Abstract
OBJECTIVE
Amylin interacts with leptin to alter metabolism. We evaluated, for the first time, amylin- and/or leptin-activated signaling pathways in human peripheral tissues (hPTs).
RESEARCH DESIGN AND METHODS
Leptin and amylin signaling studies were performed in vitro in human primary adipocytes (hPAs) and human peripheral blood mononuclear cells (hPBMCs) and ex vivo in human adipose tissue (hAT) from male versus female subjects, obese versus lean subjects, and subjects with subcutaneous versus omental adipose tissue.
RESULTS
The long form of leptin receptor was expressed in human tissues and cells studied in ex vivo and in vitro, respectively. Leptin and amylin alone and in combination activate signal transducer and activator of transcription 3 (STAT3), AMP-activated protein kinase, Akt, and extracellular signal-regulated kinase signaling pathways in hAT ex vivo and hPAs and hPBMCs in vitro; all phosphorylation events were saturable at leptin and amylin concentrations of ∼50 and ∼20 ng/ml, respectively. The effects of leptin and amylin on STAT3 phosphorylation in hPAs and hPBMCs in vitro were totally abolished under endoplasmic reticulum stress and/or in the presence of a STAT3 inhibitor. Results similar to those in the in vitro studies were observed in hAT studied ex vivo.
CONCLUSIONS
Leptin and amylin activate overlapping intracellular signaling pathways in humans and have additive, but not synergistic, effects in signaling pathways studied in hPTs in vitro and ex vivo.
Leptin is an adipocyte-secreted hormone that plays a major role in energy homeostasis and weight balance (1). Leptin not only activates central nervous system networks that suppress appetite (1) but also acts in the periphery to alter immune function and metabolism (2). Leptin administration to leptin-deficient (ob/ob) mice has been shown to reduce food intake and body mass and improve insulin resistance even before body weight is reduced (3). Moreover, leptin improves insulin resistance in leptin-deficient lipoatrophic mice and humans with the metabolic syndrome and insulin resistance (3). Amylin is a 37-amino acid peptide hormone that is cosecreted with insulin from pancreatic β-cells (4). The physiological effects of amylin receptor agonism include decreased food intake (4) and reduction of postprandial glucagon release in a glucose-dependent manner (5). Moreover, it has been proposed that treatment with a combination of amylin and leptin may be more effective than leptin or amylin alone for obesity treatment in both animals (2) and humans (5).
Comprehensive pharmacological studies demonstrated that concurrent amylin and leptin infusion synergistically reduce body weight and adiposity in diet-induced obesity in rats (6). It has also been demonstrated that acute amylin infusion amplifies central leptin signaling, and with sustained treatment amylin and leptin elicit synergistic weight and fat loss (6). Amylin-treated rats demonstrate a trend for a greater number of phosphorylated (p)-signal transducer and activator of transcription (STAT) 3–positive cells within the arcuate nucleus than vehicle-treated controls, an effect similar to that of leptin, whereas rats treated with both amylin and leptin have significantly more p-STAT3–positive cells than vehicle-, amylin-, or leptin-treated rats (7). Furthermore, this study also demonstrated that leptin receptor mRNA was significantly increased by amylin/leptin coinfusion both centrally and peripherally, i.e., in white adipose tissue and liver (7). Moreover, it has been shown that peripheral leptin injection in rodents increases STAT3 and mitogen-activated protein kinase (MAPK) phosphorylation in liver and adipose tissue (8). No previous study has evaluated leptin and amylin signaling in human peripheral tissues (hPTs) nor investigated how amylin interacts with leptin to alter signaling in hPTs.
We performed ex vivo and in vitro signaling studies to clarify the role of leptin and amylin in activating signaling pathways in hPTs as well as their potential interactions. We first investigated in vitro leptin and amylin signaling in human primary adipocytes (hPAs) and human peripheral blood mononuclear cells (hPBMCs), known cell targets of leptin action. We then performed ex vivo leptin and amylin signaling studies in human adipose tissue (hAT) from subjects with subcutaneous versus omental adipose tissue, male versus female subjects, and obese versus lean subjects.
RESEARCH DESIGN AND METHODS
We used hAT from subjects undergoing laparoscopic adjustable gastric banding, hernioplasty, liposuction, or abdominoplasty. The study protocol was approved by the Institutional Review Board at the Beth Israel Deaconess Medical Center, and subjects gave written informed consent to participate. All subjects were otherwise healthy, had no evidence of immunological or endocrine disease based on physical examination and routine blood tests, and had no history of recent infection. The subjects' age, vital signs, and BMI were recorded. We collected tissue samples from obese (six men and six women aged 31–54 years old with BMI 42–44 kg/m2) and lean (three men and four women aged 22–32 years old with BMI 21–23 kg/m2) subjects.
Materials
All primary and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Leptin human recombinants were purchased from ProSpec-Tany TechnoGene (Rehovot, Israel). Amylin human recombinants were purchased from Phoenix Pharmaceuticals (Burlingame, CA). FBS and FCS were provided by Gibco Life Technologies. BSA, α-minimal essential medium (α-MEM), RPMI 1640, NaHCO3, HEPES, biotin, pantothenate, human transferrin, gentamicin, insulin, cortisol, triiodothyronine, dithiothreitol (DTT), tunicamycin (TUN), and ciglitazone were purchased from Sigma-Aldrich (St. Louis, MO).
Real-time PCR
The long form of leptin receptor (ObRb) expression was detected with real-time (RT)-PCR. RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA), and first-strand cDNA synthesis was performed using SuperScript III (Invitrogen), according to the manufacturer's protocol. For RT-PCR, 100 ng of cDNA/25-μl reaction was amplified using a TaqMan Gene Expression system (Applied Biosystems, Foster City, CA), specific primers and a FAM-tagged probe set (Applied Biosystems), and the standard real-time 7500 protocol (Applied Biosystems), with an initial polymerase activation step at 95°C for 10 min and 40 cycles. This included a 15-s melting step at 95°C and a 1-min annealing-elongation step at 60°C. The analysis of relative gene expression was based on ΔCt values obtained from RT-PCR (9).
Ex vivo signaling study in hAT
The ex vivo culture was established according to the method described by Kim et al. (8). In brief, 5–7 g of fresh subcutaneous and/or omental hAT was placed into Krebs-Ringer-HEPES buffer (20 mmol/l, pH 7.4) with 2.5% BSA and 200 nmol/l adenosine at 37°C in the operating room and immediately taken to the laboratory for further analysis. In the laboratory, the tissue samples were minced into pieces of ∼1 mm in diameter, and any nonadipose and nonmuscle tissue was removed by washing with fresh buffer. The samples were aliquoted and incubated at 37°C either with or without amylin and leptin.
hPA culture
The hPA culture was established according to the method described by Ribet et al. (10). In brief, subcutaneous and omental hAT samples were obtained from lean (35–41 years old, BMI 22–25 kg/m2) and obese (34–48 years old, BMI 39–50 kg/m2) men and women, respectively. The hAT was then digested with PBS/collagenase solution (3 mg collagenase/g tissue and 1 ml PBS/1 mg collagenase) + 3.5% fatty acid–free BSA and then filtered using a filter bottle unit (sterile funnel with double-layered gauze), and the solution was centrifuged at 1200 rpm for 10 min. The pellets were resuspended in α-MEM supplemented with 15 mmol/l NaHCO3, 15 mmol/l HEPES, 33 μmol/l biotin, 17 μmol/l pantothenate, 10 mg/ml human transferrin, 0.05 mg/ml gentamicin, and 10% FBS and then were plated overnight. After confluence, α-MEM was removed, and the cells were washed once or twice with Hanks' balanced salt solution. To induce adipocyte differentiation, the cells were exposed to differentiation medium containing 66 nmol/l insulin, 100 nmol/l cortisol, 0.2 nmol/l triiodothyronine, and 1 μg/ml ciglitazone. The medium was changed every 2 days, and cells were kept in culture for 28 days.
hPBMC culture
hPBMC was isolated by density gradient sedimentation on Ficoll-Paque (Pharmacia, Uppsala, Sweden) as described previously (11). The cells were washed twice in PBS and resuspended in medium appropriate for cell culture (RPMI 1640 supplemented with 25 mmol/l HEPES, 2 mmol/l l-glutamine, 100 μU/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B.
Endoplasmic reticulum stress induction
The induction of endoplasmic reticulum stress was established according to the method described by Hagiwara et al. (12). In brief, to induce endoplasmic reticulum stress, the cells were pretreated with TUN (3 μg/ml) and/or DTT (1 mmol/l) for 5 h and subsequently treated with leptin and/or amylin.
Protein extraction
For total cell extracts, collected cells were suspended in a lysis buffer containing 20 mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl, 5 mmol/l EDTA, 0.1 mmol/l phenylmethylsulfonyl fluoride, 0.05% aprotinin, and 0.1% Igepal and then incubated for 30 min at 4°C. The suspension was centrifuged for 25min at 14,240g, and the supernatant was saved as the total extract. Next, the pellet was resuspended in a lysis buffer containing 50 mmol/l HEPES-NaOH (pH 7.8), 50 mmol/l KCl, 300 mmol/l NaCl, 0.1 mmol/l EDTA, 1 mmol/l DTT, 0.1 mmol/l phenylmethylsulfonyl fluoride, and 10% (v/v) glycerol. The suspension was mixed for 30 min at 4°C and centrifuged for 15 min at 890g; the supernatant was saved as the nuclear extract.
Western blotting
For Western blotting, proteins were loaded in each lane. After SDS-PAGE, proteins were blotted onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH). The membranes were blocked for 1 h in Tris-buffered saline (TBS) containing 5% nonfat dry milk and 0.1% Tween 20. Incubation with primary antibodies was performed overnight in TBS containing 5% nonfat dry milk, and then incubation was continued with horseradish peroxidase secondary antibodies for 2 h. After incubation with antibodies, membranes were washed with TBS containing 0.1% Tween 20. Enhanced chemiluminescence was used for detection. Measurement of signal intensity on nitrocellulose membranes after Western blotting with various antibodies was performed using ImageJ processing and analysis software.
Statistical analysis
Data were analyzed using one-way ANOVA followed by a post hoc test for multiple comparisons. Analyses were performed using SPSS (version 11.5; SPSS, Chicago, IL).
RESULTS
Leptin receptor expression in hAT ex vivo and in hPAs and hPBMCs in vitro
ObRb was expressed in human tissues and cells studied ex vivo and in vitro, respectively (data not shown). We did not observe any increase in ObRb expression as a result of leptin, amylin, and/or leptin+amylin administration for 30 min compared with the control. In addition, we did not observe any difference in ObRb expression in subjects with subcutaneous versus omental hAT, male versus female subjects, and obese versus lean subjects (data not shown) in the limited number of subjects studied.
Leptin and amylin signaling: dose- and time-response curves in hAT ex vivo and in hPAs and hPBMCs in vitro from obese female subjects
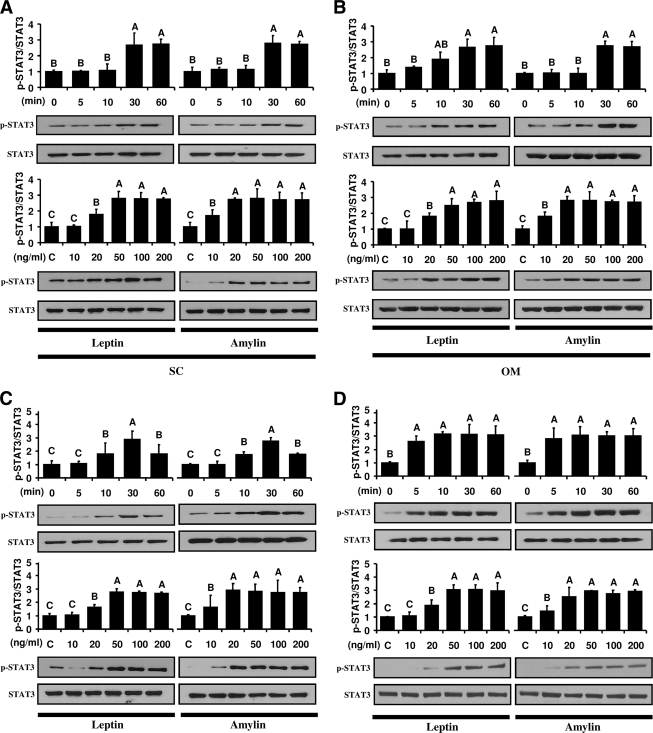
Ex vivo and in vitro leptin and amylin administration significantly induced phosphorylation of STAT3 by ∼2.8-fold higher than control at 30 min in hAT (Fig. 1A and B) ex vivo and in hPAs (Fig. 1C) and hPBMCs (Fig. 1D) in vitro. Importantly, all leptin and amylin signaling pathways were activated in a dose-dependent manner, but activations of all signaling pathways were saturable at a leptin concentration of ∼50 ng/ml and amylin concentration of ∼20 ng/ml. We did not observe any difference in STAT3 activation in subjects with subcutaneous versus omental hAT, obese versus lean subjects, and male versus female subjects (data not shown).
Figure 1.
Leptin and amylin signaling. Dose- and time-response curves in hAT ex vivo and in hPAs and hPBMCs in vitro from obese female subjects. Ex vivo and in vitro leptin and amylin administration in hAT (A and B), hPAs (C), and hPBMCs (D) were performed as described in detail in research design and methods. A–D: The tissues and cells were incubated with leptin and/or amylin (50 ng/ml of leptin and 20 ng/ml of amylin for the time-response study and 30 min incubation for the dose-response study). All tissue lysates (whole protein extraction) were examined by Western blot with primary p-STAT3 and STAT3 antibodies. The secondary antibody used was horseradish peroxidase-conjugated anti-mouse and anti-goat antibodies. All figures showing quantitative analysis include data from at least three independent experiments. All density values for each protein band of interest are expressed as a fold increase. All data were analyzed using one-way ANOVA followed by post hoc test for multiple comparisons. Values are means ± SD. Means with different letters are significantly different, P < 0.05.
Activation of STAT3 signaling by administration of leptin and amylin alone or in combination in hAT ex vivo and in hPAs and hPBMCs in vitro from obese female subjects
Based on the above results (Fig. 1), we chose a representative administration time (30 min) and leptin (50 ng/ml) and amylin (20 ng/ml) concentrations. Either leptin or amylin stimulated phosphorylation of STAT3 by ∼2.8-fold in subcutaneous and omental hAT ex vivo (supplementary Fig. 1A, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0518/DC1), by ∼2.9-fold in hPAs in vitro (supplementary Fig. 1B), and by ∼2.8-fold in hPBMCs (supplementary Fig. 1C) in vitro. In addition, coadministration of leptin and amylin activated STAT3 signaling in hAT ex vivo by ∼4.7-fold, in hPAs in vitro by ∼5.1-fold, and in hPBMCs in vitro by ∼4.9-fold. Moreover, nuclear translocation of STAT3 was increased by leptin, and this effect was more enhanced by coadministration of amylin in hPAs and hPBMCs in vitro (data not shown). In contrast, increased STAT3 phosphorylation by leptin+amylin administration was totally abolished by pretreatment with AG490, a STAT3 inhibitor, in hPAs (supplementary Fig. 1D) and hPBMCs (supplementary Fig. 1E) in vitro. Importantly, we observed an additive, but not synergistic, effect of amylin on leptin-treated tissues and/or cells. There was no difference in STAT3 activation in experiments comparing subjects with subcutaneous versus omental hAT, obese versus lean subjects, and male versus female subjects (data not shown).
Activation of extracellular signal-regulated kinase signaling by administration of leptin and amylin alone or in combination in hAT ex vivo and in hPAs and hPBMCs in vitro from obese female subjects
Extracellular signal-regulated kinase (ERK) signaling was activated in leptin-treated (by ∼2.3-fold) and amylin-treated (by ∼2.2-fold) hAT ex vivo, and this activation was further increased in response to coadministration of amylin and leptin by ∼3.4-fold (supplementary Fig. 2A, available in an online appendix). As expected, ERK signaling was activated in leptin- and amylin-treated hPAs in vitro by ∼2.9- and ∼2.8-fold (supplementary Fig. 2B) and in hPBMCs in vitro by ∼2.8- and ∼2.7-fold, respectively (supplementary Fig. 2C). In addition, activation of ERK signaling in response to leptin and amylin coadministration in hPAs in vitro was increased by ∼5.1-fold and in hPBMCs in vitro by ∼4.8-fold, but leptin+amylin-activated ERK signaling was totally blocked by pretreatment with an ERK inhibitor (supplementary Fig. 2, B and C). We did not observe any difference in ERK activation in comparing cells or tissues from subjects with subcutaneous versus omental hAT, obese versus lean subjects, and male versus female subjects (data not shown).
Activation of Akt and AMPK signaling by administration of leptin and amylin alone or in combination in hAT ex vivo and in hPAs and hPBMCs in vitro from obese female subjects
Leptin activated Akt and AMPK signaling in hAT ex vivo by ∼1.9- and ∼3.5-fold (supplementary Fig. 3A, available in an online appendix), in hPAs in vitro by ∼2.6- and ∼2.8-fold (supplementary Fig. 3B), and in hPBMCs in vitro by ∼3.0- and ∼3.2-fold (supplementary Fig. 3C), respectively. Amylin also activated Akt and AMPK signaling in hAT ex vivo by ∼2.0- and ∼3.6-fold (supplementary Fig. 3A), in hPAs in vitro by ∼2.6- and ∼2.8-fold (supplementary Fig. 3B), and in hPBMCs in vitro by ∼3.0- and ∼3.1-fold (supplementary Fig. 3C), respectively. Moreover, activation of Akt and AMPK signaling by leptin and amylin alone in hAT ex vivo and in hPAs and hPBMCs in vitro was increased in response to leptin and amylin coadministration. In contrast, activation of Akt and AMPK signaling in leptin+amylin-stimulated cells was blocked by pretreatment with each inhibitor. We did not observe any difference in Akt and/or AMPK activation in cells or tissues from subjects with subcutaneous versus omental hAT, obese versus lean subjects, and male versus female subjects (data not shown).
Inhibition of STAT3 signaling by endoplasmic reticulum stress in hPAs and hPBMCs in vitro from obese female subjects
Stimulation of the cells with leptin+amylin led to a marked and significant increase in phosphorylation of STAT3 in hPAs by ∼5.0-fold (supplementary Fig. 4A, available in an online appendix) and in hPBMCs by ∼4.8-fold (supplementary Fig. 4B), but, when challenged with endoplasmic reticulum stress inducers, TUN and/or DTT, the leptin+amylin-activated STAT3 phosphorylation was totally abolished, suggesting that amylin could not overcome endoplasmic reticulum stress–induced inhibition of leptin-activated STAT3 signaling. We did not observe any difference in endoplasmic reticulum stress–blocked STAT3 activation in hPAs and hPBMCs from obese versus lean and male versus female subjects (data not shown).
CONCLUSIONS
Leptin, the prototype adipocyte-secreted adipocytokine, regulates food intake and body weight, acting primarily in the hypothalamus (1). It may also act in the periphery to modulate immune and metabolic functions (2). Peripheral leptin administration has been demonstrated to phosphorylate STAT3 and MAPK in rodent adipose tissue and liver (8). Similar effects have not yet been explored in humans. Amylin, a hormone secreted from the pancreas, functions to decrease food intake, gastric emptying. and glucagon secretion (4). Although amylin has been proposed to amplify leptin signaling in the hypothalamus, thereby synergistically effecting weight and fat loss in animal models (6), the molecular mechanisms underlying these effects remain to be fully clarified. We have previously demonstrated that altering circulating leptin levels in humans does not alter circulating amylin levels (13). Whether the action of amylin can increase leptin signaling in human tissues remains to be fully elucidated. More specifically, there have been no leptin and amylin signaling studies evaluating hPTs, including hAT and hPBMCs. Hence, we performed ex vivo and in vitro signaling studies to investigate the role of amylin and leptin in activating signaling pathways in hPTs as well as their potential interaction.
STAT3 mediates the expression of a variety of genes in response to cell stimuli and thus plays a key role in many cellular processes such as cell growth and apoptosis (8). In fact, disrupting the ability of the leptin receptor to activate the STAT3 pathway in mice leads to severe obesity and several other neuroendocrine abnormalities (14). The STAT3 pathway is the first identified signaling mechanism found to be associated with the leptin receptor (8). Leptin induces phosphorylation of STAT3 in keratinocytes, endometrial cancer cells, hypothalamic cells, murine adipocytes, and mouse adipose tissue (3,8,15,16). Amylin, similar to leptin, has been shown to increase the number of p-STAT3 cells in the arcuate nucleus of rats (7), and this effect was significantly greater with coadministration of leptin and amylin compared with individual treatments (7). Based on these results, we checked for the first time whether leptin signaling interacts with amylin in humans and whether cotreatment of amylin and leptin could further increase leptin-stimulated STAT3 signaling in hPT. We observed that leptin and amylin increased STAT3 signaling in hAT ex vivo and hPAs and hPBMCs in vitro, and the effect of these two hormones was similar in magnitude and peaked around the same time. Our data in peripheral tissues are consistent with rodent studies previously demonstrating that amylin-treated rats tend to have a greater number of p-STAT3–positive cells than vehicle controls in hypothalami (7). Although it is important to study the effects of leptin and amylin in human hypothalamic or other central nervous system cells, our data suggest that amylin-increased STAT3 signaling in leptin-stimulated hAT and cells may have important clinical implications.
ERK, a member of the MAPK family, is an additional pathway downstream of the leptin receptor (16). Leptin has been shown to activate ERK1/2 in a time- and dose-dependent manner in several cultured mouse cells in vitro as well as rodent tissues in vivo (17). The ERK pathway has been reported to mediate the effects of leptin on human PBMCs in vitro as well as rat kidney and rat adipose tissue (8,17). Based on these previous studies, we further investigated whether amylin could activate ERK signaling in hPTs, because no relevant data in humans have been published to date. We observed that amylin increases ERK activation alone and in leptin-stimulated hAT ex vivo as well as in hPAs and hPBMCs in vitro. Because ERK is a significant downstream target for the physiological effects of leptin (15,16), we suggest that amylin may also have a beneficial effect on human physiology and pathophysiology.
AMPK is an evolutionarily conserved serine/threonine protein kinase central to the regulation of energy balance at both the cellular and whole-body level (17). In its classic role as an intracellular metabolic stress-sensing kinase, AMPK switches on fatty acid oxidation and glucose uptake, while switching off hepatic gluconeogenesis, and exerts a broader role in metabolism by controlling appetite (17). In fact, AMPK is involved in cellular energy homeostasis by AMPK phosphorylation and inhibition of acetyl-CoA carboxylase and thus stimulates fatty acid oxidation (17,18). However, whether leptin can activate AMPK and/or whether amylin can activate AMPK to induce fatty acid oxidation in hPTs has not yet been demonstrated. We checked leptin and amylin signaling in hPTs in vitro and ex vivo and observed that leptin and amylin increase AMPK activation in all cells. Moreover, we observed that leptin and amylin administration directly increases AMPK activation in hAT ex vivo. Because AMPK is associated with fatty acid oxidation, which plays a key role in the pathophysiology of insulin resistance (17), leptin- and amylin-activated AMPK signaling in hAT and other peripheral tissues offers further support to the notion that the use of these hormones, alone or in combination, could be a viable strategy in treating obesity and type 2 diabetes.
The Akt signaling pathway is responsible for cellular processes such as cell growth, proliferation, and glucose metabolism (19). It has been shown that obesity induces increased levels of leptin, insulin, insulin-like growth factor, tumor necrosis factor-α, and interleukin-6 and reduces adiponectin levels (19). These altered factors change Akt signaling activity, which, in turn, regulates downstream targets, leading to increased cell survival and cell growth and promotion of the cell cycle (19). Based on these previous results, we further investigated whether leptin and/or amylin could activate Akt signaling in hPTs in vitro and ex vivo. We observed that leptin and amylin alone and in combination increase Akt phosphorylation in hAT ex vivo as well as hPAs and hPBMCs in vitro suggesting that leptin and amylin, alone or in combination, could have beneficial effects on cell survival.
We observed that amylin activates Jak2 signaling pathways in hAT ex vivo and in hPAs and hPBMCs in vitro (data not shown). Because OBRb has the capacity to activate Jak2 (20), we suggest that amylin-mediated intracellular signaling pathways ex vivo and in vitro might be activated not only through cGMP (21) but also potentially through Jak2 signaling. This is the first report not only on amylin interacting with Jak2 signaling in human peripheral tissues but also on amylin signaling in human peripheral tissues. It has been suggested that amylin may act through upregulation of the leptin receptor and that this induces STAT3 activation in hypothalami in rodent in vivo (7). We did not observe any difference in ObRb expression in human peripheral tissues treated with leptin, amylin, and leptin+amylin, but the treatment time was only 30 min. A longer exposure to amylin and leptin as well as a receptor knockdown experiment would also be useful in determining whether amylin could act through ObRb in human peripheral tissues. Because it is impossible to perform the receptor knockdown studies in humans, alternative methods such as ObRb knockdown in vivo rodent or mouse studies may have to be performed. In any case, the lack of ObRb upregulation in our studies excludes this potential mechanism and suggests that most probably amylin may activate signaling pathways downstream of Jak2. This original observation has to be replicated by other studies in the future.
It is generally accepted that obese versus lean subjects have differences in leptin responsiveness (3). We also expected that there would be differences between the obese versus lean group in terms of leptin responsiveness. Despite minor differences in the timing of signaling activation, we did not observe any differences in several leptin-activated signaling pathways in obese versus lean subjects in the tissues studied. There are a number of potential explanations for this observation. First, although there are differences between obese versus lean subjects in response to leptin accumulation in the medium of cultured omental and subcutaneous adipose tissue in the early differentiation stages in vitro (22), leptin and leptin mRNA levels are not different in obese versus lean subjects after full differentiation (23). The effect of leptin on lipolysis is not different between cultured obese and lean human adipocytes in vitro (24). These results demonstrate that there are major quantitative changes in leptin responsiveness between obese and lean adipocytes during differentiating stages, but not differentiated stages, of hAT in vitro. We have used differentiated adipocytes herein. Signaling interactions of leptin and amylin in various differentiation stages remain to be studied. Second, although we studied the signaling pathways considered to be primary targets of leptin, on the basis of current evidence deriving mainly from rodent studies, we have not looked at all signaling pathways potentially mediating the action of leptin in the periphery. Moreover, there might be other, totally unknown, pathways activated by leptin because there have been no prior studies on leptin signaling in human tissues. Much more needs to be learned in the future. Third, it is also possible that signaling pathways mediating leptin actions in peripheral tissues (and resistance thereof) could be totally different from those in hypothalami and/or other central nervous system areas (25). Because it has been proposed that centrally administered leptin affects insulin sensitivity and metabolism in peripheral tissues, it is possible that differences in central nervous system signaling between lean and obese subjects are much more important than leptin-activated signaling pathways in the periphery. Fourth, leptin and amylin act not only in adipose tissue but also in several other metabolically active peripheral tissues, including but not limited to liver and reproductive organs (26). Because leptin signaling could differ between lean and obese subjects in these tissues and because this difference could have direct clinical implications in metabolism, more studies, focusing on tissues other than the ones studied herein, need to be performed. Finally, it is possible that signaling pathways in humans may be different from those in animals, which may be analogous to the different effect of leptin in regulating neuroendocrine response to starvation that we have previously shown in mice (27) versus humans (28).
Endoplasmic reticulum stress has recently been shown to play a role in the development of leptin resistance in the hypothalamus of rodents (13), and it has been suggested that endoplasmic reticulum capacity is directly related to leptin sensitivity (13,29). This finding resulted in suggestions that endoplasmic reticulum stress reversal could be used as a strategy to sensitize obese mice and by extension, humans, to leptin. These previous studies have shown that the reduction in endoplasmic reticulum function creates endoplasmic reticulum stress, blocks leptin action, and generates leptin resistance in mice, suggesting that endoplasmic reticulum stress inhibits at an upstream step of STAT3 phosphorylation and provides a potential mechanism in which increased endoplasmic reticulum stress antagonizes STAT3-mediated leptin signaling (29). However, whether activation of endoplasmic reticulum stress interferes with leptin and/or amylin signaling in hPTs has not yet been demonstrated. Here, we report for the first time that TUN and also DTT pretreatment of human cells completely blocks leptin- and amylin-stimulated STAT3 activation in hPAs and hPBMCs in vitro. Our data demonstrate that amylin does not function as a leptin sensitizer to bypass endoplasmic reticulum stress–induced leptin resistance and suggests that increased ER stress in obese people may induce leptin and also amylin resistance. Because in vivo leptin and amylin actions may differ in comparison to in vitro, studies of in vivo leptin and amylin signaling in humans are needed to prove or disprove this hypothesis. It is currently impossible to perform human in vivo endoplasmic reticulum stress studies, however, and thus specific methods for endoplasmic reticulum stress induction in humans need to be developed.
We believe that our data are important from the physiological point of view. This is the very first attempt to map the intracellular signaling pathways downstream of leptin and amylin in human tissues. This first report, if followed by other similar reports, will eventually allow full characterization of signaling pathways in all peripheral tissues in humans as well as the comparative evaluation of human versus animal signaling pathways. This new knowledge in human physiology will consequently lead to the very first attempt to also study pathophysiology and later therapeutics in humans. Although we are initiating this effort with the current ground-breaking report, much more needs to be done (additional tissues, other signaling pathways, and other physiological conditions) in the future. Despite these limitations, our initial data in human tissues suggest 1) that leptin tolerance (not resistance) exists in the tissues studied in human and 2) that unlike experiments in rodents showing a synergistic effect of amylin on leptin-modulated food intake, the effects of amylin and leptin in human peripheral tissues are additive and not synergistic.
In summary, despite minor differences in the timing of signaling activation in the different tissues and cells studied, we did not observe major differences in the magnitude of STAT3 activation in response to leptin and amylin administration in hAT ex vivo and in hPAs and hPBMCs in vitro. In addition, we did not observe any differences in STAT3, ERK, and AMPK phosphorylation when we compared male versus female and obese versus lean subjects. Importantly, leptin and amylin signaling pathways were saturable at a level of ∼50 ng/ml (leptin) and ∼20 ng/ml (amylin), suggesting that no additional signaling effect can be observed at doses higher than the above doses. Although leptin and amylin in combination increased phosphorylation of the pathways studied herein, we observed not a multiplicative, but rather an additive, effect of amylin- on leptin-stimulated hPTs ex vivo and in vitro. The present study adds to our recent clinical data demonstrating that leptin administration does not alter circulating amylin levels (13) and provides preclinical evidence that leptin and amylin administration activate overlapping intracellular signaling pathways in human peripheral tissues. These hormones have an additive effect in hPTs, further suggesting that leptin and amylin may play an additive role in regulating obesity and type 2 diabetes in humans.
Acknowledgments
Funding was received from the National Institute of Diabetes and Digestive and Kidney Diseases grants DK58785, DK79929, and DK81913 and AG032030. The Mantzoros Laboratory is also supported by a discretionary grant from Beth Israel Deaconess Medical Center.
No potential conflicts of interest relevant to this article were reported.
H.-S.M. participated in the study design, performance, and coordination and wrote the manuscript. C.S.M. wrote the manuscript. J.P.C., K.N.D., C.G.F., F.Z., and B.S. participated in the study design, performance, and coordination. C.S.M. conceived and planned the study and participated in the study design, performance, and coordination.
We thank Dr. Young-Bum Kim, Division of Endocrinology, Diabetes, and Metabolism, Beth Israel Deaconess Medical Center, Harvard Medical School, for technical assistance in the ex vivo study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Badman MK, Flier JS: The gut and energy balance: visceral allies in the obesity wars. Science 2005;307:1909–1914 [DOI] [PubMed] [Google Scholar]
- 2.Matarese G, Moschos S, Mantzoros CS: Leptin in immunology. J Immunol 2005;174:3137–3142 [DOI] [PubMed] [Google Scholar]
- 3.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F: Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995;269:540–543 [DOI] [PubMed] [Google Scholar]
- 4.Roth JD, Maier H, Chen S, Roland BL: Implications of amylin receptor agonism: integrated neurohormonal mechanisms and therapeutic applications. Arch Neurol 2009;66:306–310 [DOI] [PubMed] [Google Scholar]
- 5.Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, Koda JE, Weyer C: Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth JD, Coffey T, Jodka CM, Maier H, Athanacio JR, Mack CM, Weyer C, Parkes DG: Combination therapy with amylin and peptide YY[3–36] in obese rodents: anorexigenic synergy and weight loss additivity. Endocrinology 2007;148:6054–6061 [DOI] [PubMed] [Google Scholar]
- 7.Turek VF, Trevaskis JL, Levin BE, Dunn-Meynell AA, Irani B, Gu G, Wittmer C, Griffin PS, Vu C, Parkes DG, Roth JD: Mechanisms of amylin/leptin synergy in rodent models. Endocrinology 2010;151:143–152 [DOI] [PubMed] [Google Scholar]
- 8.Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB: In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology 2000;141:2328–2339 [DOI] [PubMed] [Google Scholar]
- 9.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 10.Ribet C, Montastier E, Valle C, Bezaire V, Mazzucotelli A, Mairal A, Viguerie N, Langin D: Peroxisome proliferator-activated receptor-alpha control of lipid and glucose metabolism in human white adipocytes. Endocrinology 2010;151:123–133 [DOI] [PubMed] [Google Scholar]
- 11.Böyum A: Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 1968;97:77–89 [PubMed] [Google Scholar]
- 12.Hagiwara S, Iwasaka H, Shingu C, Matsumoto S, Hasegawa A, Asai N, Noguchi T: Heat shock protein 72 protects insulin-secreting β cells from lipopolysaccharide-induced endoplasmic reticulum stress. Int J Hyperthermia 2009;25:626–633 [DOI] [PubMed] [Google Scholar]
- 13.Hwang JJ, Chan JL, Ntali G, Malkova D, Mantzoros CS: Leptin does not directly regulate the pancreatic hormones amylin and pancreatic polypeptide: interventional studies in humans. Diabetes Care 2008;31:945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Brink GR, O'Toole T, Hardwick JC, van den Boogaardt DE, Versteeg HH, van Deventer SJ, Peppelenbosch MP: Leptin signaling in human peripheral blood mononuclear cells, activation of p38 and p42/44 mitogen-activated protein (MAP) kinase and p70 S6 kinase. Mol Cell Biol Res Commun 2000;4:144–150 [DOI] [PubMed] [Google Scholar]
- 15.Catalano S, Giordano C, Rizza P, Gu G, Barone I, Bonofiglio D, Giordano F, Malivindi R, Gaccione D, Lanzino M, De Amicis F, Andò S: Evidence that leptin through STAT and CREB signaling enhances cyclin D1 expression and promotes human endometrial cancer proliferation. J Cell Physiol 2009;218:490–500 [DOI] [PubMed] [Google Scholar]
- 16.Rahmouni K, Sigmund CD, Haynes WG, Mark AL: Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 2009;58:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjørbaek C, Kahn BB: Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res 2004;59:305–331 [DOI] [PubMed] [Google Scholar]
- 18.Muoio DM, Dohm GL, Fiedorek FT, Jr, Tapscott EB, Coleman RA, Dohn GL: Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 1997;46:1360–1363 [DOI] [PubMed] [Google Scholar]
- 19.Huang XF, Chen JZ: Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev 2009;10:610–616 [DOI] [PubMed] [Google Scholar]
- 20.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM: Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 1996;14:95–97 [DOI] [PubMed] [Google Scholar]
- 21.Riediger T, Schmid HA, Lutz T, Simon E: Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. Am J Physiol Regul Integr Comp Physiol 2001;281:R1833–R1843 [DOI] [PubMed] [Google Scholar]
- 22.Trujillo ME, Lee MJ, Sullivan S, Feng J, Schneider SH, Greenberg AS, Fried SK: Tumor necrosis factor α and glucocorticoid synergistically increase leptin production in human adipose tissue: role for p38 mitogen-activated protein kinase. J Clin Endocrinol Metab 2006;91:1484–1490 [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Jenkins JR, Trayhurn P: Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-α. Am J Physiol Endocrinol Metab 2005;288:E731–E740 [DOI] [PubMed] [Google Scholar]
- 24.Aprath-Husmann I, Röhrig K, Gottschling-Zeller H, Skurk T, Scriba D, Birgel M, Hauner H: Effects of leptin on the differentiation and metabolism of human adipocytes. Int J Obes Relat Metab Disord 2001;25:1465–1470 [DOI] [PubMed] [Google Scholar]
- 25.Mantzoros CS, Frederich RC, Qu D, Lowell BB, Maratos-Flier E, Flier JS: Severe leptin resistance in brown fat-deficient uncoupling protein promoter-driven diphtheria toxin A mice despite suppression of hypothalamic neuropeptide Y and circulating corticosterone concentrations. Diabetes 1998;47:230–238 [DOI] [PubMed] [Google Scholar]
- 26.Mantzoros CS, Cramer DW, Liberman RF, Barbieri RL: Predictive value of serum and follicular fluid leptin concentrations during assisted reproductive cycles in normal women and in women with the polycystic ovarian syndrome. Hum Reprod 2000;15:539–544 [DOI] [PubMed] [Google Scholar]
- 27.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS: Role of leptin in the neuroendocrine response to fasting. Nature 1996;382:250–252 [DOI] [PubMed] [Google Scholar]
- 28.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS: The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 2003;111:1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U: Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9:35–51 [DOI] [PubMed] [Google Scholar]