Abstract
OBJECTIVE
Data on latent autoimmune diabetes in adults (LADA) from population-based studies are sparse. We sought to investigate the prevalence and correlates of LADA.
RESEARCH DESIGN AND METHODS
A total of 8,109 participants, who were aged ≥15 years and living in Tianjin, China, were assessed to identify individuals with type 2 diabetes (American Diabetes Association Criteria, 1997) and further to detect patients with LADA. LADA was ascertained by 1) the presence of type 2 diabetes and age ≥35 years, 2) the lack of a requirement for insulin at least 6 months after the diagnosis of type 2 diabetes, and 3) serum GAD antibody positivity. Data were analyzed using multinomial logistic regression with adjustment for potential confounders.
RESULTS
Of all participants, 498 (6.1%) were patients with type 2 diabetes. Of them, 46 (9.2%) were found to have LADA. The prevalence of LADA was 0.6% (46 of 8,109), and tended to increase with age up to 50–59 years in all participants. The odds ratios (95% CI) of LADA related to hypertension, family history of diabetes, waist-to-hip ratio ≥0.85, and major stressful events were 1.93 (1.02–3.65), 17.59 (9.08–34.06), 5.37 (2.31–12.49), and 4.09 (1.75–9.52), respectively.
CONCLUSIONS
The prevalence of LADA is ∼9% in patients with type 2 diabetes. Hypertension, family history of diabetes, central obesity, and major stressful events may be associated with the occurrence of LADA.
Latent autoimmune diabetes in the adult (LADA) is a slowly progressive form of autoimmune diabetes and is characterized by diabetes-associated autoantibody positivity (1). Patients with LADA have an insidious onset of hyperglycemia and clinical presentation similar to that of type 2 diabetes at onset (2,3). Epidemiological studies suggest that LADA may account for 2–12% of all cases of diabetes (4).
The prevalence of LADA in western countries varies from 2.8 to 10% in patients with type 2 diabetes (5–8). The Diabetes Outcomes Progression Trial recently reported that GAD antibody (GADA) positivity is 4.2% in North America and 3.7% in Europe among individuals with type 2 diabetes (9). In China, two clinical studies have shown that the estimated prevalence of LADA in patients with type 2 diabetes is ∼7% (10,11). The etiology of LADA is unclear, and it is not known whether LADA is due to the same underlying disease process as childhood type 1 diabetes. A recent study has suggested that the patients with LADA share genetic features with both type 1 and type 2 diabetic patients (12). In addition, several studies have reported that metabolic disorders, such as hypertension and obesity, and a family history of diabetes are associated with the risk of LADA (10,13,14).
Accumulating clinical evidence has shown significant overlap between type 1 and type 2 diabetes. The disease process in patients with classic type 1 diabetes is believed to be autoimmune in nature, whereas the disease process in classic type 2 diabetes is not. The treatment for patients with LADA may need to be different from that used for patients with type 2 diabetes (15). Because LADA is frequently misdiagnosed as type 2 diabetes, identification of LADA is of clinical importance. In this population-based cross-sectional study, we sought to investigate the prevalence of LADA and further to explore whether familial, vascular, and psychosocial factors are associated with the occurrence of LADA.
RESEARCH DESIGN AND METHODS
The study population was derived from the inhabitants of Tianjin, which is the third largest city and located in the east coastal area in China. The city is composed of six urban districts, six suburbs, and three newly developed areas. Each area contains 8–10 residential communities, and each community consisted of 8–10 blocks. The population of the city is ∼11 million. In June 2005, a multiphase stratified cluster sampling method was implemented, and a random sample of the city was drawn for the study population. The strategy of sampling in this study was designed on the basis of socioeconomic status and was in line with the sampling scheme for the Chinese National Dietary and Nutrition Study in Tianjin. In brief, a three-step randomized sampling procedure was performed as follows: first, two urban districts and one suburb were drawn as the first-stage sample; second, three communities were selected as the second-level unit; and, finally, three neighborhoods were chosen as third-level unit. Thereafter, a total of 18 urban and nine suburb blocks were included in the study. All inhabitants (N = 8,540) who had lived in the selected blocks for >5 years, who were aged ≥15 years, and who were type 1 diabetes free were initially invited to participate in the study. In July 2005, a two-phase survey consisting of a screening phase and further blood testing phase was implemented. The screening phase included a health interview, physical examination, and fasting peripheral blood glucose test for 8,109 (95.0%) participants. In the second phase, all subjects who had positive screening results (fasting peripheral blood glucose ≥5.4 mmol/l) were invited to provide fasting and prandial blood samples. Informed consent was received from all participants. The ethics committee at the Tianjin Medical University approved the study.
Data collection
Data on age, sex, education, lifestyle, health status, and family history of chronic disease and major stressful events were collected from participants at the screening phase through the interview following a structured questionnaire. Education was categorized by the maximum years of formal schooling and was dichotomized (≥9 vs. <9 years). Family history of diabetes was defined as having diabetes in any of the following family members: parents, grandparents (either paternal and maternal), and siblings. Height and weight were measured without shoes and heavy clothes. BMI was calculated as weight in kilograms divided by the square of height in meters. Waist circumference was measured between the lower rib margin and the anterior superior iliac spine, and the hip circumference was measured over the maximum of the buttocks. Waist-to-hip ratio was calculated as waist circumference (centimeters) divided by hip circumference (centimeters) and dichotomized (<0.85 vs. ≥0.85) based on its distribution. Alcohol drinking was also dichotomized (drinking at least once per week vs. former drinker or never drinking). Smoking status was categorized as never, low (1–20 cigarettes/day), and high (>20 cigarettes/day). Experience of major stressful events was defined as job or close relatives lost, frequent spousal conflict, or accidents in the past 10 years. Physical activity participation was dichotomized as “regular” (two times or more per week for at least 1 year) and “nonregular.”
Diagnosis of type 2 diabetes and LADA
Fasting peripheral blood glucose was measured with a OneTouch ULTRA2 meter (Life Scan) at the screening phase for all participants, and fasting and 2-h postprandial venous blood samples were taken for subjects who had fasting peripheral blood glucose ≥5.4 mmol/l at the second phase. Fasting plasma glucose and 2-h postprandial plasma glucose were measured using a glucose oxidase procedure. Type 2 diabetes was ascertained as fasting plasma glucose ≥7.0 mmol/l or postprandial 2-h plasma glucose ≥11.1 mmol/l according to the American Diabetes Association diagnostic criteria (1997). LADA was identified based on Immunology of Diabetes Society criteria (16) as follows: 1) the presence of type 2 diabetes and age ≥35 years; 2) a lack of requirement for insulin at least 6 months after the diagnosis of type 2 diabetes; and 3) serum GADA positivity as tested by radioligand assay (17).
Statistical analyses
The characteristics of participants in different groups were compared using χ2 tests for categorical variables and one-way ANOVA for continuous variables. The prevalence rates were calculated as the number of subjects with LADA divided by number of subjects and standardized by the structure of total population in the city in 2005. Multinomial logistic regression analyses were used to estimate the odds ratios (ORs) and 95% CI of LADA and type 2 diabetes in relation to age, sex, education, hypertension, family history of diabetes, smoking, alcohol drinking, physical activity, BMI, waist-to-hip ratio, and major stressful events. LADA and type 2 diabetes were used as separate outcomes, and diabetes-free subjects were used as the referent group in the multinomial logistic regression models. The analyses were performed in participants aged ≥35 years (n = 6,137) because of the absence of LADA in subjects aged 15–34 years. All statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL).
RESULTS
The 8,109 participants consisted of 3,878 (47.8%) men and 4,231 (52.2%) women (χ2 = 15.37, P < 0.001). Age and sex distributions in our study population were similar to the distributions in the source population in Tianjin based on data from the Annual Statistic Report, Tianjin, 2005. Among all subjects, 498 (6.1%) had type 2 diabetes, which included 268 (53.8%) with previously diagnosed diabetes and 230 (46.2%) with newly detected diabetes. Of the 498 patients with type 2 diabetes, 46 (9.2%) were found to have LADA. Patients with LADA were more likely to be older and obese, to have hypertension, and to have a family history of diabetes and stressful events but showed less alcohol drinking, compared with diabetes-free subjects. The three groups did not differ significantly in terms of education and smoking (Table 1).
Table 1.
Characteristics of the study participants by type 2 diabetes and LADA
| Characteristics | Nondiabetes | Type 2 diabetes | LADA | P value |
|---|---|---|---|---|
| n (%) | 7,611 (93.8) | 452 (5.6) | 46 (0.6) | |
| Age (years) | 45.8 ± 15.3 | 55.9 ± 10.4 | 53.9 ± 7.8 | <0.001 |
| Female sex | 3,932 (51.7) | 275 (60.8) | 24 (52.2) | 0.001 |
| Education (≥9 years) | 2,504 (33.0) | 130 (28.8) | 15 (32.6) | 0.182 |
| Smoking | 2,770 (36.4) | 168 (37.3) | 21 (45.7) | 0.406 |
| Alcohol drinking* | 2,076 (28.0) | 86 (19.5) | 11 (23.9) | <0.001 |
| Hypertension* | 1,265 (17.1) | 191 (42.5) | 16 (34.8) | <0.001 |
| Family history of diabetes* | 181 (2.7) | 137 (30.4) | 15 (32.6) | <0.001 |
| Major stressful events* | 237 (3.1) | 84 (18.8) | 7 (15.2) | <0.001 |
| BMI* | 24.0 ± 3.4 | 26.2 ± 3.5 | 25.7 ± 3.4 | <0.001 |
| Underweight (<20 kg/m2) | 788 (10.4) | 11 ( 2.5) | 1 ( 2.2) | |
| Normal (20–24.99 kg/m2) | 4,094 (53.8) | 164 (36.7) | 17 (37.0) | |
| Overweight (25–29.99 kg/m2) | 2,361 (31.0) | 216 (48.3) | 23 (50.0) | |
| Obese (≥30 kg/m2) | 368 ( 4.8) | 56 (12.5) | 5 (10.9) | <0.001 |
| Waist-to-hip ratio (≥0.85)* | 3,825 (50.3) | 347 (78.2) | 38 (82.6) | <0.001 |
Data are n (%) or means ± SD. n = 8,109.
*Numbers of subjects with missing values were 195 for alcohol drinking, 162 for hypertension, 803 for family history of diabetes, 43 for major stressful events, 5 for BMI, and 11 for waist-to-hip ratio.
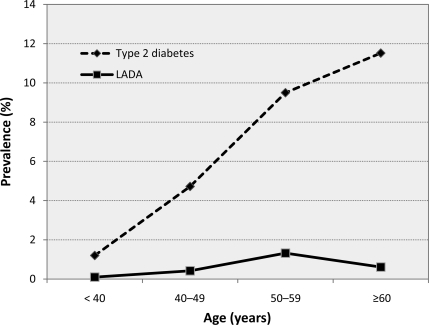
Table 2 shows that the prevalence of LADA was 9.2% (8.0% in women and 11.1% in men, P = 0.253) in individuals with type 2 diabetes. Among all participants, the prevalence of LADA was 0.6% for both men and women and was 0.7% in participants aged ≥35 years. The standardized prevalence of LADA was 0.69% (0.68% in men and 0.70% in women), which was based on the source population aged ≥35 in Tianjin in 2005. With age, the prevalence of LADA slowly increased up to 50–59 years and seemed to decline thereafter. However, the prevalence of type 2 diabetes was dramatically elevated with age and tended to continuously increase after age 60 years (Fig. 1).
Table 2.
Prevalence of LADA in patients with type 2 diabetes and all participants
| Age | LADA in patients with type 2 diabetes |
LADA in all participants |
||||
|---|---|---|---|---|---|---|
| Male | Female | Total | Male | Female | Total | |
| n | 498 | 8,109 | ||||
| <40 years | 1/12 (8.3) | 1/20 (5.0) | 2/32 (6.3) | 1/1,221 (0.1) | 1/1,372 (0.1) | 2/2,593 (0.1) |
| 40–49 years | 2/33 (6.1) | 6/57 (10.5) | 8/90 (8.9) | 2/931 (0.2) | 6/975 (0.6) | 8/1,906 (0.4) |
| 50–59 years | 12/77 (15.6) | 14/110 (12.7) | 26/187 (13.9) | 12/948 (1.3) | 14/1,021 (1.4) | 26/1,969 (1.3) |
| ≥60 years | 7/77 (9.1) | 3/112 (2.7) | 10/189 (5.3) | 7/778 (0.9) | 3/863 (0.3) | 10/1,641 (0.6) |
| Total | 22/199 (11.1) | 24/299 (8.0) | 46/498(9.2) | 22/3,878 (0.6) | 24/4,231 (0.6) | 46/8,109 (0.6) |
Data are n (%) unless otherwise indicated.
Figure 1.
Prevalence of LADA and type 2 diabetes in all participants.
In multinomial logistic regression, hypertension, family history of diabetes, high waist-to-hip ratio, and major stressful events were significantly associated with both LADA and type 2 diabetes, compared with diabetes-free subjects. Age, female sex, and high BMI (≥25 kg/m2) were positively and alcohol drinking was negatively related to type 2 diabetes. Although these factors seemed to be associated with LADA, the association did not reach statistical significance because of the small number of patients with LADA (Table 3). Regular physical activity (OR 0.95 [95% CI 0.49–1.84] for LADA and 0.91 [0.64–1.29] for type 2 diabetes) and education (≥9 years) (0.99 [0.53–1.83] for LADA and 0.82 [0.67–1.01] for type 2 diabetes) were not significantly associated with either LADA or type 2 diabetes. Compared with subjects who never smoked, subjects who smoked low and high numbers of cigarettes had ORs of 1.02 (95% CI 0.56–1.88) and 1.89 (0.44–8.02) for LADA and 0.89 (0.72–1.10) and 1.50 (0.88–2.56) for type 2 diabetes, respectively. These results suggest that LADA and type 2 diabetes might share risk factors.
Table 3.
Factors and ORs of prevalent LADA and type 2 diabetes
| Factors | LADA |
Type 2 diabetes |
||
|---|---|---|---|---|
| n | OR (95% CI)* | n | OR (95% CI)* | |
| n | 46 | 436 | ||
| Age (years)† | 46 | 1.00 (0.97–1.03) | 436 | 1.03 (1.02–1.05) |
| Female sex | 24 | 1.77 (0.85–3.67) | 265 | 2.54 (1.90–3.39) |
| Alcohol drinking | 11 | 0.66 (0.30–1.49) | 84 | 0.71 (0.52–0.98) |
| BMI | ||||
| <20 kg/m2 | 1 | 0.62 (0.08–4.82) | 10 | 0.54 (0.26–1.16) |
| 20–24.99 kg/m2 | 17 | 1 (Referent) | 160 | 1 (Referent) |
| 25–29.99 kg/m2 | 23 | 1.72 (0.90–3.29) | 211 | 1.68 (1.30–2.17) |
| ≥30 kg/m2 | 5 | 1.86 (0.66–5.26) | 55 | 2.18 (1.46–3.25) |
| Waist-to-hip ratio | ||||
| <0.85 | 8 | 1 (Referent) | 93 | 1 (Referent) |
| ≥0.85 | 38 | 5.37 (2.31–12.49) | 343 | 4.60 (3.39–6.23) |
| Hypertension | 16 | 1.93 (1.02–3.65) | 187 | 2.50 (1.96–3.19) |
| Family history of diabetes | 15 | 17.59 (9.08–34.06) | 133 | 16.06 (11.71–22.03) |
| Major stressful events | 7 | 4.09 (1.75–9.52) | 81 | 4.98 (3.51–7.07) |
*Adjusted for age, sex, alcohol drinking, BMI, waist-to-hip ratio, hypertension, family history of diabetes, and major stressful events if applicable.
†As a continuous variable.
A standardized workup for the GADA assay was performed in 50 patients with newly diagnosed type 1 diabetes and 100 control subjects (mean age 32, range 4–80 years). The results showed that the median area under the receiver operator characteristic curve was 0.95 (86% sensitivity and 95% specificity).
CONCLUSIONS
In this large population-based cross-sectional study, we found that 1) the prevalence of LADA is 9.2% among individuals with type 2 diabetes and 0.6% in all participants, 2) the prevalence slowly increased with age up to 60 years and was high in individuals aged 50–59 years, and 3) hypertension, family history of diabetes, high waist-to-hip ratio, and experience of stressful events are related to the occurrence of LADA. Given the high prevalence of diabetes worldwide, our findings are of clinical importance.
The prevalence of LADA varies according to the populations involved, criteria used, and antibodies analyzed. It has become clear that LADA accounts for ∼10% of initial diagnoses of type 2 diabetes (18). Epidemiological studies have shown conflicting results on the differences in LADA prevalence in terms of age and sex among people with type 2 diabetes. One study showed a male predominance of LADA, whereas other studies found a higher prevalence among women (6,19). Another study reported that the prevalence of GADA positivity is 0.9% in a Spanish population aged 18–65 years (20). In this population-based cross-sectional study, we found that 1) the prevalence of LADA is 9.2% among individuals with type 2 diabetes and 0.6% in all participants and 2) the prevalence is not sex specific but is high in individuals aged 50–59 years.
Autoimmunity is the major cause of type 1 diabetes and is assumed to be the cause of LADA, which shares the biochemical marker of β-cell–directed autoimmunity with classic type 1 diabetes (21). Thus, LADA is considered a “mild” form of type 1 diabetes, which shows slow progression to insulin dependence. The fact that HLA-DR4-DQ8 antigens are more widespread among patients with classic type 1 diabetes than among those with LADA may offer an explanation as to why patients with LADA do not develop insulin dependence as quickly as patients with classic type 1 diabetes (22).
The risk factors for LADA are less well understood than those for type 1 and type 2 diabetes. Age and overweight are important risk factors for type 2 diabetes. Whether these factors also increase the risk of LADA is unclear. Previous studies based on cross-sectional data have shown that patients with LADA were younger and less obese than type 2 diabetic subjects (5) or found these variables to be similar in those with type 2 diabetes. A population-based prospective study has shown the risk effect of age, overweight, and physical inactivity on LADA (14). A cross-sectional study indicated that family history of diabetes is a strong risk factor for LADA because of a combination of shared genetic and environmental factors (13). In addition, a recent clinical study reported patients with LADA having more metabolic disorders such as hypertension and overweight (10). In the present study, we found that hypertension, family history of diabetes, high waist-to-hip ratio, and experience of major stressful events are related to both LADA and type 2 diabetes, suggesting that LADA and type 2 diabetes might share risk factors. However, the statistical power was limited for the analyses of other factors for LADA because of the small number of patients with LADA in our study. Further population-based longitudinal studies are warranted to clarify the temporality for the relationships between these factors and LADA risk.
The main strengths of our study are the large population-based sample, the randomized sampling procedure, the diagnosis of diabetes based on both fasting and prandial plasma glucose level, and the identification of individuals with LADA by GADA positivity. However, some limitations need to be pointed out. First, we used fasting peripheral blood glucose rather than fasting plasma glucose as the screening test; thus, patients with diabetes might have been misclassified in the nondiabetes group, which would attenuate our results. In addition, individuals with a severe or long-standing chronic disease, e.g., diabetes, might have refused to participate in this study because of their unhealthy condition. On the other hand, it is possible that individuals with mild type 2 diabetes might be more interested in participation than those without this condition. However, the participation rate was high (95%), and the prevalence of type 2 diabetes in this study is comparable to that in previous reports. Thus, the participation bias might not be substantial. Second, the definition used for LADA as age ≥35 years might be arbitrary. Yet, in our study, all patients with type 2 diabetes who were aged <35 years were GADA negative. Third, information on the presence of current and past disease and family history of diabetes was mainly based on self-report. Subjects with diabetes are probably more likely to be aware of relatives with diabetes; therefore, recall bias may not be ruled out. However, the prevalence of smoking and hypertension in our study was similar to the prevalence reported by other studies. In addition, information on several diabetes-related diseases such as cerebrovascular disease and coronary heart disease was not available. Finally, the temporality of the given associations is unclear because of the cross-sectional design of this study.
In summary, our results show that the prevalence of LADA is ∼9% among individuals with type 2 diabetes and high in individuals aged 50–59 years. Hypertension, family history of diabetes, high waist-to-hip ratio, and experiences of stressful events are associated with the occurrence of LADA, suggesting the involvement of both genetic and environmental factors in LADA. Given the high prevalence of type 2 diabetes worldwide, our findings highlight the need to identify patients with LADA for proper treatment. Further population-based longitudinal studies are required to verify the risk factors for the development of LADA.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant 30471490).
No potential conflicts of interest relevant to this article were reported.
X.Q. initiated and designed the study, ran the analyses, wrote the manuscript, and reviewed/edited the manuscript. J.S. ran the analyses, researched data, wrote the manuscript, and reviewed/edited the manuscript. Jin.W. researched data and reviewed/edited the manuscript. P.P.W. reviewed/edited the manuscript. Z.X. researched data and reviewed/edited the manuscript. M.M. reviewed/edited the manuscript. J.J. researched data and reviewed/edited the manuscript. Jia.W. researched data and reviewed/edited the manuscript. Y.X. researched data and reviewed/edited the manuscript. W.X. contributed to the data interpretation and reviewed/edited the manuscript.
We gratefully acknowledge Gan Huang, Helai Jin, and Xia Wang at the Institute of Metabolism and Endocrinology, Second Xiangya Hospital, Central South University, Changsha, People's Republic of China, for their laboratory support of our study.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Tiberti C, Giordano C, Locatelli M, Bosi E, Bottazzo GF, Buzzetti R, Cucinotta D, Galluzzo A, Falorni A, Dotta F: Identification of tyrosine phosphatase 2(256–760) construct as a new, sensitive marker for the detection of islet autoimmunity in type 2 diabetic patients: the Non-Insulin Requiring Autoimmune Diabetes (NIRAD) Study 2. Diabetes 2008;57:1276–1283 [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ: Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 3.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 4.Naik RG, Palmer JP: Latent autoimmune diabetes in adults (LADA). Rev Endocr Metab Disord 2003;4:233–241 [DOI] [PubMed] [Google Scholar]
- 5.Tuomi T, Carlsson A, Li H, Isomaa B, Miettinen A, Nilsson A, Nissén M, Ehrnström BO, Forsén B, Snickars B, Lahti K, Forsblom C, Saloranta C, Taskinen MR, Groop LC: Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes 1999;48:150–157 [DOI] [PubMed] [Google Scholar]
- 6.Falorni A, Brozzetti A: Diabetes-related antibodies in adult diabetic patients. Best Pract Res Clin Endocrinol Metab 2005;19:119–133 [DOI] [PubMed] [Google Scholar]
- 7.Ruige JB, Batstra MR, Aanstoot HJ, Bouter LM, Bruining GJ, De Neeling JN, Heine RJ: Low prevalence of antibodies to GAD65 in a 50- to 74-year-old general Dutch population. The Hoorn Study. Diabetes Care 1997;20:1108–1110 [DOI] [PubMed] [Google Scholar]
- 8.Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, Shattock M, Bottazzo GF, Holman R: UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet 1997;350:1288–1293 [DOI] [PubMed] [Google Scholar]
- 9.Zinman B, Kahn SE, Haffner SM, O'Neill MC, Heise MA, Freed MIADOPT Study Group Phenotypic characteristics of GAD antibody-positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes 2004;53:3193–3200 [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Ma XJ, Bao YQ, Pan XP, Lu W, Hu C, Xiang KS, Jia WP: Study on prevalence of latent autoimmune diabetes in adults and its relationship with metabolic syndrome. Zhonghua Yi Xue Za Zhi 2009;89:1250–1254[in Chinese] [PubMed] [Google Scholar]
- 11.Li X, Zhou Z, Huang G, Su H, Yan X, Yang L: Metabolic syndrome in adult-onset latent autoimmune diabetes. Metab Syndr Relat Disord 2005;3:174–180 [DOI] [PubMed] [Google Scholar]
- 12.Cervin C, Lyssenko V, Bakhtadze E, Lindholm E, Nilsson P, Tuomi T, Cilio CM, Groop L: Genetic similarities between latent autoimmune diabetes in adults, type 1 diabetes, and type 2 diabetes. Diabetes 2008;57:1433–1437 [DOI] [PubMed] [Google Scholar]
- 13.Carlsson S, Midthjell K, Grill V: Influence of family history of diabetes on incidence and prevalence of latent autoimmune diabetes of the adult: results from the Nord-Trøndelag Health Study. Diabetes Care 2007;30:3040–3045 [DOI] [PubMed] [Google Scholar]
- 14.Carlsson S, Midthjell K, Tesfamarian MY, Grill V: Age, overweight and physical inactivity increase the risk of latent autoimmune diabetes in adults: results from the Nord-Trøndelag Health Study. Diabetologia 2007;50:55–58 [DOI] [PubMed] [Google Scholar]
- 15.Brophy S, Brunt H, Davies H, Mannan S, Williams R: Interventions for latent autoimmune diabetes (LADA) in adults. Cochrane Database Syst Rev 2007;3:CD006165. [DOI] [PubMed] [Google Scholar]
- 16.Fourlanos S, Dotta F, Greenbaum CJ, Palmer JP, Rolandsson O, Colman PG, Harrison LC: Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia 2005;48:2206–2212 [DOI] [PubMed] [Google Scholar]
- 17.Huang G, Zhou ZG, Peng J, Yan X, Zhu XP, Yang L, Li X, Wang JP, Jiang TJ: Detection of GAD-Ab index in diabetic patients using 35S labeled recombinant human GAD65 antigen. Chin J Nucl Med 2003;23:82–86 [Google Scholar]
- 18.van Deutekom AW, Heine RJ, Simsek S: The islet autoantibody titres: their clinical relevance in latent autoimmune diabetes in adults (LADA) and the classification of diabetes mellitus. Diabet Med 2008;25:117–125 [DOI] [PubMed] [Google Scholar]
- 19.Genovese S, Bazzigaluppi E, Gonçalves D, Ciucci A, Cavallo MG, Purrello F, Anello M, Rotella CM, Bardini G, Vaccaro O, Riccardi G, Travaglini P, Morenghi E, Bosi E, Pozzilli P: Clinical phenotype and β-cell autoimmunity in Italian patients with adult-onset diabetes. Eur J Endocrinol 2006;154:441–447 [DOI] [PubMed] [Google Scholar]
- 20.Soriguer-Escofet F, Esteva I, Rojo-Martinez G, Ruiz de Adana S, Catalá M, Merelo MJ, Aguilar M, Tinahones F, García-Almeida JM, Gómez-Zumaquero JM, Cuesta-Muñoz AL, Ortego J, Freire JM: Prevalence of latent autoimmune diabetes of adults (LADA) in Southern Spain. Diabetes Res Clin Pract 2002;56:213–220 [DOI] [PubMed] [Google Scholar]
- 21.Radtke MA, Midthjell K, Nilsen TI, Grill V: Heterogeneity of patients with latent autoimmune diabetes in adults: linkage to autoimmunity is apparent only in those with perceived need for insulin treatment: results from the Nord-Trøndelag Health (HUNT) study. Diabetes Care 2009;32:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schranz DB, Bekris L, Landin-Olsson M, Törn C, Niläng A, Toll A, Sjöström J, Grönlund H, Lernmark A: Newly diagnosed latent autoimmune diabetes in adults (LADA) is associated with low level glutamate decarboxylase (GAD65) and IA-2 autoantibodies. Diabetes Incidence Study in Sweden (DISS). Horm Metab Res 2000;32:133–138 [DOI] [PubMed] [Google Scholar]