Abstract
OBJECTIVE
To test the effects of two Mediterranean diet (MedDiet) interventions versus a low-fat diet on incidence of diabetes.
RESEARCH DESIGN AND METHODS
This was a three-arm randomized trial in 418 nondiabetic subjects aged 55–80 years recruited in one center (PREDIMED-Reus, northeastern Spain) of the Prevención con Dieta Mediterránea [PREDIMED] study, a large nutrition intervention trial for primary cardiovascular prevention in individuals at high cardiovascular risk. Participants were randomly assigned to education on a low-fat diet (control group) or to one of two MedDiets, supplemented with either free virgin olive oil (1 liter/week) or nuts (30 g/day). Diets were ad libitum, and no advice on physical activity was given. The main outcome was diabetes incidence diagnosed by the 2009 American Diabetes Association criteria.
RESULTS
After a median follow-up of 4.0 years, diabetes incidence was 10.1% (95% CI 5.1–15.1), 11.0% (5.9–16.1), and 17.9% (11.4–24.4) in the MedDiet with olive oil group, the MedDiet with nuts group, and the control group, respectively. Multivariable adjusted hazard ratios of diabetes were 0.49 (0.25–0.97) and 0.48 (0.24–0.96) in the MedDiet supplemented with olive oil and nuts groups, respectively, compared with the control group. When the two MedDiet groups were pooled and compared with the control group, diabetes incidence was reduced by 52% (27–86). In all study arms, increased adherence to the MedDiet was inversely associated with diabetes incidence. Diabetes risk reduction occurred in the absence of significant changes in body weight or physical activity.
CONCLUSIONS
MedDiets without calorie restriction seem to be effective in the prevention of diabetes in subjects at high cardiovascular risk.
The increasing incidence of type 2 diabetes throughout the world, closely linked to westernized dietary patterns, physical inactivity, and raising rates of obesity, is a challenging health problem. Lifestyle changes are effective measures to prevent diabetes, and weight loss is the main predictor of success (1). Five clinical trials that examined the effects of reputedly healthy, energy-restricted diets together with increased physical activity in individuals with impaired glucose tolerance, a prediabetic stage, showed risk reductions between 30 and 70% (2–6). The results of these studies provide convincing evidence that lifestyle modification reduces the incidence of diabetes among high-risk individuals. In four of these studies (2–5), diabetes rates decreased in relation to substantial reductions in body weight, whereas in the Indian trial (6) lifestyle intervention was successful despite no weight loss. Observational studies have also shown that diets rich in vegetables and low in red meat and whole-fat dairy products are associated with a decreased risk of diabetes, whereas dietary patterns rich in red meats, processed foods, refined grains, and sweets increase diabetes risk (7).
The traditional Mediterranean diet (MedDiet), characterized by high consumption of vegetables, legumes, grains, fruits, nuts, and olive oil, moderate consumption of fish and wine, and low consumption of red and processed meat and whole-fat dairy products, is widely recognized as a healthy dietary pattern (8). Two prospective studies from Southern Europe suggested a lower incidence of diabetes with increasing adherence to the MedDiet in previously healthy individuals (9) or myocardial infarction survivors (10). Recently, a clinical trial showed that, compared with a low-fat diet, a MedDiet allowed better glycemic control and delayed the need for antidiabetes drug treatment in patients with newly diagnosed diabetes (11). However, the role of the MedDiet in the prevention of diabetes has not been tested in a clinical trial.
We conducted a randomized controlled trial to compare the effect on diabetes incidence of three non–calorie-restricted nutritional interventions: a low-fat diet (control diet), a MedDiet enriched with virgin olive oil, and a MedDiet enriched with mixed nuts.
RESEARCH DESIGN AND METHODS
The Prevención con Dieta Mediterránea (PREDIMED) study is a multicenter, randomized, parallel group primary prevention trial conducted in Spain to assess the effects of two MedDiets, supplemented with either extra virgin olive oil or mixed nuts, versus a low-fat control diet on cardiovascular and other chronic disease outcomes in individuals at high cardiovascular risk. Full details of the PREDIMED protocol have been published elsewhere (12) and are available at www.predimed.org and www.predimed.es. Recruitment took place between October 2003 and June 2008, including 7,232 participants randomly assigned to the three interventions.
In only one of the PREDIMED centers (PREDIMED-Reus) was a yearly oral glucose tolerance test (OGTT) in nondiabetic participants part of the protocol. The present report represents a nested substudy with the aim of assessing the effects of the three interventions on the incidence of diabetes using a yearly OGTT as a diagnostic tool. The local institutional review board approved the study protocol, and all participants provided written informed consent.
Candidates for the study were community-dwelling men aged 55–80 years and women aged 60–80 years without prior cardiovascular disease but having at least three cardiovascular risk factors, namely smoking, hypertension, dyslipidemia, overweight (BMI ≥25 kg/m2), and family history of premature cardiovascular disease (≤55 years in men and ≤60 years in women). Participants with prevalent diabetes were excluded from the present analysis. Other exclusion criteria were any severe chronic illness, alcohol or drug abuse, BMI ≥40 kg/m2, and history of allergy or intolerance to olive oil or nuts (12).
A behavioral intervention promoting the MedDiet was implemented, as described previously (12). In brief, on the basis of the initial assessment of individual scores of adherence using a 14-item questionnaire, dietitians gave personalized dietary advice to participants randomly assigned to both MedDiets, with instructions directed to scale up the score, including, among others, 1) abundant use of olive oil for cooking and dressing, 2) increased consumption of fruit, vegetables, legumes, and fish, 3) reduction in total meat consumption, recommending white meat instead of red or processed meat, 4) preparation of homemade sauce with tomato, garlic, onion, and spices with olive oil to dress vegetables, pasta, rice, and other dishes, 5) avoidance of butter, cream, fast food, sweets, pastries, and sugar-sweetened beverages, and 6) in alcohol drinkers, moderate consumption of red wine.
At inclusion and quarterly thereafter, dietitians administered both individual interviews and group sessions, separately for each group. Sessions consisted of informative talks and delivery of written material with elaborate descriptions of typical foods for each dietary pattern, seasonal shopping lists, meal plans, and recipes. Participants assigned to MedDiet groups were given free allotments of either virgin olive oil (1 liter/week) or mixed nuts (30 g/day). Participants assigned to the low-fat diet received recommendations to reduce all types of fat, from both animal and vegetable sources, but no free foods. Instead, to encourage adherence, at quarterly visits they were given small gifts, such as oil dispensers, aprons, shopping bags, or cookbooks. Energy restriction was not advised, nor was physical activity promoted.
At baseline and at each annual visit we administered 1) a short-questionnaire about lifestyle variables, medical conditions, and medication use, 2) a 14-item questionnaire of adherence to the MedDiet (12), 3) a 137-item validated food frequency questionnaire (13), and 4) the validated Spanish version of the Minnesota Leisure-Time Physical Activity questionnaire (14). Staff involved in collecting questionnaires and physical measures were unblinded to intervention group. Energy and nutrient intakes were calculated from Spanish food composition tables as described previously (12). At yearly visits, weight was recorded, samples of fasting blood were taken, and an OGTT was scheduled. Plasma glucose concentrations were centrally analyzed by the glucose-oxidase method. Laboratory technicians were blinded to intervention group.
We assessed the proportion of participants in each group attaining prespecific lifestyle goals on at least 50% of the follow-up visits. Goals included 1) improved adherence to the MedDiet (≥10 points in the 14-point score), 2) a high (≥2) monounsaturated fatty acid (MUFA)-to-saturated fatty acid (SFA) ratio, 3) high olive oil consumption (≥20 g/1,000 kcal/day), 4) high nut consumption (≥10 g/1,000 kcal/day), 5) high dietary fiber intake (≥14 g/1,000 kcal/day), 6) substantial weight loss (≥5% of initial body weight), and 7) high physical activity (≥395 kcal/day, the top tertile). Changes in weight and physical activity were not intervention goals but were assessed because of their well-known association with diabetes.
The primary outcome was new-onset diabetes, diagnosed according to American Diabetes Association criteria (15), namely fasting plasma glucose ≥7.0 mmol/l or 2-h plasma glucose ≥11.1 mmol/l after a 75-g oral glucose load, measured yearly. A second test using the same criteria was required for confirmation. Case ascertainment was done by the PREDIMED Clinical Event Committee, whose members were blinded to intervention group. When diabetes was diagnosed, participants and their primary care physicians were informed and no further OGTTs were scheduled. Every effort was made to retain participants and to ascertain vital status, including telephone calls and home visits by PREDIMED investigators if necessary.
Statistical analysis
Comparisons among groups for qualitative variables were done with the χ2 test. We fitted Cox regression models to assess the relative risk of diabetes by allocation group, estimating hazard ratios and 95% CIs. The time variable was the interval between randomization and the date of last follow-up, death, or diabetes diagnosis, whichever occurred first. Participants who were free of diabetes or who were lost during follow-up were censored at the date of the last visit. The assumption of proportional hazards was tested using time-dependent covariates. In all analyses, we fitted a Cox regression model adjusted for age and sex. In a subsequent model, we adjusted additionally for baseline energy intake, BMI, waist circumference, physical activity, smoking status, fasting serum glucose, use of lipid-lowering drugs, MedDiet score, and weight change during the study. The last model was repeated after merging the two MedDiet groups into a single category. Multiplicative interaction (effect modification) between the intervention (“Mediterranean diets,” i.e., the two groups merged into one category) and age, sex, BMI, and baseline fasting glucose were assessed using the likelihood ratio test for multiplicative product terms introduced in fully adjusted Cox models. Kaplan-Meier survival curves were plotted to estimate the probability of remaining free of diabetes during follow-up. Analyses were based on the intention-to-treat principle. All P values are two-tailed at the <0.05 level. Statistical analysis were performed with SPSS (version 17.0; SPSS, Chicago, IL) software.
RESULTS
Of 1,125 eligible candidates, 870 fulfilled the inclusion criteria and entered the trial. Of these, 452 were excluded because of a prior diagnosis of diabetes. A total of 418 nondiabetic volunteers were randomly assigned into the three groups (supplementary Fig. 1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-1288/DC1). The first participant entered the study in October 2003 and the last one in June 2008. Most participants (98.8%) were enrolled for at least 1 year, 88.3% were enrolled for ≥3 years, and 19.9% were enrolled for ≥6 years. The median follow-up was 4.0 years (interquartile range, 3.0–5.0). Attrition rates were low and were almost exclusively due to major disease events or death.
Table 1 shows baseline characteristics of participants according to intervention arm. The mean age was 67.3 years, and 58.4% of participants were women. The groups were well balanced with respect to most relevant variables. However, participants in the control group used fewer lipid-lowering drugs and had a lower MedDiet score than the other two groups. Participants refused the OGTT on 17% of the scheduled occasions, and 41 (9.8%) of them had none performed.
Table 1.
Characteristics of the study population at baseline
| MedDiet with VOO group | MedDiet with nuts group | Control diet group | |
|---|---|---|---|
| n | 139 | 145 | 134 |
| Age (years) | 67.4 ± 6.1 | 66.6 ± 5.8 | 67.8 ± 6.1 |
| Male sex (%) | 40 | 47 | 38 |
| Current smoker (%) | 11 | 15 | 15 |
| Weight (kg) | 75.3 ± 10.3 | 76.1 ± 10.5 | 76.2 ± 11.3 |
| BMI (kg/m2) | 29.7 ± 3.3 | 29.6 ± 3.1 | 30.0 ± 3.3 |
| Waist circumference(cm) | 101.1 ± 8.6 | 100.3 ± 8.5 | 102.2 ± 9.4 |
| Leisure-time physical activity (kcal/day) | 372 ± 280 | 389 ± 267 | 338 ± 209 |
| Plasma biomarkers | |||
| LDL cholesterol (mmol/l) | 3.7 ± 0.9 | 3.5 ± 0.8 | 3.7 ± 0.9 |
| HDL cholesterol (mmol/l) | 1.5 ± 0.3 | 1.5 ± 0.4 | 1.5 ± 0.4 |
| Triglycerides (mmol/l) | 1.5 ± 0.6 | 1.6 ± 0.8 | 1.6 ± 0.8 |
| Non–HDL cholesterol (mmol/l) | 4.3 ± 0.9 | 4.2 ± 0.9 | 4.4 ± 1.0 |
| Fasting glucose (mmol/l) | 5.5 ± 0.8 | 5.5 ± 0.9 | 5.5 ± 0.9 |
| 2-h postload glucose (mmol/l)* | 7.1 ± 2.6 | 6.9 ± 2.4 | 7.4 ± 2.9 |
| Fasting insulin (μU/ml)† | 5.8 ± 3.6 | 5.4 ± 3.2 | 6.2 ± 4.4 |
| HOMA-IR† | 1.41 ± 0.87 | 1.34 ± 0.87 | 1.60 ± 1.17 |
| Medication use (%) | |||
| Lipid-lowering drugs | 46.8 | 47.6 | 40.3 |
| Antihypertensive medication | 82.0 | 82.1 | 78.4 |
| Estrogen replacement therapy | 2.4 | 0.0 | 2.4 |
| Energy, food, and nutrient intake | |||
| Total energy (kcal/day) | 2,320 ± 579 | 2,365 ± 570 | 2,314 ± 580 |
| Carbohydrate (% energy) | 41 ± 6 | 40 ± 6 | 41 ± 7 |
| Protein (% energy) | 16 ± 3 | 16 ± 2 | 16 ± 2 |
| Fat (% energy) | 41 ± 6 | 41 ± 6 | 40 ± 7 |
| MUFA-to-SFA ratio | 1.9 ± 0.4 | 2.0 ± 0.4 | 1.9 ± 0.5 |
| Total fiber (g/day) | 23.7 ± 7.6 | 23.6 ± 8.0 | 23.0 ± 7.7 |
| Olive oil (g/day) | 41.2 ± 17.7 | 42.0 ± 16.5 | 40.1 ± 20.4 |
| Nuts (g/day) | 13.1 ± 15.0 | 14.4 ± 15.4 | 9.3 ± 12.1 |
| Vegetables (g/day) | 309 ± 129 | 310 ± 141 | 286 ± 117 |
| Fruits (g/day) | 298 ± 185 | 315 ± 164 | 286 ± 168 |
| Legumes (g/day) | 18 ± 7.9 | 19 ± 9.0 | 18 ± 8.4 |
| Cereals (g/day) | 248 ± 98 | 245 ± 99 | 251 ± 105 |
| Red meat and meat products (g/day) | 80 ± 44 | 86 ± 46 | 84 ± 47 |
| Milk and dairy products (g/day) | 355 ± 201 | 348 ± 183 | 346 ± 207 |
| Seafood (g/day) | 107 ± 42 | 104 ± 43 | 99 ± 41 |
| Alcohol (g/day) | 8 ± 11 | 11 ± 14 | 9 ± 12 |
| Red wine (ml/day) | 57 ± 95 | 75 ± 101 | 64 ± 89 |
| Score of adherence to the MedDiet | 8.4 ± 1.9 | 8.4 ± 1.9 | 7.9 ± 1.9 |
Data are means ± SD or %. VOO, virgin olive oil.
*Data were available for 263 participants.
†Data were available for 307 participants.
The diets were well tolerated. Up to 3% of participants in each treatment arm reported difficulties in following the prescribed diets, which were solved in all cases by the dietitians through individual counsel, negotiation, and small diet adjustments. The number of participants in each group who reported improved bowel motions (5.2–8.1%) was approximately twice the number of those who complained of newly developed constipation (3.3–3.7%). Supplementary Table 1 (available in an online appendix) shows the proportion of participants in each group who attained prespecified goals during the trial. The goals of higher MUFA-to-SFA ratios, higher intakes of total olive oil, nuts, fruit and vegetables, legumes, and fish, and a MedDiet score ≥10 were achieved more frequently by participants allocated to the two MedDiets than by those in the control group, whereas a small proportion of participants in each group reached substantial dietary fiber intakes. Only 21% of participants in the control group achieved the goal specific for this group of total fat intake <35% of energy. Weight changes among the 418 participants at the end of follow-up were −0.2 ± 4.6 kg for the olive oil diet group, −0.6 ± 4.2 kg for the nut diet group, and −0.6 ± 4.3 kg for the low-fat diet group (P = 0.74 for the comparison between groups). Likewise, physical activity changes were similar in the three groups: −17.4 ± 336, −58.8 ± 297, and −35.8 ± 257 kcal/day, respectively (P = 0.50). As shown in supplementary Table 1, at the end of the study, participants sustained weight loss >5% to a similar extent in the three groups, and a lower proportion of those in the control group were in the top tertile of physical activity. There were few changes in medication during the trial. Hypolipidemic and antihypertensive therapy was initiated by 15.6 and 6.1% of participants and discontinued by 5.4 and 3.2%, respectively, with a similar distribution among groups.
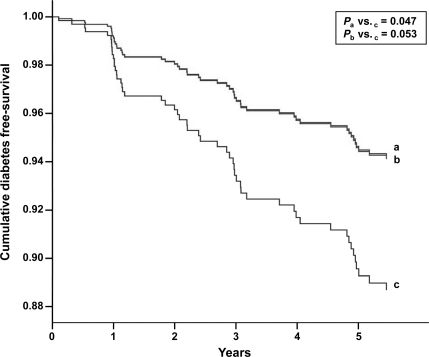
During the study, 54 individuals developed new-onset diabetes. Supplementary Table 2 (available in an online appendix) shows that the rate per 1,000 person-years of diabetes incidence was 24.6 (95% CI, 13.5–40.8) for the MedDiet with virgin olive oil group, 26.8 (15.3–43.0) for the MedDiet with nuts group, and 46.6 (30.1–68.5) for the control group. Cumulative incidence of diabetes was 10.1 (5.1–15.1) in the MedDiet with olive oil group and 11.0 (5.9–16.1) in the MedDiet with nuts group, whereas it was 17.9 (11.4–24.4) in the control group. Figure 1 shows that cumulative diabetes-free survival was lower in the control group compared with both MedDiet groups. After adjustment for various confounders, incident diabetes was reduced by 51% in the MedDiet with olive oil group and by 52% in the MedDiet with nuts group in comparison with the control group (Table 2). Thus, when the two MedDiet groups were merged into a single category in a similarly adjusted model, diabetes incidence was reduced by 52%. In multivariable regression models, sex, age, baseline obesity, and baseline fasting glucose were unrelated to outcomes.
Figure 1.
Cumulative diabetes free-survival by group of intervention. Cox regression models with outcome of diabetes onset and exposure to MedDiet intervention group vs. control diet group, adjusted by sex, age, baseline energy intake, BMI, waist circumference, physical activity, smoking status, fasting serum glucose, use of lipid-lowering drugs, Mediterranean diet score, and weight change during the study. a, MedDiet and virgin olive oil group; b, MedDiet and nuts group; c, control diet group.
Table 2.
Hazard ratios (95% CI) of diabetes by intervention group
| MedDiet with VOO vs. control diet | MedDiet with nuts vs. control diet | Both MedDiets vs. control diet | |
|---|---|---|---|
| Crude model | 0.53 (0.27–1.09) | 0.58 (0.31–1.10) | 0.55 (0.32–0.95) |
| Age- and sex-adjusted model | 0.52 (0.27–1.00) | 0.55 (0.29–1.00) | 0.53 (0.31–0.92) |
| Multivariate adjusted model* | 0.49 (0.25–0.97) | 0.48 (0.24–0.96) | 0.48 (0.27–0.86) |
| Sex† | |||
| Male | 0.48 (0.16–1.46) | 0.65 (0.21–2.00) | 0.55 (0.21–1.43) |
| Female | 0.47 (0.19–1.17) | 0.32 (0.11–0.93) | 0.40 (0.18–0.90) |
| Age† | |||
| ≤67 years | 0.50 (0.18–1.39) | 0.65 (0.26–1.61) | 0.58 (0.26–1.31) |
| >67 years | 0.26 (0.08–0.83) | 0.27 (0.07–0.98) | 0.26 (0.09–0.76) |
| BMI† | |||
| ≤30 kg/m2 | 0.56 (0.21–1.49) | 0.52 (0.19–1.41) | 0.54 (0.24–1.22) |
| >30 kg/m2 | 0.50 (0.18–1.42) | 0.62 (0.22–1.76) | 0.56 (0.23–1.43) |
| Fasting glucose† | |||
| ≤6.1 mmol/l | 0.44 (0.16–1.25) | 0.60 (0.24–1.50) | 0.53 (0.23–1.20) |
| >6.1 mmol/l | 0.29 (0.09–0.95) | 0.39 (0.11–1.37) | 0.32 (0.11–0.98) |
Cox regression models to assess the relative risk of diabetes by allocation group, estimating the hazard ratios (95% CI), were performed. Pinteraction (MedDiet × sex) = 0.496; Pinteraction (MedDiet × age) = 0.195; Pinteraction (MedDiet × BMI) = 0.592; Pinteraction (MedDiet × fasting glucose) = 0.932. VOO, virgin olive oil.
*Adjusted for sex, age, baseline energy intake, BMI, waist circumference, physical activity, smoking status, fasting serum glucose, use of lipid-lowering drugs, Mediterranean diet score, and weight changes during the study.
†Adjusted for the same variables as in footnote *, except for the variable of interest.
Diabetes incidence was lower in participants assigned to the two MedDiets (considered together), who attained ≥4 of the 7 prespecified goals or achieved a MedDiet score ≥10 (supplementary Fig. 2, available in an online appendix). Thus, 6.3% of participants in the MedDiet groups developed diabetes if they attained ≥4 goals compared with 15.0% of those who reached <4 goals (P = 0.02). Rates depending on attainment or not of a high score (≥10) of adherence to the MedDiet were 9.4 vs. 20.6% (P = 0.07), respectively, in the control group. Changes in weight or physical activity did not differ among participants in each intervention arm developing or not developing diabetes at the end of the study. In the control group, however, subjects developing diabetes had sustained a mean weight gain of 1.8 ± 3.3 kg, whereas those remaining diabetes-free had an average weight loss of 1.1 ± 4.4 kg, a nearly significant difference (P = 0.10).
CONCLUSIONS
In this nutrition intervention study we found that a non–calorie-restricted traditional MedDiet enriched with high-fat foods of vegetable origin decreased the incidence of diabetes in individuals at high cardiovascular risk after a median follow-up of 4.0 years. Diabetes rates were reduced by 51 and 52% by the consumption of MedDiets supplemented with virgin olive oil or mixed nuts, respectively, compared with a control diet consisting of advice on a low-fat diet. When the results of the two MedDiet groups were merged, risk reduction was 52%. These results extend those of prior studies showing that lifestyle interventions can substantially reduce the incidence of diabetes in individuals at high risk (2–6). However, in these studies, the interventions consisted of advice on a calorie-restricted diet plus physical activity and, except for one study (6), weight loss was a major driving force in reducing the incidence of diabetes. Of note, in our study, diabetes risk reduction occurred in the absence of significant changes in body weight or physical activity.
Our estimate of the magnitude of the effect of the MedDiet can be viewed as conservative because the data were analyzed by intention to treat, even though some participants in all treatment arms might not have been fully compliant with the intended dietary modifications. As described in other reports of the PREDIMED study (12,16), a sizable proportion of participants in the control group, despite being advised to follow the low-fat diet, did not substantially reduce total fat intake (supplementary Table 1), because of a long-lasting preference for using olive oil in the kitchen and at the table in Mediterranean cultures. In fact, diabetes risk was reduced to a similar extent in participants of all treatment arms who reported higher scores of adherence to the MedDiet (supplementary Fig. 2).
Our results are consistent with prior evidence suggesting a protective effect of the MedDiet against diabetes (7,9–11). Characteristically, the MedDiet is a high-fat, high-unsaturated fat dietary pattern, a feature that was maximized in our study by the free provision of virgin olive oil (rich in MUFAs) and mixed nuts (rich in MUFAs and polyunsaturated fatty acids) to participants in the MedDiet groups. As suggested by the known associations among subtypes of dietary fat and diabetes risk (17), the increased unsaturated fat load of our MedDiets was probably instrumental in achieving diabetes risk reduction.
The results of a prior PREDIMED study report (12) support the protective role of olive oil and nuts against diabetes risk, as both MedDiets were associated with improved fasting glucose in diabetic participants and decreased insulin resistance in those without diabetes after a 3-month follow-up, again in the absence of weight loss. In the same study (12), a reduction in circulating inflammatory biomarkers was observed in the two MedDiet groups. Because chronic low-grade inflammation is a pathogenetic factor in diabetes, synergy among the anti-inflammatory properties of the MedDiet and those specific to virgin olive oil (18) and nuts (19) might also be relevant to diabetes risk reduction. Regarding nuts, reports from large prospective studies suggest that usual intake relates inversely to future diabetes risk in women (20) but not in men (21). No such data are available for olive oil consumption and risk of diabetes. However, a former report of the PREDIMED trial (16) showed that, compared with the control diet, both MedDiets, particularly the nut-enriched diet, had a favorable effect on metabolic syndrome status after intervention for 1 year. The fact that in our study participants in any treatment arm who were more compliant with the MedDiet had two to three times lower incidence of diabetes than those with lesser scores supports a beneficial effect of the whole MedDiet pattern.
There are some limitations to our study. First, the Mediterranean cohort studied was older in age and at high risk for cardiovascular disease. The generalization of our findings to younger and/or healthier individuals from other geographical locations is uncertain. Nevertheless, it is plausible that the beneficial effect of the MedDiet on diabetes risk may be reproduced in other populations, as it has been shown for all-cause mortality, cardiovascular disease incidence, and cancer mortality in U.S. populations (22). Second, the lifestyle score used in our study to determine whether changes in dietary goals related to diabetes incidence could not reflect the totality of dietary changes, thus making difficult to show significant differences among interventions. Third, some participants did not undergo an OGTT, thus limiting an eventual diagnosis of diabetes to a fasting blood glucose ≥7.0 mmol/l confirmed by a second test, which might have falsely lowered overall incident rates. Finally, our sample size was relatively small, because the results are based on fewer than 55 incident cases, and the CIs for our estimates are wide. Longer follow-up of the PREDIMED cohort may eventually provide stronger evidence of diabetes prevention by the MedDiet.
In summary, the results show that a non–energy-restricted traditional MedDiet high in unsaturated fat can be a useful tool for preventing diabetes. Because other studies have shown that the benefit of lifestyle modification in reducing diabetes risk extends beyond the termination of active intervention (23–25), education of the population on the MedDiet might be a safe public health approach to delay or prevent development of diabetes as well as that of other prevalent chronic diseases (22). Further research is needed to elucidate the mechanisms leading to diabetes risk reduction independently of weight loss.
Acknowledgments
This study was funded, in part, by the Spanish Ministry of Health (Instituto de Salud Carlos III) (projects PI051839, PI070240, PI1001407, G03/140, and RD06/0045), Fondo Europeo de Desarrollo Regional, and the Public Health Division of the Department of Health of the Autonomous Government of Catalonia in collaboration with Merck Sharp & Dohme. The Fundación Patrimonio Comunal Olivarero and Hojiblanca SA (Málaga, Spain), California Walnut Commission (Sacramento, CA), Borges SA (Reus, Spain), and Morella Nuts SA (Reus, Spain) donated the olive oil, walnuts, almonds, and hazelnuts, respectively, used in the study.
J.S.-S. has received research funding from the International Nut Council (Reus, Spain) and is a nonpaid member of the Scientific Advisory Board of the International Nut Council. E.R. has received research funding from the California Walnut Commission (Sacramento, CA) and is a nonpaid member of its Scientific Advisory Committee.
No other potential conflicts of interest relevant to this article were reported.
None of the funding sources played a role in the design, collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication. CIBER Fisiopatologia de la Obesidad y Nutrición is an initiative of Instituto de Salud Carlos III, Spain.
J.S-S. conceived the study concept and design, obtained funding, acquired data, analyzed and interpreted data, wrote the manuscript, and reviewed/edited the manuscript. M.B. conceived the study concept and design, acquired data, analyzed and interpreted data, and reviewed/edited the manuscript. N.B. acquired data, analyzed and interpreted data, performed statistical analysis, wrote the manuscript, and reviewed/edited the manuscript. M.A.M.-G. conceived the study concept and design, obtained funding, analyzed and interpreted data, performed statistical analysis, wrote the manuscript, and reviewed/edited the manuscript. N.I.-J. acquired data, analyzed and interpreted data, and reviewed/edited the manuscript. J.B. conceived the study concept and design, acquired data, and reviewed/edited the manuscript. R.E. conceived the study concept and design, obtained funding, analyzed and interpreted data, wrote the manuscript, and reviewed/edited the manuscript. M.I.C. conceived the study concept and design and reviewed/edited the manuscript. D.C. conceived the study concept and design and reviewed/edited the manuscript. F.A. conceived the study concept and design and reviewed/edited the manuscript. V.R.-G. conceived the study concept and design, obtained funding, and reviewed/edited the manuscript. E.R. conceived the study concept and design, obtained funding, and reviewed/edited the manuscript.
We thank the participants for their enthusiastic collaboration, the PREDIMED personnel for excellent assistance, and the personnel of all affiliated primary care centers.
Footnotes
Clinical trial reg. no. ISRCTN35739639, ISRCTN.org.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.American Diabetes Association, Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML: Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31:S61–S78 [DOI] [PubMed] [Google Scholar]
- 2.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV: Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa MFinnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 4.Kosaka K, Noda M, Kuzuya T: Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract 2005;67:152–162 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DMDiabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay VIndian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 7.Kastorini CM, Panagiotakos DB: Dietary patterns and prevention of type 2 diabetes; from research to clinical practice; a systematic review. Curr Diabetes Rev 2009;5:221–227 [DOI] [PubMed] [Google Scholar]
- 8.Martínez-González MA, Bes-Rastrollo M, Serra-Majem L, Lairon D, Estruch R, Trichopoulou A: Mediterranean food pattern and the primary prevention of chronic disease: recent developments. Nutr Rev 2009;67(Suppl. 1):S111–S116 [DOI] [PubMed] [Google Scholar]
- 9.Martínez-González MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, Basterra-Gortari FJ, Beunza JJ, Vazquez Z, Benito S, Tortosa A, Bes-Rastrollo M: Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ 2008;336:1348–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Marfisi R, Levantesi G, Silletta MG, Tavazzi L, Tognoni G, Valagussa F, Marchioli R: Incidence of new-onset diabetes and impaired fasting glucose in patients with recent myocardial infarction and the effect of clinical and lifestyle risk factors. Lancet 2007;370:667–675 [DOI] [PubMed] [Google Scholar]
- 11.Esposito K, Maiorino MI, Ciotola M, Di Palo C, Scognamiglio P, Gicchino M, Petrizzo M, Saccomanno F, Beneduce F, Ceriello A, Giugliano D: Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med 2009;151:306–314 [DOI] [PubMed] [Google Scholar]
- 12.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, Fiol M, Gómez-Gracia E, López-Sabater MC, Vinyoles E, Arós F, Conde M, Lahoz C, Lapetra J, Sáez G, Ros EPREDIMED Study Investigators Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 2006;145:1–11 [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Ballart JD, Piñol JL, Zazpe I, Corella D, Carrasco P, Toledo E, Perez-Bauer M, Martínez-González MA, Salas-Salvadó J, Martín-Moreno JM: Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 2010;103:1808–1816 [DOI] [PubMed] [Google Scholar]
- 14.Elosua R, Marrugat J, Molina L, Pons S, Pujol E: Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am J Epidemiol 1994;139:1197–1209 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31(Suppl. 1):S55–S60 [DOI] [PubMed] [Google Scholar]
- 16.Salas-Salvadó J, Fernández-Ballart J, Ros E, Martínez-González MA, Fitó M, Estruch R, Corella D, Fiol M, Gómez-Gracia E, Arós F, Flores G, Lapetra J, Lamuela-Raventós R, Ruiz-Gutiérrez V, Bulló M, Basora J, Covas MIPREDIMED Study Investigators Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: one-year results of the PREDIMED randomized trial. Arch Intern Med 2008;168:2449–2458 [DOI] [PubMed] [Google Scholar]
- 17.Riserus U, Willett WC, Hu FB: Dietary fats and prevention of type 2 diabetes. Progr Lipid Res 2009;48:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covas MI, Konstantinidou V, Fitó M: Olive oil and cardiovascular health. J Cardiovasc Pharmacol 2009;54:477–482 [DOI] [PubMed] [Google Scholar]
- 19.Ros E: Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr 2009;89:1649S–1656S [DOI] [PubMed] [Google Scholar]
- 20.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB: Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002;288:2554–2560 [DOI] [PubMed] [Google Scholar]
- 21.Kochar J, Gaziano JM, Djoussé L: Nut consumption and risk of type II diabetes in the Physicians' Health Study. Eur J Clin Nutr 2010;64:75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A: Adherence to Mediterranean diet and health status: meta-analysis. BMJ 2008;11:337:a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto JFinnish Diabetes Prevention Study Group Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 24.Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roglic G, Hu Y, Bennett PH: The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 25.Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM: 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]