Abstract
OBJECTIVE
To determine the effect of treatment with insulin aspart compared with NPH insulin, together with metformin/placebo and rosiglitazone/placebo. The hypothesis was that combined correction of major pathogenetic defects in type 2 diabetes would result in optimal glycemic control.
RESEARCH DESIGN AND METHODS
This study was a 2-year investigator-driven randomized partly placebo-controlled multicenter trial in 371 patients with type 2 diabetes on at least oral antiglycemic treatment. Patients were assigned to one of eight treatment groups in a factorial design with insulin aspart at mealtimes versus NPH insulin once daily at bedtime, metformin twice daily versus placebo, and rosiglitazone twice daily versus placebo. The main outcome measurement was change in A1C.
RESULTS
A1C decreased more in patients treated with insulin aspart compared with NPH (−0.41 ± 0.10%, P < 0.001). Metformin decreased A1C compared with placebo (−0.60 ± 0.10%, P < 0.001), as did rosiglitazone (−0.55 ± 0.10%, P < 0.001). Triple therapy (rosiglitazone, metformin, and any insulin) resulted in a greater reduction in A1C than rosiglitazone plus insulin (−0.50 ± 0.14%, P < 0.001) and metformin plus insulin (−0.45 ± 0.14%, P < 0.001). Aspart was associated with a higher increase in body weight (1.6 ± 0.6 kg, P < 0.01) and higher incidence of mild daytime hypoglycemia (4.9 ± 7.5 vs. 1.7 ± 5.4 number/person/year, P < 0.001) compared with NPH.
CONCLUSIONS
Insulin treatment of postprandial hyperglycemia results in lower A1C than treatment of fasting hyperglycemia, at the expense of higher body weight and hypoglycemic episodes. However, insulin therapy has to be combined with treatment of both peripheral and liver insulin resistance to normalize blood glucose, and in this case, the insulin regimen is less important.
Only a few long-term studies have focused on different insulin treatment modalities and the blinded combination with different oral antidiabetic drugs. Theoretically, the best antiglycemic treatment is to aim at restoring the main pathophysiological defects in type 2 diabetes: decreased first-phase insulin secretion, peripheral insulin resistance, and elevated hepatic gluconeogenesis. These defects may theoretically be partly corrected pharmacologically by a rapid-acting insulin analog before meals, mimicking the lacking postprandial insulin peak; by an insulin sensitizer improving peripheral insulin action; and by metformin reducing hepatic glucose production (1).
The South Danish Diabetes Study is an investigator-driven 2-year randomized controlled clinical trial testing the following hypotheses: 1) the more physiological insulin profile obtained with insulin aspart treatment at meals (without long-acting insulin at night) is more effective than the conventional use of NPH insulin given once daily at bedtime; 2) addition of metformin or rosiglitazone to insulin treatment will further improve the glucose control; and 3) combination of insulin, metformin, and rosiglitazone (triple therapy) will result in the most optimal glycemic control.
RESEARCH DESIGN AND METHODS
Subjects
Patients aged 30–70 years with type 2 diabetes were included at eight hospital centers in the southern region of Denmark. Eligible patients had the following characteristics: BMI >25 kg/m2 and fasting plasma C-peptide >300 pmol/l, treatment for at least 3 months with stable doses of oral antidiabetic medications and/or insulin, and A1C >7.0%. Prior insulin treatment could be any insulin regimen, but most subjects were treated with long-acting insulin. The exclusion criteria were congestive heart failure, impaired renal function, and known intolerance to metformin or rosiglitazone and/or treatment with glitazones <30 days before randomization.
Study design
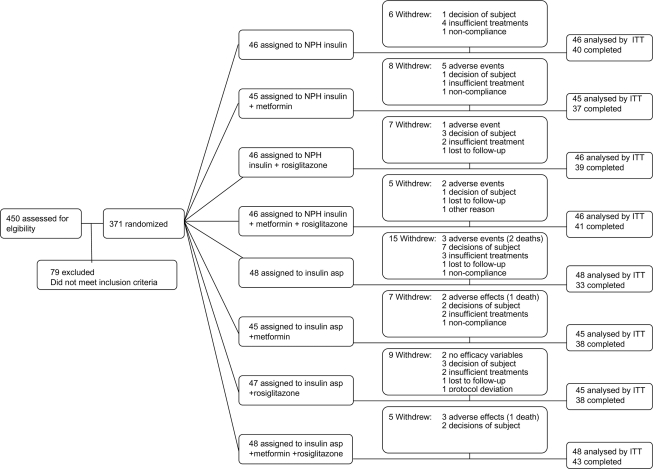
A total of 450 subjects were included. After a 4-week run-in period, all 371 eligible patients were randomized to one of eight treatment groups in a factorial design with NPH insulin versus insulin aspart, metformin versus placebo, and rosiglitazone versus placebo (Fig. 1). After a 3-month insulin titration period, patients were followed every 3 months for 2 years.
Figure 1.
Enrollment and outcomes. The number of participants enrolled in the study is shown. The intention-to-treat (ITT) population included 369 patients, since 2 were withdrawn before first efficacy evaluation. The per-protocol population included 251 patients. asp, aspart.
Intervention
All prior antidiabetic treatments were stopped. Patients allocated to NPH insulin at bedtime measured fasting blood glucose (FBG) every day and received a starting dose of 12 IU. Patients already on insulin received 50% of their prior total daily dose. In a treat-to-target algorithm, insulin dose was increased by 2 IU if FBG was >5.6 mmol/l, 4 IU if FBG was >8.0 mmol/l, and 6 IU if FBG was >12.0 mmol/l on 3 consecutive days until FBG was ≤5.5 mmol/l and A1C was <6.5%, provided no unacceptable hypoglycemic episodes.
Patients allocated to insulin aspart measured postprandial blood glucose three times daily 90 min after each main meal and received a starting dose of 4 IU just before each main meal. Patients already on insulin received 50% of their prior total daily dose divided into three doses. In a treat-to-target algorithm, insulin dose at each meal was increased by 1 IU if postprandial blood glucose was ≥7.5 mmol/l, 2 IU if postprandial blood glucose was ≥9.0 mmol/l, 3 IU if postprandial blood glucose was ≥11.0 mmol/l on 3 consecutive days until postprandial blood glucose was <7.5 mmol/l and A1C was <6.5%, provided no limiting hypoglycemic episodes.
After a 3-month intensive insulin titration period, patients were instructed to continue using the algorithm, but daily blood glucose monitoring was not requested if the treatment goals were achieved.
Metformin or placebo was given from the start of the study as one tablet of 500 mg twice daily during the first 4 weeks succeeded by two tablets twice daily, and rosiglitazone or placebo was given as one tablet of 4 mg once daily in the first 8 weeks succeeded by one tablet twice daily.
An increase of A1C by >2.0% (absolute) or A1C exceeding 12.0% (absolute), measured twice over a 6-month period, were considered treatment failures.
Biochemical and clinical measurements
A1C was measured every 3 months. Patients monitored capillary blood glucose daily (One Touch Ultra, LifeScan) and performed two eight-point 24-h glucose profiles before each visit.
Safety assessments
Any adverse event was recorded. Hypoglycemic episodes were registered by the patients every day in a diary and were defined as either mild (blood glucose >2.8 mmol/l and symptoms consistent with hypoglycemia) or moderate (blood glucose ≤2.8 mmol/l with or without symptoms). Serious hypoglycemia was defined as any hypoglycemic episode requiring assistance.
Protocol oversight
The protocol was in accordance with the Declaration of Helsinki and approved by the regional committee on Biomedical Research Ethics (M-2417-02). GCP monitoring was performed by the local GCP unit and a contract company. Statistical analysis was performed by an independent statistician. The statistical analysis plan was completed before the database was locked and unblinded. Safety data were reviewed unblinded during the study by an independent academic diabetologist. The randomization code was developed by an independent statistician using a computer random number generator to select random blocks of eight. Randomization to insulin type was open, whereas allocation to other treatments was double-blinded.
Sample size
The primary outcome variable was A1C. Assuming a minimal relevant difference between the two insulin treatment arms of 0.4% and a standard deviation of 1.15, a total of 176 in each of the pooled insulin treatment groups were calculated as necessary to provide the study with 90% power to detect a difference of this magnitude (P < 0.05). Assuming a dropout rate of 10%, a total of around 400 subjects were planned to be included.
Statistical analysis
The factorial design of the study allowed us to compare the effect of each component in the antidiabetic treatment evaluated but not to compare every eight treatment groups with each other. In accordance with the hypothesis, we compared insulin aspart with NPH insulin, determined the effect of adding metformin and/or rosiglitazone to insulin treatment, and paid special attention to the effect of triple therapy (insulin plus metformin plus rosiglitazone). All data are presented as means ± SD or SEM. Statistical analysis was on an intention-to-treat basis and last observation carried forward. A per-protocol analysis for the primary end point was also performed. The efficacy analysis (A1C) was performed by ANCOVA on changes from baseline to the mean of A1C for 12–24 months (inclusive) with the three treatments and center as fixed main effects and baseline A1C value as a covariate. The patient was a random effect in the model. First-order interactions and each of the following baseline covariates were also included as fixed effects in the statistical model: fasting plasma C-peptide, interaction between fasting plasma C-peptide and treatment, and previous insulin use. Treatment differences in the number of patients with A1C ≤7.0% were tested using logistic linear regression, with the three treatments and their interactions included in the model. Plasma glucose profiles were analyzed by a repeated-measures ANOVA, performed after logarithmic transformation. Hypoglycemic episodes were analyzed using a generalized linear model based on the negative binomial distribution. The number of patients experiencing at least one hypoglycemic episode was compared with the groups using the Fisher exact test.
RESULTS
Patient characteristics
There were no clinically important differences in baseline demographic and clinical characteristics between treatment groups in the study population (Table 1).
Table 1.
Baseline clinical characteristics of the study population
| NPH + placebo | NPH + metformin | NPH + rosiglitazone | NPH + both | NPH total | ASP + placebo | ASP + metformin | ASP + rosiglitazone | ASP + both | ASP total | |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 46 | 45 | 46 | 46 | 183 | 48 | 45 | 47 | 48 | 188 |
| Sex (F/M) | 13/33 | 19/26 | 18/28 | 16/30 | 66/117 | 25/23 | 17/28 | 20/27 | 13/34 | 76/112 |
| Age (years) | 55.8 ± 7.7 | 55.4 ± 8.5 | 57.3 ± 8.9 | 57.3 ± 8.2 | 56.5 ± 8.3 | 57.1 ± 8.5 | 56.1 ± 8.2 | 56.1 ± 8.3 | 55.3 ± 9.1 | 56.2 ± 8.5 |
| Diabetes duration (years) | 7.3 ± 4.3 | 8.2 ± 4.0 | 9.2 ± 6.9 | 8.1 ± 5.3 | 8.2 ± 5.2 | 9.1 ± 5.5 | 8.7 ± 4.5 | 9.4 ± 6.3 | 9.0 ± 5.8 | 9.1 ± 5.5 |
| Body weight (kg) | 100.2 ± 19.8 | 105.1 ± 17.7 | 100.9 ± 16.5 | 101.1 ± 19.3 | 102.1 ± 18.3 | 98.3 ± 16.6 | 100.5 ± 17.9 | 95.6 ± 14.6 | 99.1 ± 16.6 | 98.3 ± 16.4 |
| BMI (kg/m2) | 34.0 ± 6.0 | 35.7 ± 6.4 | 34.0 ± 5.7 | 34.4 ± 7.0 | 34.5 ± 6.3 | 33.7 ± 5.0 | 33.7 ± 6.1 | 32.7 ± 4.7 | 32.9 ± 4.4 | 33.2 ± 5.0 |
| A1C (%) | 8.7 ± 1.3 | 8.9 ± 1.2 | 8.7 ± 1.2 | 8.5 ± 1.1 | 8.7 ± 1.3 | 8.5 ± 1.2 | 8.5 ± 1.2 | 8.3 ± 1.0 | 8.5 ± 1.2 | 8.5 ± 1.1 |
| FPG (mmol/l) | 11.2 ± 2.6 | 11.3 ± 2.8 | 10.5 ± 2.4 | 10.2 ± 2.6 | 10.8 ± 2.6 | 10.7 ± 2.5 | 10.0 ± 2.3 | 9.9 ± 2.1 | 10.3 ± 2.6 | 10.2 ± 2.4 |
| Fasting C-peptide concentration (pmol/l) | 1,063 ± 495 | 1,115 ± 505 | 1,026 ± 554 | 1,099 ± 548 | 1,076 ± 523 | 1,070 ± 528 | 967 ± 460 | 975 ± 440 | 1,000 ± 467 | 1,003 ± 474 |
| Treatment at inclusion* | ||||||||||
| Metformin (%) | 78 | 84 | 70 | 67 | 75 | 71 | 69 | 70 | 63 | 68 |
| Sulfonylurea (%) | 65 | 58 | 46 | 56 | 56 | 48 | 29 | 51 | 52 | 45 |
| Insulin (%) | 29 | 31 | 46 | 30 | 34 | 48 | 53 | 51 | 42 | 48 |
Data are means ± SD unless otherwise stated.
*No patients received glitazone or DDP-4 inhibitor treatment before the study. ASP, insulin aspart; both, metformin + rosiglitazone.
Glycemic control
The overall difference between the reduction in A1C between the aspart and NPH groups (n = 175 vs. 182) was −0.41 ± 0.10% (P < 0.001) (i.e., insulin aspart was associated with a larger reduction in A1C than observed with NPH insulin). Moreover, in all patients, metformin versus placebo treatment (n = 179 vs. 178) was associated with a decrease in A1C of −0.60 ± 0.10% (P < 0.001) and rosiglitazone versus placebo treatment (n = 179 vs. 178) with a decrease of −0.55 ± 0.10% (P < 0.001). When only the per-protocol population was examined, similar results were observed.
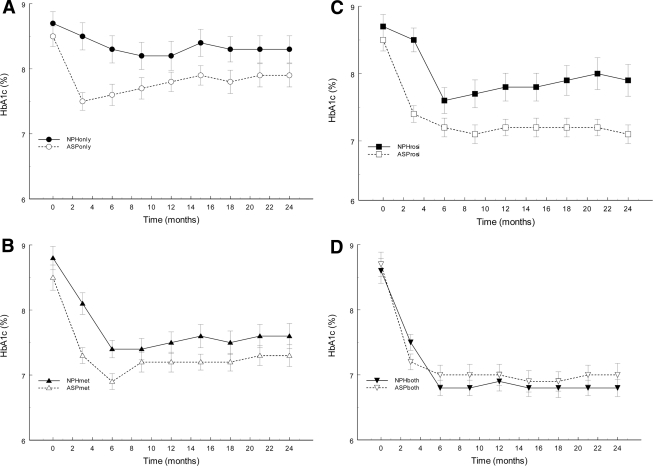
As illustrated in Fig. 2 and Table 2, A1C decreased in all eight study groups but most during addition of oral antidiabetic agents to insulin. There was no difference between the two triple therapy groups (aspart versus NPH plus metformin and rosiglitazone) (P = 0.15). Triple therapy, with any insulin, resulted in the greatest reduction in A1C compared with any insulin plus placebo (−1.14 ± 0.13%, P < 0.001), any insulin plus rosiglitazone (−0.50 ± 0.14%, P < 0.001), and any insulin plus metformin (−0.45 ± 0.14%, P < 0.001).
Figure 2.
Mean ± SE observed A1C values during the 2-year intervention period in patients randomized to treatment with either NPH insulin (black symbols) or insulin aspart (open symbols) in combination with placebo (A, P < 0.001), metformin (B, P = 0.15), rosiglitazone (C, P < 0.02), or metformin and rosiglitazone (D, P = 0.15).
Table 2.
Observed A1C, percentage of patients with A1C ≤7.0%, fasting venous plasma glucose concentration, insulin dose, body weight, and hypoglycemic episodes after 2 years of treatment
| NPH + placebo | NPH + metformin | NPH + rosiglitazone | NPH + both | ASP + placebo | ASP + metformin | ASP + rosiglitazone | ASP + both | |
|---|---|---|---|---|---|---|---|---|
| A1C (%) | 8.3 ± 1.4 | 7.6 ± 1.3 | 7.9 ± 1.6 | 6.8 ± 0.9 | 7.9 ± 1.2 | 7.3 ± 1.1 | 7.1 ± 0.9 | 7.0 ± 1.2 |
| A1C ≤7% (%) | 20 | 42 | 39 | 67 | 24 | 51 | 52 | 64 |
| Fasting glucose (mmol/l) | 7.3 ± 2.5 | 6.6 ± 2.0 | 6.6 ± 2.6 | 5.7 ± 1.5 | 12.2 ± 3.9 | 9.6 ± 3.1 | 9.3 ± 2.6 | 8.9 ± 3.3 |
| Insulin dose (IU) | 100.4 ± 64.6 | 80.1 ± 55.5 | 55.3 ± 41.1 | 39.0 ± 34.4 | 89.6 ± 58.9 | 61.2 ± 38.6 | 53.6 ± 41.3 | 46.7 ± 34.7 |
| Body weight (kg) | 105.5 ± 20.2 | 108.0 ± 19.6 | 108.5 ± 21.1 | 105.7 ± 20.9 | 104.4 ± 18.3 | 104.4 ± 19.2 | 106 ± 20.3 | 105.3 ± 19.6 |
| Hypoglycemia [Any (%)] | 35 (76) | 33 (73) | 37 (80) | 32 (70) | 43 (91) | 36 (82) | 39 (85) | 42 (88) |
| Daytime year 1 | ||||||||
| Mild | 2.3 ± 8.2 | 1.0 ± 2.7 | 1.4 ± 3.9 | 2.0 ± 4.8 | 10.1 ± 12.2 | 9.1 ± 10.5 | 9.2 ± 14.6 | 10.4 ± 12.4 |
| Moderate + severe | 0.4 ± 1.4 | 0.3 ± 1.1 | 0.2 ± 0.6 | 0.3 ± 1.2 | 3.1 ± 6.3 | 2.9 ± 4.9 | 3.6 ± 10.9 | 3.9 ± 7.7 |
| Daytime year 2 | ||||||||
| Mild | 2.6 ± 8.1 | 1.1 ± 2.9 | 1.9 ± 5.8 | 1.1 ± 2.9 | 7.2 ± 9.7 | 4.1 ± 5.5 | 4.8 ± 8.7 | 3.8 ± 5.4 |
| Moderate + severe | 0.3 ± 0.9 | 0.1 ± 0.4 | 0.2 ± 0.7 | 0.2 ± 0.7 | 2.5 ± 4.4 | 1.5 ± 2.6 | 1.6 ± 5.2 | 1.4 ± 4.0 |
| Nighttime year 1 | ||||||||
| Mild | 2.8 ± 6.1 | 2.8 ± 4.8 | 3.2 ± 5.2 | 4.3 ± 10.0 | 1.4 ± 3.1 | 1.4 ± 3.1 | 0.6 ± 1.4 | 0.5 ± 1.5 |
| Moderate + severe | 0.5 ± 1.9 | 0.4 ± 1.2 | 1.1 ± 3.0 | 1.1 ± 3.0 | 0.3 ± 1.2 | 0.2 ± 0.6 | 0.2 ± 0.7 | 0.1 ± 0.3 |
| Nighttime year 2 | ||||||||
| Mild | 2.8 ± 4.5 | 1.5 ± 2.8 | 3.3 ± 5.5 | 2.6 ± 4.9 | 0.5 ± 2.0 | 0.7 ± 3.0 | 0.1 ± 0.4 | 0.1 ± 0.5 |
| Moderate + severe | 1.1 ± 2.9 | 0.5 ± 1.3 | 1.3 ± 3.0 | 0.4 ± 1.2 | 0.2 ± 0.6 | 0.2 ± 0.7 | 0.1 ± 0.3 | 0.0 ± 0.2 |
Data are mean ± SD unless otherwise indicated. Hypoglycemic episodes were recorded as the total number of episodes in the first and second year of the intervention period. Moderate episodes were those with either plasma glucose concentrations <2.8 mmol/l or when assistance from another person was needed. Unit for daytime and nighttime hypoglycemic episodes is number/person/year. ASP, insulin aspart; both, metformin + rosiglitazone.
The percentages of patients reaching the A1C target of ≤7.0% in all patients were as follows: aspart versus NPH, 48 vs. 42% (P = 0.25); metformin versus placebo, 56 vs. 34% (P < 0.001); and rosiglitazone versus placebo, 56 vs. 34% (P = 0.002). The percentages reaching the same goal in the two triple therapy groups, aspart versus NPH, were 64 versus 67% (P = 0.15).
Self-monitored plasma glucose profiles were significantly lower using insulin aspart versus NPH insulin (P = 0.005), metformin versus placebo (P < 0.001), and rosiglitazone versus placebo (P < 0.001) (supplementary Fig. 1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0531/DC1).
Insulin dose
Insulin dose was highest in the groups treated by insulin alone, whereas addition of either metformin or rosiglitazone resulted in a decrease in total daily insulin dose and addition of both metformin and rosiglitazone resulted in the lowest insulin dose (Table 2).
Hypoglycemia
Overall, more patients using insulin aspart reported at least one hypoglycemic episode compared with the patients using NPH insulin (160 vs. 137, P = 0.005), with no difference between the metformin and placebo groups (143 vs. 154, NS) or between the rosiglitazone and placebo groups (150 vs. 147, NS).
During the last year of intervention, treatment with insulin aspart was associated with more total daytime hypoglycemic episodes compared with NPH insulin (6.7 ± 9.9 vs. 1.9 ± 5.7, P < 0.001). However, the total number of nocturnal hypoglycemic episodes was higher in the NPH insulin group than in the aspart group (3.0 ± 6.3 vs. 0.5 ± 2.1, P < 0.001). During the entire intervention period, eight patients in the NPH insulin group experienced eight episodes of severe hypoglycemia and 11 patients in the aspart group experienced 13 episodes.
Body weight
Body weight increased in all treatment groups (Table 2). Overall, insulin aspart was associated with an increase in body weight of 1.6 ± 0.6 kg (P = 0.009) compared with NPH insulin, rosiglitazone with an increase of 2.3 ± 0.6 kg (P < 0.001) compared with non-rosiglitazone treatment, and metformin with a decrease in body weight of 2.8 ± 0.6 kg (P < 0.001) compared with non-metformin treatment.
Adverse events
When the NPH and insulin aspart groups were compared, statistically significant more adverse events were found in the insulin aspart group (861 vs. 723) (P < 0.003). There was no statistically significant differences between specific adverse events between the two groups and no difference in number of serious adverse events was found (51 vs. 56) or death (4 vs. 0, Fig. 1). No difference in adverse events, either in adverse event or in serious adverse event was found comparing the metformin and placebo group (serious adverse event: 53 vs. 54) or the rosiglitazone and placebo group (serious adverse event: 54 vs. 56). (The details of the specific adverse events can be found in supplementary Table 1).
CONCLUSIONS
Effect of insulin treatment on blood glucose control
The study provides evidence that monotherapy with NPH insulin at bedtime is not an optimal way of treating hyperglycemia in type 2 diabetes, although this treatment has been recommended until recently (2,3). It could be argued that a long-acting insulin analog would have performed better, but in the LANMET treat-to-target trial comparing NPH insulin and insulin glargine (4), the two insulin treatments combined with metformin gave identical A1C values.
Treatment with insulin aspart resulted in better blood glucose control than NPH insulin, even though aspart was not combined with any basal insulin. The difference between aspart and NPH treatment on A1C disappeared when combined with metformin or both metformin and rosiglitazone. The benefit of prandial insulin treatment was also found during the first year of the 4T study (5) but disappeared in the 2-year follow-up (6) when basal-bolus insulin treatments were combined.
Recently, another study showed a nonsignificant difference in favor of insulin lispro compared with insulin glargine of ∼0.2% in A1C (7), which is supported by an older study also comparing insulin lispro with insulin glargine and showing a significant reduction in A1C of 0.8% in the prandial insulin lispro group (8).
It has been shown that postprandial hyperglycemia is an important determinant for the level of A1C, especially the closer the A1C is to the treatment goal (9). This result supports the fact that treatment aimed at replacing first-phase insulin secretion is important. Furthermore, an advantage of the solely prandial insulin regimens is that, during the night, patients are only covered by endogenous insulin production and therefore do not develop nocturnal hypoglycemia (10).
Effect of treatment with oral hypoglycemic agents on blood glucose control
Both metformin and rosiglitazone as add-ons to insulin treatment improved the metabolic control significantly and lowered the insulin dose, indicating that it is important not only to give insulin, but also to improve insulin action in peripheral tissues and in the liver. Several previous studies have addressed the issue of combining insulin and metformin treatment and have indicated a clear advantage of doing so (11–15).
Most studies on combination treatment with glitazone and insulin have found an improvement in glycemic control compared with insulin given in monotherapy (15–19). Moreover, glitazones also have an insulin-sparing effect (15–18). However, the combination treatment is associated with an increase in body weight (15–19).
Effect of triple therapy treatment on blood glucose control
The most optimal treatment with respect to glycemic control in our study was triple therapy using insulin, rosiglitazone, and metformin. Around 66% of our patients treated with triple therapy reached the A1C target of <7.0%. A similar high proportion of responders have only rarely been achieved in other randomized trials of insulin treatment in type 2 diabetes besides in the advanced insulin regimens groups in the 4T trial (6). The same combination therapy has been investigated in a few short-term studies previously (10,20–23).
The triple concept gave identical results no matter what insulin regimen was used. The reason for this seems to be that insulin aspart only replaces first-phase insulin secretion and NPH insulin only basal insulin secretion, thus not covering the full 24-h period adequately. Based on the 4T follow-up study (6), it may be speculated that the combined effect of prandial aspart and basal NPH insulin is the best treatment.
Hypoglycemia
The overall numbers of clinically relevant hypoglycemic episodes were low, being comparable to the numbers reported in the 4T trial (5). Despite the surplus of hypoglycemic episodes in the insulin aspart group, the majority of episodes were during the daytime, which may be more acceptable than nocturnal episodes seen more often in the NPH insulin group. Moreover, it can be speculated if the measurements of postprandial glucose concentration thrice daily in the aspart groups made this group more prone to register hypoglycemic episodes during the day.
Body weight
It is well known that improvement in blood glucose control often increases body weight. Insulin treatment in combination with glitazones may result in more weight gain than other treatments (24). However, interestingly, if we compared the weight gain using triple therapy with insulin, metformin, and rosiglitazone, it was comparable to that observed with insulin treatment alone, as also seen in the 4T study (5).
Adverse effects
There is no clear explanation for the increased number of adverse events found in the aspart-treated groups. It was seen in all system organ classes but not in the number of serious adverse events or death. All adverse events were noted by the patients in their diabetes diary, and it may be speculated if the thrice-daily measurement and notation of blood glucose made them more prone to report adverse events than the NPH group only measuring FBG.
The recently published ACCORD, ADVANCE, and VADT studies show no benefit in relation to reduction in cardiovascular disease risk with improvements in glycemic control. It is important to realize that our study was not a study designed to address this issue. Moreover, it seems from the recent follow-up study of the UK Prospective Diabetes Study (25) that normalization of glycemic control should be obtained when the diagnosis of type 2 diabetes is made and further that the benefits of improved glycemic control are maintained many years after the study intervention (25).
In conclusion, treatment of postprandial hyperglycemia with insulin aspart results in lower A1C than treatment of fasting hyperglycemia with NPH insulin, but with more side effects. For optimal treatment, insulin must be combined with treatment of both peripheral and liver insulin resistance, and, in this case, the choice of insulin regimen is less important.
Acknowledgments
The counties of southern Denmark and the Danish Medical Research Council are acknowledged for financial support. Novo Nordisk is acknowledged for supplying insulin and financial support for data management and statistical expertise, and GlaxoSmithKline is acknowledged for providing blinded tablets containing rosiglitazone/placebo and metformin/placebo. LifeScan donated the blood glucose meters.
No other potential conflicts of interest relevant to this article were reported.
J.G. and J.E.H. planned the study, researched data, and wrote the manuscript. E.G., H.J., and T.B.H. planned the study, researched data, and edited the manuscript. C.C. researched data and edited the manuscript. K.Y., H.G., H.M.H., V.V., and J.H. planned the study, researched data, and edited the manuscript. H.B.-N. planned the study, researched data, and wrote the manuscript.
Footnotes
Clinical trial registry no.: NCT00121966, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Beck-Nielsen H, Groop LC: Metabolic and genetic characterization of prediabetic states: sequence of events leading to non-insulin-dependent diabetes mellitus. J Clin Invest 1994;94:1714–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B: Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2006;49:1711–1721 [DOI] [PubMed] [Google Scholar]
- 3.Yki-Jarvinen H, Ryysy L, Nikkila K, Tulokas T, Vanamo R, Heikkila M: Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus: a randomized, controlled trial. Ann Intern Med 1999;130:389–396 [DOI] [PubMed] [Google Scholar]
- 4.Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, Vahatalo M, Virtamo H, Nikkila K, Tulokas T, Hulme S, Hardy K, McNulty S, Hanninen J, Levanen H, Lahdenpera S, Lehtonen R, Ryysy L: Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 2006;49:442–451 [DOI] [PubMed] [Google Scholar]
- 5.Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, Levy JC: Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, Paul SK: Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]
- 7.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T: Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084 [DOI] [PubMed] [Google Scholar]
- 8.Kazda C, Hulstrunk H, Helsberg K, Langer F, Forst T, Hanefeld M: Prandial insulin substitution with insulin lispro or insulin lispro mid mixture vs. basal therapy with insulin glargine: a randomized controlled trial in patients with type 2 diabetes beginning insulin therapy. J Diabetes Complications 2006;20:145–152 [DOI] [PubMed] [Google Scholar]
- 9.Monnier L, Lapinski H, Colette C: Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881–885 [DOI] [PubMed] [Google Scholar]
- 10.Poulsen MK, Henriksen JE, Hother-Nielsen O, Beck-Nielsen H: The combined effect of triple therapy with rosiglitazone, metformin, and insulin aspart in type 2 diabetic patients. Diabetes Care 2003;26:3273–3279 [DOI] [PubMed] [Google Scholar]
- 11.Ponssen HH, Elte JW, Lehert P, Schouten JP, Bets D: Combined metformin and insulin therapy for patients with type 2 diabetes mellitus. Clin Ther 2000;22:709–718 [DOI] [PubMed] [Google Scholar]
- 12.Avilès-Santa L, Sinding J, Raskin P: Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 1999;131:182–188 [DOI] [PubMed] [Google Scholar]
- 13.Kvapil M, Swatko A, Hilberg C, Shestakova M: Biphasic insulin aspart 30 plus metformin: an effective combination in type 2 diabetes. Diabetes Obes Metab 2006;8:39–48 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch IB: Metformin added to insulin therapy in poorly controlled type 2 diabetes. Diabetes Care 1999;22:854. [DOI] [PubMed] [Google Scholar]
- 15.Strowig SM, viles-Santa ML, Raskin P: Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and troglitazone in type 2 diabetes. Diabetes Care 2002;25:1691–1698 [DOI] [PubMed] [Google Scholar]
- 16.Raskin P, Rendell M, Riddle MC, Dole JF, Freed MI, Rosenstock J: A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care 2001;24:1226–1232 [DOI] [PubMed] [Google Scholar]
- 17.Schwartz S, Raskin P, Fonseca V, Graveline JF: Effect of troglitazone in insulin-treated patients with type II diabetes mellitus: Troglitazone and Exogenous Insulin Study Group. N Engl J Med 1998;338:861–866 [DOI] [PubMed] [Google Scholar]
- 18.Buse JB, Gumbiner B, Mathias NP, Nelson DM, Faja BW, Whitcomb RW: Troglitazone use in insulin-treated type 2 diabetic patients: the Troglitazone Insulin Study Group. Diabetes Care 1998;21:1455–1461 [DOI] [PubMed] [Google Scholar]
- 19.Rosenstock J, Einhorn D, Hershon K, Glazer NB, Yu S: Efficacy and safety of pioglitazone in type 2 diabetes: a randomised, placebo-controlled study in patients receiving stable insulin therapy. Int J Clin Pract 2002;56:251–257 [PubMed] [Google Scholar]
- 20.Home PD, Bailey CJ, Donaldson J, Chen H, Stewart MW: A double-blind randomized study comparing the effects of continuing or not continuing rosiglitazone + metformin therapy when starting insulin therapy in people with type 2 diabetes. Diabet Med 2007;24:618–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strowig SM, Raskin P: Combination therapy using metformin or thiazolidinediones and insulin in the treatment of diabetes mellitus. Diabetes Obes Metab 2005;7:633–641 [DOI] [PubMed] [Google Scholar]
- 22.Strowig SM, viles-Santa ML, Raskin P: Improved glycemic control without weight gain using triple therapy in type 2 diabetes. Diabetes Care 2004;27:1577–1583 [DOI] [PubMed] [Google Scholar]
- 23.Raskin P, Rogelio B, Schwartz S, Chaykin L, Chu P, Wynne A: Over 75% of patients with type 2 diabetes reached target A1C by adding biphasic insulin aspart 70/30 to optimized metformin and pioglitazone treatment (Abstract). Diabetes 55(Suppl. 1):A131, 2006 [Google Scholar]
- 24.Yki-Jarvinen H: Combination therapies with insulin in type 2 diabetes. Diabetes Care 2001;24:758–767 [DOI] [PubMed] [Google Scholar]
- 25.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR: Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359:1565–1576 [DOI] [PubMed] [Google Scholar]