23.Eriksson M, Tynelius P, Rasmussen F: Associations of birthweight and infant growth with body composition at age 15—the COMPASS study. Paediatr Perinat Epidemiol 2008;22:379–388 [DOI] [PubMed] [Google Scholar]
- 24.Wells JC, Fewtrell MS: Measuring body composition. Arch Dis Child 2006;91:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adegboye AR, Heitmann B: Accuracy and correlates of maternal recall of birthweight and gestational age. BJOG 2008;115:886–893 [DOI] [PMC free article] [PubMed] [Google Scholar]