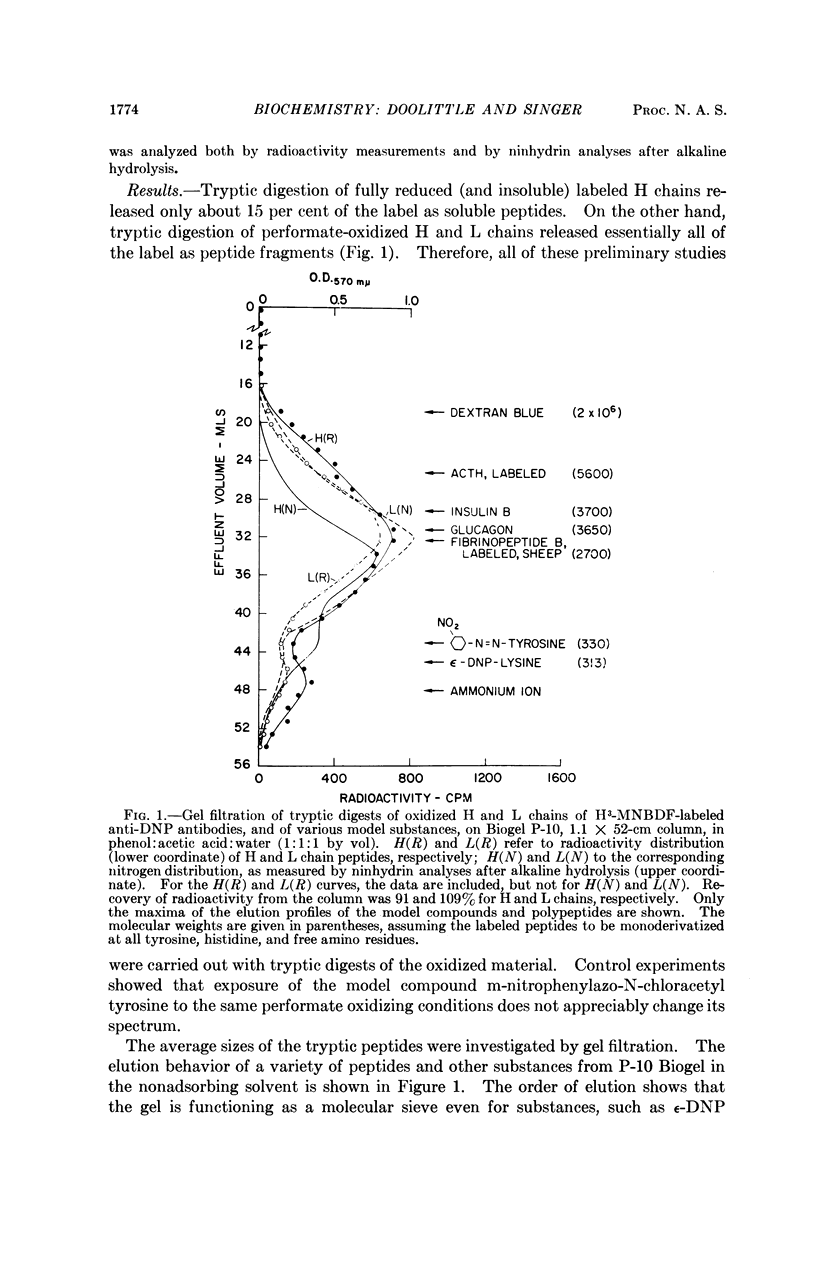
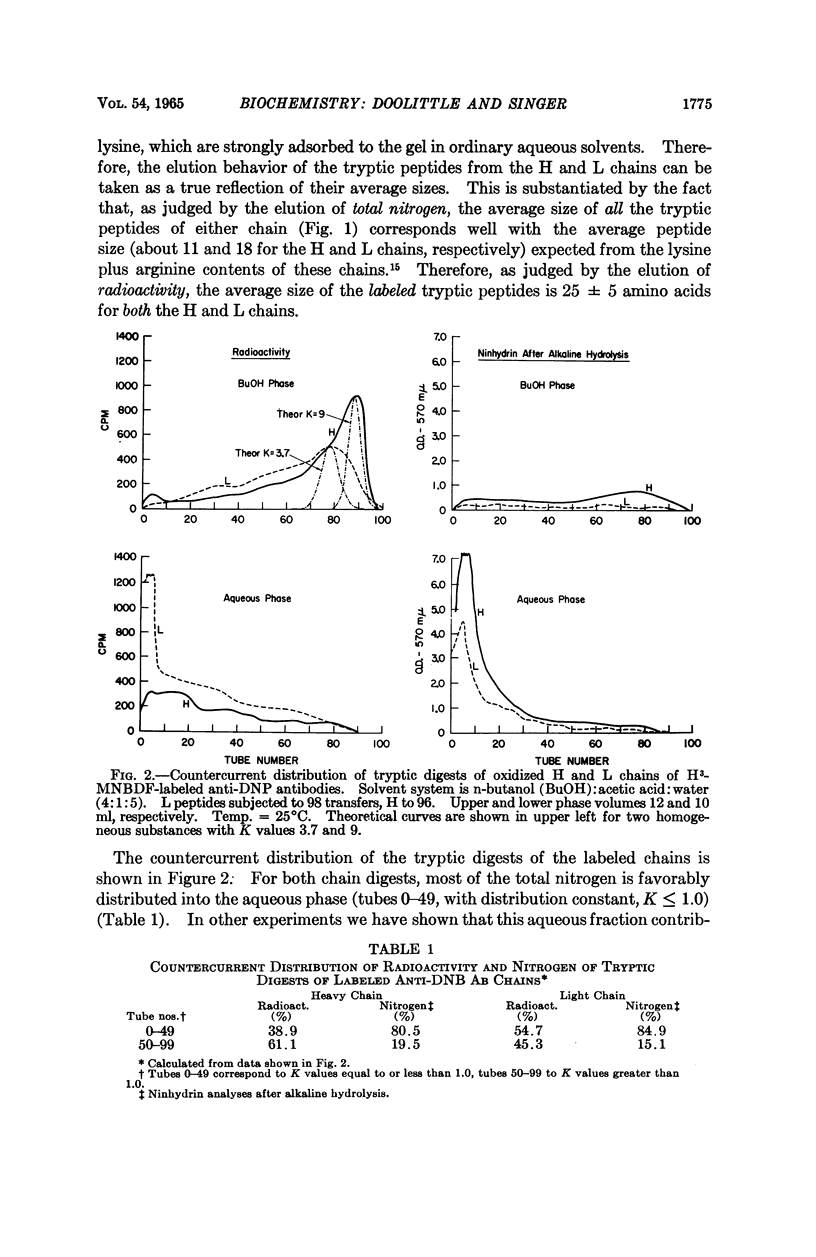
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT J. C., HOOD L. E., DREYER W. J., POTTER M. EVIDENCE FOR AMINO ACID SEQUENCE DIFFERENCES AMONG PROTEINS RESEMBLING THE L-CHAIN SUBUNITS OF IMMUNOGLOBULINS. J Mol Biol. 1965 May;12:81–87. doi: 10.1016/s0022-2836(65)80284-4. [DOI] [PubMed] [Google Scholar]
- DAY L. A., STURTEVANT J. M., SINGER S. J. The kinetics of the reactions between antibodies to the 2,4 dinitrophenyl group and specific haptens. Ann N Y Acad Sci. 1963 May 8;103:611–625. doi: 10.1111/j.1749-6632.1963.tb53721.x. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., GALLY J. A. The nature of Bence-Jones proteins. Chemical similarities to polypetide chains of myeloma globulins and normal gamma-globulins. J Exp Med. 1962 Aug 1;116:207–227. doi: 10.1084/jem.116.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- FARAH F. S., KERN M., EISEN H. N. The preparation and some properties of purified antibody specific for the 2,4-dinitrophenyl group. J Exp Med. 1960 Dec 1;112:1195–1210. doi: 10.1084/jem.112.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Singer S. J. Affinity labeling of antibodies to the p-azophenyltrimethylammonium hapten and a structural relationship among antibody active sites of different specificities. Biochem Biophys Res Commun. 1965 Jul 26;20(3):315–320. doi: 10.1016/0006-291x(65)90366-9. [DOI] [PubMed] [Google Scholar]
- GITLIN D., MERLER E. A comparison of the peptides released from related rabbit antibodies by enzymatic hydrolysis. J Exp Med. 1961 Aug 1;114:217–230. doi: 10.1084/jem.114.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIVOL D., SELA M. ISOLATION AND FRAGMENTATION OF ANTIBODIES TO POLYTYROSYL GELATIN. Biochemistry. 1964 Mar;3:444–451. doi: 10.1021/bi00891a024. [DOI] [PubMed] [Google Scholar]
- GOLD E. F., KNIGHT K. L., HAUROWITZ F. PEPTIDE MAPS OF ANTIBODIES AGAINST AN ANTIGEN CONTAINING TWO DIFFERENT DETERMINANT GROUPS. Biochem Biophys Res Commun. 1965 Jan 4;18:76–80. doi: 10.1016/0006-291x(65)90885-5. [DOI] [PubMed] [Google Scholar]
- GROFF J. L., HAUROWITZ F. COMPARISON OF THE PEPTIDE MAPS OF ANTIBODIES AGAINST AN ACIDIC AND A BASIC DETERMINANT GROUP. Immunochemistry. 1964 Apr;1:31–36. doi: 10.1016/0019-2791(64)90053-9. [DOI] [PubMed] [Google Scholar]
- HABER E. RECOVERY OF ANTIGENIC SPECIFICITY AFTER DENATURATION AND COMPLETE REDUCTION OF DISULFIDES IN A PAPAIN FRAGMENT OF ANTIBODY. Proc Natl Acad Sci U S A. 1964 Oct;52:1099–1106. doi: 10.1073/pnas.52.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- Hilschmann N., Craig L. C. Amino acid sequence studies with Bence-Jones proteins. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1403–1409. doi: 10.1073/pnas.53.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSHLAND M. E., ENGLBERGER F. M. Differences in the amino acid composition of two purified antibodies from the same rabbit. Proc Natl Acad Sci U S A. 1963 Jul;50:61–68. doi: 10.1073/pnas.50.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZGER H., MANNIK M. RECOMBINATION OF ANTIBODY POLYPEPTIDE CHAINS IN THE PRESENCE OF ANTIGEN. J Exp Med. 1964 Nov 1;120:765–782. doi: 10.1084/jem.120.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZGER H., WOFSY L., SINGER S. J. AFFINITY LABELING OF THE ACTIVE SITES OF ANTIBODIES TO THE 2,4-DINITROPHENYL HAPTEN. Biochemistry. 1963 Sep-Oct;2:979–988. doi: 10.1021/bi00905a014. [DOI] [PubMed] [Google Scholar]
- METZGER H., WOFSY L., SINGER S. J. THE PARTICIPATION OF A AND B POLYPEPTIDE CHAINS IN THE ACTIVE SITES OF ANTIBODY MOLECULES. Proc Natl Acad Sci U S A. 1964 Apr;51:612–618. doi: 10.1073/pnas.51.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- SEIJEN H. G., GRUBER M. STRUCTURE OF GAMMA-GLOBULINS AND ANTIBODIES. J Mol Biol. 1963 Aug;7:209–211. doi: 10.1016/s0022-2836(63)80047-9. [DOI] [PubMed] [Google Scholar]
- Titani K., Whitley E., Jr, Avogardo L., Putnam F. W. Immunoglobulin structure: partial amino acid sequence of a Bence Jones protein. Science. 1965 Sep 3;149(3688):1090–1092. doi: 10.1126/science.149.3688.1090. [DOI] [PubMed] [Google Scholar]
- Velick S. F., Parker C. W., Eisen H. N. EXCITATION ENERGY TRANSFER AND THE QUANTITATIVE STUDY OF THE ANTIBODY HAPTEN REACTION. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1470–1482. doi: 10.1073/pnas.46.11.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOFSY L., METZGER H., SINGER S. J. Affinity labeling-a general method for labeling the active sites of antibody and enzyme molecules. Biochemistry. 1962 Nov;1:1031–1039. doi: 10.1021/bi00912a013. [DOI] [PubMed] [Google Scholar]



