Abstract
OBJECTIVE
Acute glycemic variability contributes to diabetic complications potentially through induction of inflammation. Our objective was to determine whether acute hyperglycemia affects urinary secretion of inflammatory cytokines/chemokines in humans with uncomplicated type 1 diabetes.
RESEARCH DESIGN AND METHODS
Blood pressure, renal hemodynamics (inulin and paraaminohippurate clearances), and urine samples were obtained after 6 h of clamped euglycemia (4–6 mmol/l) and hyperglycemia (9–11 mmol/l) on two consecutive days in subjects with type 1 diabetes (n = 25). Forty-two urinary cytokines/chemokines were measured using a Luminex platform.
RESULTS
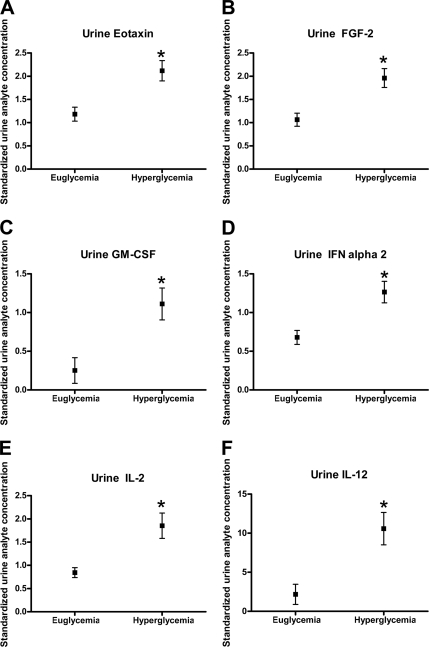
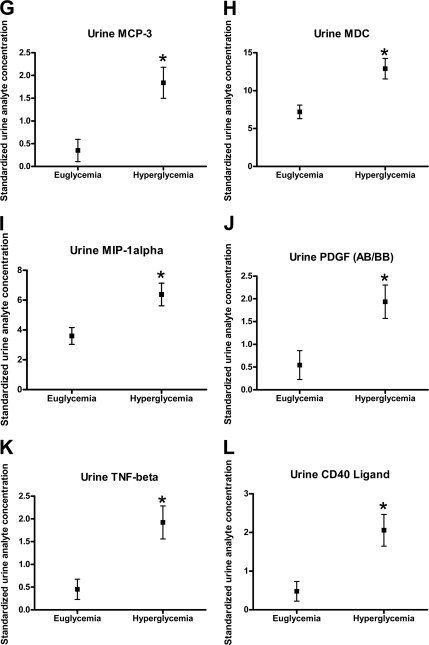
Clamped hyperglycemia produced an expected increase in glomerular filtration rate (131 ± 4 to 148 ± 8 ml/min/1.73 m2). Clamped hyperglycemia was associated with significant increases in urinary eotaxin, fibroblast growth factor-2, granulocyte-macrophage colony-stimulating factor, interferon-α 2, interleukin-2 and -12, monocyte chemoattractant protein-3, macrophage-derived chemokine, macrophage inflammatory protein-1α, platelet-derived growth factor, tumor necrosis factor-α, and CD40 ligand (P < 0.05).
CONCLUSIONS
Acute hyperglycemia results in increased urinary excretion of inflammatory cytokines/chemokines in humans with uncomplicated type 1 diabetes, and this may contribute to kidney injury.
Emerging evidence suggests that acute glycemic excursions may significantly contribute to microvascular end-organ injury in patients with diabetes, independent of long-term glycemic control (1). While the mechanisms accounting for this observation are not completely understood, evidence suggests that acute hyperglycemia affects systemic and renal microvascular hemodynamic function and activates systemic inflammatory pathways (2–6). The effect of acute clamped hyperglycemia on renal inflammatory mediators, however, is not completely understood (7–9). Accordingly, our goal was to characterize the earliest effects of acute hyperglycemia on the activation of renal inflammatory pathways in patients with uncomplicated type 1 diabetes in order to better understand how acute glycemic excursions may independently contribute to long-term kidney injury.
RESEARCH DESIGN AND METHODS
Recruitment of a subset of subjects in this cohort has been described in detail elsewhere (supplementary Table A1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-1219/DC1) (10). Subjects adhered to a sodium replete (>140 mmol/day) and moderate protein (<1.5 g/kg/day) diet during the 7-day period before each experiment (10,11). A 24-h urine collection on day 6 was used to evaluate dietary adherence through the determination of urinary sodium and urea excretion. Protein intake was calculated from the urea excretion using standard methods (10,11).
On two consecutive days, brachial artery blood pressure, renal hemodynamic parameters, and a spot urine sample were obtained after a 6-h modified clamp, during euglycemia (day 1, 4–6 mmol/l), and hyperglycemia (day 2, 9–11 mmol/l) (10,11). In the left arm, a peripheral venous cannula was inserted for infusion of glucose and insulin, and a second cannula was inserted for blood sampling more distally. At the same time, a third intravenous infusion was inserted into the right arm and connected to a syringe infusion pump to measure renal hemodynamic function.
Blood pressure measurements were obtained with an automated Dinamap sphygmomanometer (Critikon, Tampa, FL). Renal hemodynamic function (glomerular filtration rate and effective renal plasma flow) were estimated by steady-state infusion of inulin and paraaminohippurate (10,11).
Urinary analytes were measured in each urine sample using the 42-Plex Primary Cytokine/Chemokine Panel Luminex Assay (Eve Technologies, Calgary, Alberta, Canada) and included: epidermal growth factor, eotaxin, fibroblast growth factor-2, Fit-3L, fractalkine, granulocyte colony stimulation factor, granulocyte-macrophage colony-stimulating factor, growth-regulated oncogene, interferon (IFN)-α2, IFN-γ, interleukin (IL)-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IP-10, monocyte chemoattractant protein (MCP)-1, MCP-3, macrophage-derived chemokine, macrophage inflammatory protein (MIP)-1α, MIP-1β, platelet-derived growth factor (PDGF)-AA, PDGF-AB/BB, RANTES, sCD40K, sIL-2Rα, transforming growth factor-α, tumor necrosis factor (TNF)-α, TNF-β, and vascular endothelial growth factor (supplementary Table A2). Analytes were corrected for the urinary creatinine concentration.
Baseline clinical parameters were analyzed using parametric methods (two independent sample t tests). Within subject responses to hyperglycemia were determined by repeated measures analysis of variance and were corrected for multiple comparisons using the Tukey-Kramer test. All statistical analyses were performed using SAS 9.2. The University Health Network and Hospital for Sick Children (Toronto, Canada) Research Ethics Boards approved the protocols, and patients gave informed consent.
RESULTS
Supplementary Table A3 describes the clinical characteristics of the cohort (n = 25). Subjects were young, normotensive, normoalbuminuric men and women with type 1 diabetes. Subjects adhered to the controlled sodium and protein diet. Six hours of clamped hyperglycemia was associated with expected increases in glomerular filtration rate and effective renal plasma flow (P < 0.05).
Clamped hyperglycemia was also associated with significant increases in urinary eotaxin (P = 0.0075), fibroblast growth factor-2 (P = 0.0026), granulocyte-macrophage colony-stimulating factor (P = 0.0192), IFN-α2 (P = 0.0045), IL-12 (P = 0.0034), MCP-3 (P = 0.0062), macrophage-derived chemokine (P = 0.0111), MIP-1α (P = 0.0201), PDGF-AB/BB (P = 0.0267), TNF-β (P = 0.0049), sCD40K (P = 0.0080) (Fig. 1A–L). For IL-3, the unadjusted P value was 0.01, which was not significant after adjustment for multiple comparisons (P = 0.058).
Figure 1.
The effect of hyperglycemia on urinary chemokines/cytokines (mean ± SD).*P < 0.05 compared with clamped euglycemia.
CONCLUSIONS
The goal of this pilot study was to determine whether acute clamped hyperglycemia activates renal inflammatory pathways in patients with uncomplicated type 1 diabetes. Our rationale was twofold: 1) acute glycemic excursions may contribute to end-organ injury in patients with diabetes, independent of long-term glycemic control; and 2) while acute hyperglycemia is associated with hemodynamic changes and activation of systemic inflammation, the acute effects on renal inflammatory pathways are not described well. We therefore determined whether acute hyperglycemia may contribute to kidney injury by activation of inflammatory pathways in patients with uncomplicated type 1 diabetes by measuring renal hemodynamic function and urinary concentrations of a broad panel of cytokines/chemokines in response to acute clamped hyperglycemia.
Our major finding was that in subjects with uncomplicated type 1 diabetes, clamped hyperglycemia is associated with significant increases in 12/42 cytokines/chemokines included in the pilot study. Urinary cytokines/chemokines excretion is increased in established diabetic nephropathy, and their excretion is influenced by renoprotective medications (7,8,12–14). Less is known about the renal excretion of inflammatory biomarkers in response to acute clamped hyperglycemia in humans with uncomplicated type 1 diabetes (15). Our findings show that acute moderate hyperglycemia induces an increase in urinary excretion of inflammatory mediators. While the factors responsible for this rise were not determined in this study, possible mechanisms include synthesis and/or release of preformed cytokines/chemokines from the kidney or possible spillover from the systemic circulation.
Given the critical role for hyperglycemia in the pathogenesis of diabetic nephropathy, the acute effect of glucose on markers of inflammation and fibrosis is important for two key reasons. First, increases in the urinary excretion of inflammatory factors may give mechanistic insights into disease pathogenesis in humans at a preclinical stage of disease. Our observations are consistent with previous work that demonstrated the deleterious effects of acute glycemic excursions on the renal microvasculature and highlight biological pathways that may be involved. Second, mediators that increase under the influence of acute hyperglycemia may act as markers that can be used to monitor the biological effects of experimental and therapeutic interventions in patients with type 1 diabetes. Although our study involved a small cohort that may never develop clinical diabetic nephropathy, our findings suggest that acute hyperglycemia induces a dynamic molecular signal in the urine that may contribute to progressive kidney injury over time. Future work should involve defining the time course and reversibility of the response and studying other populations, including nondiabetic control subjects and patients with overt nephropathy.
In conclusion, acute moderate hyperglycemia is associated with significant increases in the urinary excretion of inflammatory cytokines/chemokines in subjects with uncomplicated type 1 diabetes.
Acknowledgments
This work was supported by operating grants from the Canadian Diabetes Association (to D.Z.I.C.). D.Z.I.C. is a recipient of a Kidney Foundation of Canada Scholarship; a Canadian Diabetes Association–Kidney Research Scientist Core Education and National Training (KRESCENT) Program Joint New Investigator Award; and receives operating support from the Canadian Institutes of Health Research (CIHR), the Canadian Diabetes Association, and the Heart and Stroke Foundation of Canada (with H.N.R.). H.N.R. is a recipient of a KRESCENT Program New Investigator Award. J.W.S. is the CIHR/AMGEN Canada Kidney Research Chair at the University Health Network, University of Toronto.
No potential conflicts of interest relevant to this article were reported.
D.Z.I.C. and H.N.R. researched data and wrote the manuscript. J.W.S., E.S., and T.J.B. contributed to discussion and reviewed/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Brownlee M, Hirsch IB: Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA 2006;295:1707–1708 [DOI] [PubMed] [Google Scholar]
- 2.Gordin D, Rönnback M, Forsblom C, Heikkilä O, Saraheimo M, Groop PH: Acute hyperglycaemia rapidly increases arterial stiffness in young patients with type 1 diabetes. Diabetologia 2007;50:1808–1814 [DOI] [PubMed] [Google Scholar]
- 3.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D: Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002;106:2067–2072 [DOI] [PubMed] [Google Scholar]
- 4.Stegenga ME, van der Crabben SN, Dessing MC, Pater JM, van den Pangaart PS, de Vos AF, Tanck MW, Roos D, Sauerwein HP, van der Poll T: Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med 2008;25:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordin D, Forsblom C, Rönnback M, Parkkonen M, Wadén J, Hietala K, Groop PH: Acute hyperglycaemia induces an inflammatory response in young patients with type 1 diabetes. Ann Med 2008;40:627–633 [DOI] [PubMed] [Google Scholar]
- 6.Miller JA: Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol 1999;10:1778–1785 [DOI] [PubMed] [Google Scholar]
- 7.Merchant ML, Perkins BA, Boratyn GM, Ficociello LH, Wilkey DW, Barati MT, Bertram CC, Page GP, Rovin BH, Warram JH, Krolewski AS, Klein JB: Urinary peptidome may predict renal function decline in type 1 diabetes and microalbuminuria. J Am Soc Nephrol 2009;20:2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert RE, Akdeniz A, Weitz S, Usinger WR, Molineaux C, Jones SE, Langham RG, Jerums G: Urinary connective tissue growth factor excretion in patients with type 1 diabetes and nephropathy. Diabetes Care 2003;26:2632–2636 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TQ, Tarnow L, Andersen S, Hovind P, Parving HH, Goldschmeding R, van Nieuwenhoven FA: Urinary connective tissue growth factor excretion correlates with clinical markers of renal disease in a large population of type 1 diabetic patients with diabetic nephropathy. Diabetes Care 2006;29:83–88 [DOI] [PubMed] [Google Scholar]
- 10.Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hébert RL, Sochett EB: The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes 2008;57:688–695 [DOI] [PubMed] [Google Scholar]
- 11.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA: Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 2006;17:1703–1709 [DOI] [PubMed] [Google Scholar]
- 12.Gilbert RE, Kim SA, Tuttle KR, Bakris GL, Toto RD, McGill JB, Haney DJ, Kelly DJ, Anderson PW: Effect of ruboxistaurin on urinary transforming growth factor-beta in patients with diabetic nephropathy and type 2 diabetes. Diabetes Care 2007;30:995–996 [DOI] [PubMed] [Google Scholar]
- 13.Andersen S, van Nieuwenhoven FA, Tarnow L, Rossing P, Rossing K, Wieten L, Goldschmeding R, Parving HH: Reduction of urinary connective tissue growth factor by Losartan in type 1 patients with diabetic nephropathy. Kidney Int 2005;67:2325–2329 [DOI] [PubMed] [Google Scholar]
- 14.Tashiro K, Koyanagi I, Saitoh A, Shimizu A, Shike T, Ishiguro C, Koizumi M, Funabiki K, Horikoshi S, Shirato I, Tomino Y: Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal 2002;16:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelikánová T, Simková R, Tesar V, Jirsa M: Effect of acute hyperglycaemia on selected plasma and urinary cytokine antagonists in type 1 diabetes mellitus. Diabetologia 2003;46:470–474 [DOI] [PubMed] [Google Scholar]