Abstract
We used a species-specific approach to treat 10 patients with cutaneous leishmaniasis diagnosed using polymerase chain reaction. Non-antimony treatments (oral miltefosine, ketoconazole, and liposomal amphotericin B) were chosen as an alternative to pentavalent antimony drugs based on likely or proven drug efficacy against the infecting species. Leishmania Viannia panamensis was diagnosed in three patients and treated successfully with oral ketoconazole. Miltefosine treatment cured two patients with L. infantum chagasi. A wide variety of Leishmania responded to liposomal amphotericin B administered for 5–7 days. Three patients with L. V. braziliensis, one patient with L. tropica, and two patients with L. infantum chagasi were treated successfully. One person with L. V. braziliensis healed slowly because of a resistant bacterial superinfection, and a second patient with L. infantum chagasi relapsed and was retreated with miltefosine. These drugs were reasonably well-tolerated. In this limited case series, alternative non-antimony–based regimens were convenient, safe, and effective.
Introduction
Leishmaniasis is a protozoal infection transmitted by the bite of an infected sandfly. Over 21 species of Leishmania cause infection in humans worldwide, resulting in three clinical phenotypes: cutaneous, mucocutaneous, and visceral disease. The clinical spectrum of cutaneous leishmaniasis (CL) varies widely and depends on the geographic site acquired, the infecting species, and the host immune status. Cutaneous disease typically begins as a papule that increases in size over weeks to months and becomes a shallow ulcer with a raised rim. Depending on the infecting Leishmania species, people may develop localized (for example, because of L. mexicana, L. major, or L. peruviana) or diffuse cutaneous involvement (for example, because of L. amazonensis) or cutaneous involvement with potential to disseminate (for example, because of L. Viannia braziliensis). Untreated lesions can self-resolve with scarring in 2–15 months (or longer) in immunocompetent persons.1 Local intralesional treatments have been recommended for small, single lesions (< 5 cm) without lymph node metastasis caused by species that do not typically disseminate to the mucosa or viscera (e.g., L. mexicana). Systemic treatment is often indicated to reduce the risk of dissemination to the mucosa or viscera for certain species (e.g., L. V. braziliensis), decrease the time to healing, limit the morbidity caused by large or persistent skin lesions, and reduce the chance of relapse. Currently, the standard, first-line treatment of CL is intravenous (i.v.) sodium stibogluconate, 20 mg/kg per day, for 21 days.2–4 Use of sodium stibogluconate, however, requires close monitoring for adverse effects such as arthralgias, myalgias, chemical pancreatitis, and elevated liver function tests, which occur in more than half of those treated.5 Moreover, a poor response to therapy has been observed in 23.5% of primary CL cases treated with pentavalent antimonials.6 Whether the poor response relates to insufficient drug levels (secondary to poor penetration into the skin or suboptimal dosing because of tolerability) or parasite resistance to drug therapy remains to be determined. Oral or short-course i.v. therapies are particularly attractive alternatives to a lengthy i.v. course of sodium stibogluconate, because it enables outpatient treatment of CL patients who feel otherwise well except for their skin lesions. Several non-antimony–based treatments (such as oral ketoconazole, oral miltefosine, and short-course i.v. liposomal amphotericin B) have the potential to be convenient, cost-effective, and less toxic alternatives to sodium stibogluconate.
A complicating feature of the treatment of CL is that the response to drugs (including pentavalent antimonials) varies depending on the infecting species and the geographic site of acquisition.7 A species-specific treatment approach has increasingly been advocated for precisely this reason.8 Commonly used methods for the diagnosis of Leishmania, such as direct microscopic examination of clinical specimens and culture, do not identify the infecting species. Isoenzyme analysis has long been considered the gold standard for diagnosis of leishmaniasis to the species level.9 This method, however, can be laborious and time-consuming, and it requires growth of Leishmania promastigotes in culture, which can take several weeks. In recent years, molecular speciation by polymerase chain reaction (PCR) has been increasingly cited as a rapid and reliable method, with greater sensitivity than microscopic methods.10,11 PCR can be performed directly on the clinical specimen, with results processed in a few hours, thus providing timely information to the clinician.10–13
In this single-center prospective case series, we used a species-specific treatment approach based on molecular identification of the infecting species by PCR in 10 patients with CL. An alternative non-antimony regimen was then chosen based on proven or likely efficacy against that species for CL or visceral disease. Our experience with this treatment approach and the efficacy and safety of these regimens is summarized here.
Methods
Patients.
Patients were evaluated for study enrollment if they had a clinical diagnosis of CL with parasitologic confirmation at the species level by PCR and/or isoenzyme analysis. Patients provided informed consent before treatment with non-antimonial regimens. The study was approved by the Institutional Review Board at the National Institutes of Allergy and Infectious Diseases.
Diagnostic tests.
Skin biopsies were taken from the edge of the lesion, and the following studies were performed: histopathology (for demonstration of amastigotes), Wright-Giemsa staining of touch preparations, and culture in modified Nicole-Novy-McNeal medium containing an overlay of RPMI 1640 and 15% heat inactivated fetal bovine serum. Additional studies (acid-fast staining, wet mount, fungal culture, routine culture, gram stain, and anaerobe culture) were performed on the biopsy specimen where appropriate to exclude other etiologies.
PCR was performed on skin tissue and blood at the National Institutes of Health (NIH) and was based on the use of a partial sequence of the 7SL RNA gene, which has been shown to differentiate clearly between Old World species (L. major, L. tropica, L. aethiopica, and L. donovani complex) and New World complexes L. Viannia and L. mexicana.12,13 To differentiate L. donovani and L. Viannia complexes further, tissue specimens were sent to the Centers for Disease Control and Prevention (CDC; Atlanta, GA) for PCR amplification and sequencing of a fragment of the ribosomal RNA (rRNA) internal transcribed spacer 2 followed by DNA sequencing analysis for identification of single-nucleotide polymorphisms and species-specific substitutions14 (de Almeida ME and others, unpublished data). Culture isolates were additionally sent to Walter Reed Army Institute of Research and/or the CDC for species confirmation by isoenzyme analysis.9,15
Definition of clinical relapse.
Patients were followed in our clinic at 1, 3, 6, and 12 months or more frequently as needed. If patients were lost to follow-up before 12 months, an attempt was made to contact them by phone. Patients were also instructed to contact us at any point during or after the study in the event of worsening or relapse. Photographs of the lesions were taken at baseline and clinic visits at the discretion of the physician caring for the patient. Measurement of lesion size was performed to the nearest centimeter of the long and short axes of each lesion by one observer. The area of the lesion was calculated by multiplying the lengths of the two axes (the lesion was assumed to be rectangular). For multiple lesions, the sum of the areas for each lesion was calculated to provide a total surface area. For infiltrative lesions without discrete borders, an approximate measurement was provided. Healing was defined as complete reepithelialization. A lesion was defined as cured by clinical criteria if it had completely healed without relapse for 6 months. Biopsies of suspicious lesions were only repeated in the event of suspected relapse or reinfection. Relapse was defined as new ulceration or satellite lesions or enlargement of ulceration after initial improvement.
Treatment.
Oral miltefosine (Caligor RX, New York, NY) was prescribed for single-patient use under an investigational new drug (IND) status (50 mg two times daily for 28 days).16,17 Ketoconazole (Teva Pharmaceuticals USA, North Wales, PA) was prescribed for 28 days by mouth (600 mg daily).18 Daily i.v. liposomal amphotericin B (AmBisome; Gilead Sciences, Inc., Deerfield, IL) was infused over 1–2 hours in a monitored setting for outpatients at a dose of 3–5 mg/kg per day for 5–7 days.19 The decision of whether to use 3 mg/kg per day or 5 mg/kg per day and the duration was largely based on clinical judgment (response of lesion to therapy and toxicity to patient). A normal saline bolus (500–1,000 mL) was administered before liposomal amphotericin. Patients were not pre-medicated before the infusion. Laboratory studies (complete blood count, chemistry, and liver function tests) were drawn at baseline and every 2 weeks for patients on miltefosine or ketoconazole and daily for patients on i.v. liposomal amphotericin B. Female patients of childbearing age were counseled regarding the potential for ketoconazole to interact with oral contraceptives and the teratogenic potential of miltefosine, and they were advised to use an additional barrier method of contraception. Serum or urine pregnancy tests were checked for female patients of childbearing age before initiation of antileishmanial therapy.
Rationale for choice of therapy.
The three non-antimonial regimens used in this study (oral ketoconazole, short-course i.v. liposomal amphotericin B, and oral miltefosine) were chosen based on their proven or likely efficacy against the infecting species in patients with CL and/or visceral leishmaniasis. The available evidence supporting the use of these particular drugs for species causing CL mentioned in this report is summarized in Table 1.
Table 1.
Systemic non-antimonial treatment of CL: evidence for use of non-antimony treatment by species*
| Species | Subspecies | Drug | Geographic site studied | Quality of evidence† | Reference |
|---|---|---|---|---|---|
| Old World | |||||
| L. tropica | Miltefosine | Afghanistan and Iran | IV | 20–23 | |
| New World | |||||
| L. Viannia complex | L. V. braziliensis | Liposomal amphotericin B | Belize, Bolivia/Peru, and Brazil | IV | 24–26 |
| Bolivia | III | 19 | |||
| Peru and Brazil | V | 27–29 | |||
| Miltefosine | Bolivia and Costa Rica | II and IV | 30,31 | ||
| L. V. panamensis | Ketoconazole | Panama | I | 18 | |
| Nicaragua, Israel, Algeria, Saudi Arabia, and Ethiopia | IV | 32–36 | |||
| L. donovani complex | L. infantum chagasi | Liposomal amphotericin B | Austria and Italy | IV | 37–39 |
| India and the Mediterranean | V | 40–44 | |||
| Miltefosine | Spain | IV | 45,46 | ||
| India and Ethiopia | V | 16,47,48 |
Evidence is summarized for use of only the following non-antimony regimens: ketoconazole, miltefosine, liposomal amphotericin B, and the Leishmania species mentioned in this report.
Quality of evidence. I = Evidence from one or more properly randomized, controlled trials; II = Evidence from one or more randomized trials in a partially representative patient group (small patient number and different species included); III = Evidence from one or more well-designed clinical trials without randomization from cohort or case-controlled analytic studies (preferably from one center), multiple time series studies, or dramatic results from uncontrolled experiments; IV = Evidence based on clinical experience and descriptive studies; V = Evidence based on efficacy in visceral or mucocutaneous leishmaniasis.
Results
Ten consecutive patients were seen at the Clinical Center of the NIH (Bethesda, MD) between 2001 and 2005, and all were enrolled in the study. All patients were immunocompetent. A summary of the cases is shown in Table 2.
Table 2.
Demographic, epidemiologic, and clinical characteristics
| Case no. | Age/sex | Geographic site lesion acquired | Location of lesion | Lesion appearance | No. of lesions | Baseline size of lesion (cm2)* | Size of lesion at 1 month (cm2)* | Duration of lesion (months) | Prior treatment | Treatment | Treatment side effects | Treatment course completed | Cure | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. infantum chagasi | ||||||||||||||
| 1 | 60/M | Spain | Right ear | Diffuse, inflammatory, non-ulcerated | Single | 8.4 | 5.67 | 11 | No | Miltefosine | Mild loose stools | Yes | Yes | 12 |
| 2 | 25/F | El Salvador | Left cheek | Nodular | Single | 4.14 | 2.25 | 36 | No | Liposomal amphotericin B | Nephrotoxicity | No | No | 11 |
| Miltefosine | Motion sickness | No | Yes | 39 | ||||||||||
| 3 | 7/M | El Salvador | Face | Nodular | Multiple | 1.06 | 0.42 | 24 | No | Liposomal amphotericin B | None | Yes | Yes | 12 |
| L. V. panamensis | ||||||||||||||
| 4 | 29/F | Costa Rica | Left arm | Ulcerative | Single | 15.21 | 10.5 | 4 | No | Liposomal amphotericin B | Flushing, shortness of breath, chest pain | No | – | – |
| Ketoconazole | None | Yes | Yes | 12 | ||||||||||
| 5 | 67/F | Panama | Right and left shoulders | Dry, crusted | Multiple | 13.73 | 0.34 | 8 | No | Ketoconazole | None | Yes | Yes | 8 |
| 6 | 65/M | Panama | Right knee, elbow, shoulder | Ulcerated, nodular | Multiple | 14.25 | 0 | 8 | No | Ketoconazole | Hepatitis | No | Yes | 8 |
| L. V. braziliensis | ||||||||||||||
| 7 | 55/M | Ecuador | Right arm | Ulcerated | Single | 0.16 | 0 | 1 | Yes | Liposomal amphotericin B | Nephrotoxicity | Yes | Yes | 12 |
| 8 | 37/F | Costa Rica | Left arm, right leg | Dry, crusted | Multiple | 6.13 | 2.69 | 1 | No | Liposomal amphotericin B | Nausea, fleeting rash | Yes | Yes | 36 |
| 9 | 40/F | Costa Rica | Left arm, right leg | Ulcerated, nodular | Multiple | 11.12 | 7.89 | 1 | No | Liposomal amphotericin B | Nausea, dizziness | Yes | Yes | 36 |
| L. tropica | ||||||||||||||
| 10 | 6/M | Afghanistan | Right cheek, left wrist | Papules | Multiple | 0.04 | 0 | 9 | Yes | Liposomal amphotericin B | Hypokalemia, nephrotoxicity | No | No† | 3 |
M = male; F = female; No. = number.
Measurement of lesion size was performed to the nearest centimeter of the long and short axes of each lesion by one observer and calculated by multiplying the lengths of the axes (assuming the lesion was rectangular). For multiple lesions, the sum of the areas for each lesion was calculated to provide a total surface area. For case 1, the lesion was infiltrative and did not have discrete borders. An approximate measurement is provided for this lesion.
Case 10 was lost to follow-up after 3 months. Therefore, clinical response to therapy could not be assessed after this time point.
Case 1.
A 60-year-old male was treated with miltefosine for an inflammatory lesion on his right earlobe caused by L. infantum chagasi. He tolerated the treatment well. The only side effect that he reported was mild loose stools, which were self-limited. The lesion showed significant improvement (50% less induration) within the first month. The lesion had almost completely healed by 6 months (with very minimal erythema), and his ear appeared completely normal at 1 year.
Case 2.
A 25-year-old female had an exophytic, erythematous, and non-ulcerated skin lesion on her left check caused by L. infantum chagasi (Figure 1A). After 5 days of treatment with liposomal amphotericin B (3 mg/kg per day), her serum creatinine increased from 0.9 to 1.5 mg/dL. Despite initial improvement (decrease in size of lesion by 46%), 6 months later, the lesion was again erythematous, possibly caused by itching and self-manipulation. The patient was treated symptomatically and serially evaluated. A repeat biopsy taken from the edge of the lesion showed a lymphocytic infiltrate and questionable presence of amastigotes, but PCR and cytopathologic examination of the touch preparations were negative. During the next few months, the lesion regressed but did not completely heal, and oral miltefosine was prescribed. Within 2 weeks, the lesion appeared flatter and less red. On day 25 of treatment, the patient stopped the miltefosine because of several episodes of motion sickness (gastrointestinal uneasiness and dizziness). Despite increased redness and swelling 2 days later, the lesion healed completely by 3 months, and there was no relapse for 2 years (Figure 1B).
Figure 1.
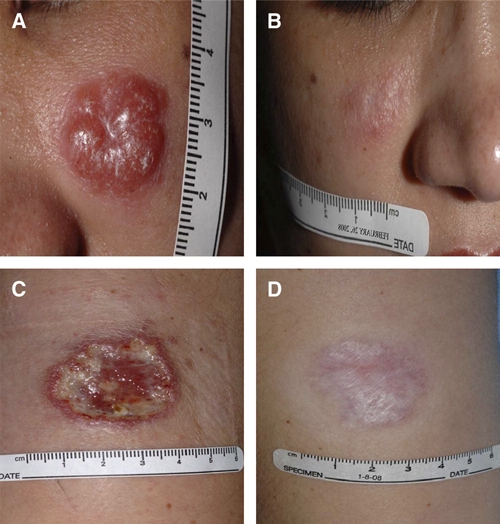
This figure shows photographs of cutaneous leishmaniasis lesions taken at baseline and final visit after completing treatment in case 2 (A and B) and case 4 (C and D). This figure appears in color at www.ajtmh.org.
Case 3.
A 7-year-old male developed nodular skin lesions on his forehead and cheek caused by L. infantum chagasi. He was treated with 5 days of i.v. liposomal amphotericin B (3 mg/kg per day) without side effects. The lesions decreased in size by 60% 1 month post-treatment, with progressive flattening and residual scarring for the next 6 months. He continued to have a complete response without relapse for 1 year.
Case 4.
A 29-year-old female developed an ulcerated skin lesion on her left arm (Figure 1C) associated with a tender cord extending from the lesion into her left axilla and axillary lymphadenopathy secondary to L. V. braziliensis complex. She was treated with liposomal amphotericin B (3 mg/kg per day) but developed flushing, shortness of breath, and chest pain within minutes of receiving the first dose. The infusion was stopped; 50 mg diphenhydramine hydrochloride (Benadryl; McNeil Consumer Healthcare, Washington, PA) and 100 mg hydrocortisone were administered intravenously, and the symptoms resolved. After a period, the organism was further identified as L. V. panamensis. She was treated with oral ketoconazole, which was tolerated very well. The lesion showed epithelialization and 31% reduction in size by 1 month; it had completely healed by 3 months. No evidence of relapse was noted for 1 year (Figure 1D).
Case 5.
A 67-year-old female developed multiple, non-ulcerated, erythematous skin lesions on both shoulders associated with multiple subcutaneous nodules caused by L. V. panamensis. She tolerated treatment with oral ketoconazole well. The size of the lesions decreased by 98% within the first month of treatment. At 8 months, the lesion was without relapse.
Case 6.
The husband of the previous patient, a 65-year-old male, developed multiple ulcerated and nodular lesions on the right knee, elbow, and shoulder caused by L. V. panamensis. Oral ketoconazole was prescribed, but it was stopped after 21 days because of increasing liver function tests and clinical hepatitis, which resolved after 1 month. Despite early discontinuation of ketoconazole, his lesions completely epithelialized at his 1-month follow-up visit and continued to show sustained improvement at follow-up 8 months later without relapse.
Case 7.
A 55-year-old male had an ulcerated lesion of his right forearm caused by L. V. braziliensis that was treated with an uncomplicated 21-day course of i.v. sodium stibogluconate at an outside institution. The lesion healed completely within 1 month of treatment; 1 year later, he was referred to us after he noticed a new ulceration with mild swelling at the edge of the scar of the previous lesion. Biopsy of the lesion confirmed L. V. braziliensis, and the patient was treated with liposomal amphotericin B (3 mg/kg per day for 7 days). During the course of therapy, the patient's lesion decreased in size, showed complete epithelialization at 1-month follow-up, and was without relapse at 1 year.
Case 8.
A 37-year-old female developed five erythematous-crusted plaques, four on her left arm and one on the right leg. A small lesion at the mucocutaneous junction of the left nares was not biopsied, because its appearance was not consistent with mucocutaneous leishmaniasis. A nodule was noted on the left elbow. The patient was treated with 7 days of i.v. liposomal amphotericin (3 mg/kg per day) for CL caused by L. V. braziliensis. The total estimated surface area of the lesions decreased in size by 56% at 1 month, and there was no relapse for 36 months.
Case 9.
A 40-year-old woman developed four painless erythematous nodules on her left arm and one on her right leg that gradually increased in size and developed raised borders with ulceration. A cord was palpable along the upper arm extending into the axilla. The patient was treated with 7 days of i.v. liposomal amphotericin (3 mg/kg per day) for L. V. braziliensis. The lesions decreased in size by 30% and became less raised within 1 week of treatment; however, 1 month later, the edge of the left-arm lesion appeared more indurated. A subcutaneous cord persisted. Pus was superficially present in multiple lesions, and no indication for incision and drainage was found. Culture of the lesions revealed heavy growth of methicillin-resistant Staphylococcus aureus. She was treated with a 10-day course of oral levofloxacin (500 mg), which led to transient improvement with subsequent return of mild purulence superficially at the site of the lesions. Thereafter, she was treated with 2% topical mupirocin ointment three times daily for 3 months. After 7 months, the left-arm lesion had completely healed, with no relapse for 36 months.
Case 10.
A 6-year-old boy developed a raised erythematous lesion with central ulceration on the right cheek and a dry lesion on the dorsum of the left wrist caused by L. tropica. The patient was treated with 21 days of i.v. sodium stibogluconate without complication. The lesions had healed 2 months later, but new papules were noted at the edge of the facial scar after 6 months. The lesion remained stable in size and appearance for 1 year but later, became larger and more erythematous. A biopsy and PCR confirmed relapse. He was treated with a 7-day course of liposomal amphotericin B (5 mg/kg per day). At follow-up 3 weeks later, the patient's papules had healed. No evidence of relapse was noted 3 months later. The patient was lost to follow-up thereafter, and for this reason, his response to treatment could not be assessed after this time period.
Discussion
A species-oriented treatment approach has been advocated for leishmaniasis given increasing recognition of interspecies differences accounting for variability in treatment response.7,8 In addition to this, a pressing need for alternatives to sodium stibogluconate has been identified because of unwanted toxicities, cumbersome administration, and treatment failures in some cases. Despite this, in most centers, molecular speciation is not routinely performed, and sodium stibogluconate remains the first-line treatment. We used a strategy that maximizes use of more convenient non-antimony regimens based on the infecting species.
Species identification.
Real-time PCR has clear advantages over microscopy in the detection and speciation of leishmaniasis.49–51 The PCR-based assays used in our study yielded rapid and accurate information on speciation that was used in a timely fashion to inform choice of therapy. Several PCR-based assays for the diagnosis of leishmaniasis have been developed to date.10–14,49–51 Different assays may use different targets for amplification (such as the ribosomal RNA gene, mini-exon gene, internal transcribed spacer regions, or kinetoplast DNA).10–14,49–51 Although these targets offer accurate taxonomic data for amplification, they vary in their specificities and species-discriminating capabilities. Clinicians using PCR-based assays must be familiar with their strengths and limitations. In our study, we used two PCR assays sequentially to adequately differentiate the L. Viannia complex further, because only CL caused by L. V. panamensis can be treated with ketoconazole. The use of multiple assays to adequately speciate leishmaniasis (and the inherent associated cost) complicates the use of a species-specific approach as shown here, particularly in resource-limited settings. Thus, a need exists for simple, comprehensive, and low-cost PCR-based assays for the adequate speciation of Leishmania.
Treatment choice and efficacy.
After the infecting species was identified to the complex or species level, a non-antimony regimen was chosen; those used in this series included ketoconazole, miltefosine, and short-course i.v. liposomal amphotericin B.
In this case series, oral ketoconazole was effective in all cases of CL caused by L. V. panamensis (N = 3). Two of these patients acquired disease in Panama, and one acquired disease in Costa Rica. Of the treatments used in this study, only ketoconazole has been proven effective in a randomized trial comparing oral ketoconazole (76% cure rate; N = 21) with intramuscular sodium stibogluconate (68% cure rate; N = 19) for the treatment of CL caused by L. V. panamensis acquired in Panama.18 Based on these findings, ketoconazole is considered by some to be the first choice for treatment of uncomplicated cutaneous lesions (i.e., single lesions without mucosal involvement) caused by this species.52 The generalizability of these data to CL caused by L. V. panamensis acquired in other regions, however, is not clearly established, and further studies are needed. Limited clinical reports suggest that ketoconazole cures CL in Nicaragua, Israel, Algeria, Saudi Arabia, and Ethiopia.32–36 Decreased cure rates have been noted in limited reports from Belize,53 although species identification beyond the complex level was not performed in most cases. For this reason, close follow-up of CL cases is necessary to monitor treatment response so that an alternative agent can be used if necessary. Another caveat to the use of ketoconazole in the treatment of CL is that speciation must be performed beyond the L. Viannia complex level, because treatment failures have been noted in cases secondary to L. V. braziliensis or L. V. guyanensis.54–56
Short-course i.v. liposomal amphotericin B was used to treat cases of CL caused by L. infantum chagasi, L. V. braziliensis, and L. tropica. Of the CL patients with L. infantum chagasi acquired in El Salvador (N = 2), one patient with multiple lesions had an excellent response to therapy. The other patient, with a single nodular lesion on the face, had a clinical but not a parasitologic relapse noticed 6 months post-treatment that was successfully retreated with miltefosine. Two patients with cutaneous disease caused by L. V. braziliensis acquired in Ecuador (N = 1) and Costa Rica (N = 1) were successfully treated. An additional patient had delayed healing attributed to bacterial superinfection caused by methicillin-resistant S. aureus that was successfully treated with oral levofloxacin and topical mupirocin. Bacterial superinfection is a known complication of CL lesions, with an incidence of 21.8% in one series.57 The lengthy time to heal in this patient with bacterial superinfection (7 months) is not unexpected, because coinfection reduces the rate of elimination of bacteria and enhances the severity of CL skin lesions.58 After the patient was treated with antibiotics, her CL lesion improved considerably, and she was without relapse for 36 months post-liposomal amphotericin B treatment.
Liposomal amphotericin B has been found to be an effective treatment of visceral leishmaniasis caused by L. infantum chagasi in the Mediterranean region and L. donovani in India,40–43 and it is currently the US Food and Drug Administration-approved drug for treatment of visceral leishmaniasis (VL) caused by these species.44 Liposomal amphotericin B has been used anecdotally in the treatment of cutaneous and mucocutaneous disease caused by L. V. braziliensis acquired in Belize, Bolivia, Peru, and Brazil as well as in immunocompromised patients.19,24,26–29,59 A prospective, non-randomized study comparing liposomal amphotericin B with sodium stibogluconate for treatment of CL caused by L. V. braziliensis found no failures in the liposomal amphotericin B-treated group and fewer side effects.19 Successful use of liposomal amphotericin B in the treatment of VL, however, does not necessarily extrapolate to cutaneous disease, because the pharmacokinetics of liposomal amphotericin B in cutaneous disease have not been well-established. Although it has been suggested that drug accumulation at the dermis level may be insufficient,60 i.v. administration of liposomal amphotericin B has been shown to be effective against experimental CL, albeit at higher doses than that required for visceral disease.61
The optimal dose and duration of liposomal amphotericin B for the treatment of CL is unknown and deserves further study. The total treatment dose used in this study was based primarily on the use of this dose with visceral disease and also on anecdotal evidence using this dose in the treatment of CL caused by L. V. braziliensis.19,44 Although these limited data are favorable for liposomal amphotericin B, the same may not be true of other lipid-based amphotericin B products such as Abelcet (amphotericin B lipid complex) or Amphotec (amphotericin B cholesteryl complex). A treatment failure has been described in one CL patient who was treated with high-dose Abelcet,62 a finding supported by animal models.63
One case of CL caused by L. tropica acquired in Afghanistan that relapsed after treatment with i.v. sodium stibogluconate was treated with liposomal amphotericin B and healed at 3 months, after which the patient was unfortunately lost to follow-up. The short duration of follow-up is inadequate for CL caused by this particular species given the known relapse rate; thus, the treatment response to liposomal amphotericin B in this patient cannot be adequately assessed. CL caused by L. tropica frequently causes a dry noduloulcerative lesion that can persist for a prolonged period of time and is frequently resistant to treatment. Cure rates of 75% have been described after at least 10 days of standard i.v. antimony therapy.64 Use of liposomal amphotericin B with CL caused by L. tropica is not well-documented. Limited clinical data suggest that miltefosine may be a viable alternative for CL caused by L. tropica.20–23
Miltefosine was used to successfully treat two patients with CL caused by L. infantum chagasi lesions acquired in Spain and El Salvador. Miltefosine has been shown to have potent leishmanicidal activity and has been used safely and effectively in the treatment of over 2,500 patients with diffuse CL, mucosal disease, and visceral disease.16,47,48 The susceptibility of L. donovani and L. infantum chagasi lesions to miltefosine has been documented by an in vivo and in vitro study.65 Of note, higher doses of miltefosine (2.5 mg/kg per day up to 150 mg/day for 28 days) than that used in our study have been used in the treatment of CL, although the optimal treatment dose has not been well-established.30,31,66
Toxicity.
Ketoconazole was tolerated without any side effects or liver function test abnormalities in two of three patients. One patient developed clinical hepatitis, which resolved after discontinuation of ketoconazole on day 21 of therapy. Ketoconazole-induced hepatitis frequently resolves after discontinuation of treatment. Serious ketoconazole-induced hepatotoxicity occurs rarely (1/15,000 exposed patients), but it can be fulminant and even fatal if treatment is continued after symptoms of hepatitis develop.67–70 More commonly, asymptomatic rises in serum transaminase activity occur during treatment with ketoconazole. Frequent monitoring of liver function tests (such as alanine aminotransferase, aspartate aminotransferase, and bilirubin) is advised every 1–2 weeks while on therapy. A progressive or greater than 3-fold rise in serum transaminases should prompt discontinuation of therapy.71 Despite the concern for liver function test abnormalities associated with use of ketoconazole, this may occur more frequently with sodium stibogluconate. Asymptomatic, mild liver function test abnormalities were less frequent with oral ketoconazole (27%) than with sodium stibogluconate (47%) in a randomized control trial of CL patients treated with either of these drugs.18
No unexpected toxicities secondary to liposomal amphotericin B use were observed in any of our patients. The side effects of liposomal amphotericin B treatment are well-described. In one of our patients, liposomal amphotericin B was discontinued because of an infusion-related reaction (chest pain, dyspnea, and flushing), and an alternative agent (ketoconazole) was successfully used instead. Infusion-related events typically occur at the start of the infusion and may be associated with fever, chills or rigors, nausea, vomiting, dyspnea, hypotension, hypertension, tachycardia, and flushing, chest discomfort, or pulmonary symptoms.72,73 Pre-medication (with diphenhydramine, corticosteroids, and/or acetaminophen) may minimize the occurrence of these reactions, although they may not be completely prevented.74 Slowing the rate of infusion may help as well.
Nephrotoxicity (defined by greater than 2-fold increase in serum creatinine or peak serum creatinine above 3.0 mg/dL) and/or electrolyte abnormalities occur less commonly in liposomal amphotericin B-treated patients than in amphotericin B-treated patients.72–74 Three of seven patients treated with liposomal amphotericin B in our series experienced an increase in serum creatinine and/or electrolyte abnormalities (hypokalemia or hypomagnesemia); treatment was discontinued in two of these patients. Recently, single-dose liposomal amphotericin B (10 mg/kg) administered intravenously for the treatment of visceral leishmaniasis was found to be effective and well-tolerated.75 The utility of this approach deserves further study in the treatment of CL, because its use could circumvent many of the dose-limiting side effects (in particular, nephrotoxicity) seen with 5 to 7 day courses of therapy.
The toxicities seen with miltefosine were mild and self-limited. One patient had mild loose stools that did not require discontinuation of therapy. The other patient had symptoms of motion sickness that led her to stop treatment 3 days early. Improved tolerance of miltefosine has previously been noted among CL patients compared with visceral patients, likely because CL patients are not systemically and acutely ill. Colombian CL patients treated with miltefosine were noted to be more likely to experience motion sickness (up to 60% of patients) than VL patients.76
Limitations.
This is a limited case series, and no treatment recommendations can be made based on our findings. None of the patients in this study were known to have immunodeficiencies. Host response is known to influence treatment response to antileishmanial drugs, and more failures are reported in an immunocompromised patient population.44 Although a strength of this case series is the lengthy duration of follow-up (12 months or longer), a few cases (N = 3) were lost to follow-up before 12 months, limiting our assessment of treatment response in these cases.
Conclusions.
The vision for the future of CL diagnosis and treatment is use of molecular methods that allow rapid and reliable identification of the infecting Leishmania species to permit use of convenient, yet effective oral-based therapies. Non-antimony regimens, as used in this study, were safe, convenient, and effective. No unexpected toxicities were observed. To facilitate the use of these regimens, future studies must better define the precise efficacy of these regimens in the management of CL based on infecting species and geographic location.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health and intramural programs of National Institute of Allergy and Infectious Diseases. The authors thank Peter J. Weina (Walter Reed Army Institute of Research, Bethesda, MD), Francis J. Steurer, and Alexandre DeSilva (Centers for Disease Control and Prevention, Atlanta, GA) for molecular sequencing of Leishmania species performed at their laboratories. The authors acknowledge the expert care provided by members of our clinical staff: Melissa Law, Cheryl Talar-Williams, Eunice Fox, and Amara Pabon. We also thank National Institute of Allergy and Infectious Diseases intramural editor Brenda Rae Marshall for assistance.
Disclaimer: Because R.R., D.P.F., S.M., and T.E.N. are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the National Institutes of Health reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. Establishment of rights outside of the United States requires a government-use license.
Footnotes
Authors' addresses: Roshan Ramanathan, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, E-mail: ramanathanr@niaid.nih.gov. Kawsar R. Talaat, International Health Center for Immunization Research, The Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: ktalaat@jhsph.edu. Daniel P. Fedorko, Department of Laboratory Medicine, National Institutes of Health, Bethesda, MD, E-mail: dfedorko@mail.nih.gov. Siddhartha Mahanty, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, E-mail: smahanty@niaid.nih.gov. Theodore E. Nash, Gastrointestinal Parasites Section, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, E-mail: tnash@niaid.nih.gov.
Reprint requests: Roshan Ramanathan, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, 4 Center Drive, Room 4/B1-05, Bethesda, MD 20892, E-mail: ramanathanr@niaid.nih.gov.
References
- 1.David CV, Craft N. Cutaneous and mucocutaneous leishmaniasis. Dermatol Ther. 2009;22:491–502. doi: 10.1111/j.1529-8019.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 2.Herwaldt BL, Berman JD. Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam) and review of pertinent clinical studies. Am J Trop Med Hyg. 1992;46:296–306. doi: 10.4269/ajtmh.1992.46.296. [DOI] [PubMed] [Google Scholar]
- 3.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 4.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 5.Wortmann G, Miller RS, Oster C, Jackson J, Aronson N. A randomized, double-blind study of the efficacy of a 10- or 20-day course of sodium stibogluconate for treatment of cutaneous leishmaniasis in United States military personnel. Clin Infect Dis. 2002;35:261–267. doi: 10.1086/341406. [DOI] [PubMed] [Google Scholar]
- 6.Tuon FF, Amato VS, Graf ME, Siqueira AM, Nicodemo AC, Amano Neto V. Treatment of New World cutaneous leishmaniasis—a systematic review with a meta-analysis. Int J Dermatol. 2008;47:109–124. doi: 10.1111/j.1365-4632.2008.03417.x. [DOI] [PubMed] [Google Scholar]
- 7.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verástegui C, Lazo M, Loayza-Muro R, De Doncker S, Maurer A, Chappuis F, Dujardin JC, Llanos-Cuentas A. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007;95:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz E, Hatz C, Blum J. New World cutaneous leishmaniasis in travelers. Lancet Infect Dis. 2006;6:342–349. doi: 10.1016/S1473-3099(06)70492-3. [DOI] [PubMed] [Google Scholar]
- 9.Kreutzer RD, Christensen HA. Characterization of Leishmania spp. by isozyme electrophoresis. Am J Trop Med Hyg. 1980;29:199–208. doi: 10.4269/ajtmh.1980.29.199. [DOI] [PubMed] [Google Scholar]
- 10.Karamian M, Motazedian MH, Fakhar M, Pakshir K, Jowkar F, Rezanezhad H. Atypical presentation of Old-World cutaneous leishmaniasis, diagnosis and species identification by PCR. J Eur Acad Dermatol Venereol. 2008;22:958–962. doi: 10.1111/j.1468-3083.2008.02674.x. [DOI] [PubMed] [Google Scholar]
- 11.Wortmann G, Hochberg L, Houng H, Sweeney C, Zapor M, Aronson N, Weina P, Ockenhouse C. Rapid identification of Leishmania complexes by a real-time PCR assay. Am J Trop Med Hyg. 2005;73:999–1004. [PubMed] [Google Scholar]
- 12.Stevenson LG, Fedorko DP, Zelazny AM. An enhanced method for the identification of Leishmania spp. using real-time polymerase chain reaction and sequence analysis of the 7SL RNA gene region. Diagn Microbiol Infect Dis. 2010;66:432–435. doi: 10.1016/j.diagmicrobio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zelazny A, Fedorko DP, Li L, Neva F, Fischer SH. Evaluation of 7SL RNA gene sequences for the identification of Leishmania spp. Am J Trop Med Hyg. 2005;72:415–420. [PubMed] [Google Scholar]
- 14.el Tai NO, Osman OF, el Fari M, Presber W, Schonian G. Genetic heterogeneity of ribosomal internal transcribed spacer in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms and sequencing. Trans R Soc Trop Med Hyg. 2000;94:575–579. doi: 10.1016/s0035-9203(00)90093-2. [DOI] [PubMed] [Google Scholar]
- 15.Kreutzer RD, Semko ME, Hendricks LD, Wright N. Identification of Leishmania spp. by multiple isozyme analysis. Am J Trop Med Hyg. 1983;32:703–715. doi: 10.4269/ajtmh.1983.32.703. [DOI] [PubMed] [Google Scholar]
- 16.Sindermann H, Engel K, Fischer C, Bommer W. Oral miltefosine for leishmaniasis in immunocompromised patients: compassionate use in 39 patients with HIV infection. Clin Infect Dis. 2004;39:1520–1523. doi: 10.1086/425359. [DOI] [PubMed] [Google Scholar]
- 17.Ritmeijer K, Dejenie A, Assefa Y, Hundie TB, Mesure J, Boots G, den Boer M, Davidson RN. A comparison of miltefosine and sodium stibogluconate for treatment of visceral leishmaniasis in an Ethiopian population with high prevalence of HIV infection. Clin Infect Dis. 2006;43:357–364. doi: 10.1086/505217. [DOI] [PubMed] [Google Scholar]
- 18.Saenz RE, Paz H, Berman JD. Efficacy of ketoconazole against Leishmania braziliensis panamensis cutaneous leishmaniasis. Am J Med. 1990;89:147–155. doi: 10.1016/0002-9343(90)90292-l. [DOI] [PubMed] [Google Scholar]
- 19.Solomon M, Baum S, Barzilai A, Scope A, Trau H, Schwartz E. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J Am Acad Dermatol. 2007;56:612–661. doi: 10.1016/j.jaad.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Keynan Y, Larios OE, Wiseman MC, Plourde M, Ouellette M, Rubinstein E. Use of oral miltefosine for cutaneous leishmaniasis in Canadian soldiers returning from Afghanistan. Can J Infect Dis Med Microbiol. 2008;19:394–396. doi: 10.1155/2008/802710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killingley B, Lamb LE, Davidson RN. Miltefosine to treat cutaneous leishmaniasis caused by Leishmania tropica. Ann Trop Med Parasitol. 2009;103:171–175. doi: 10.1179/136485909X398177. [DOI] [PubMed] [Google Scholar]
- 22.Alborzi A, Pouladfar GR, Fakhar M, Motazedian MH, Hatam GR, Kadivar MR. Isolation of Leishmania tropica from a patient with visceral leishmaniasis and disseminated cutaneous leishmaniasis, southern Iran. Am J Trop Med Hyg. 2008;79:435–437. [PubMed] [Google Scholar]
- 23.Tappe D, Müller A, Stich A. Resolution of cutaneous old world and new world leishmaniasis after oral miltefosine treatment. Am J Trop Med Hyg. 2010;82:1–3. doi: 10.4269/ajtmh.2010.09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Rosal T, Artigao FB, Miguel MJ, de Lucas R, del Castillo F. Successful treatment of childhood cutaneous leishmaniasis with liposomal amphotericin B: report of two cases. J Trop Pediatr. 2010;56:122–124. doi: 10.1093/tropej/fmp073. [DOI] [PubMed] [Google Scholar]
- 25.Brown M, Noursadeghi M, Boyle J, Davidson RN. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol. 2005;153:203–205. doi: 10.1111/j.1365-2133.2005.06670.x. [DOI] [PubMed] [Google Scholar]
- 26.Campos-Muñoz L, Quesada-Cortés A, Martín-Díaz MA, Rubio-Flores C, de Lucas-Laguna R. Leishmania braziliensis: report of a pediatric imported case with response to liposomal amphotericin B. Actas Dermosifiliogr. 2007;98:42–44. [PubMed] [Google Scholar]
- 27.Sampaio SAP, Castro RM, Dillon NL, Martins JEC. Treatment of mucocutaneous (American) leishmaniasis with amphotericin B: report of 70 cases. Int J Dermatol. 1971;10:179–181. doi: 10.1111/j.1365-4362.1971.tb01694.x. [DOI] [PubMed] [Google Scholar]
- 28.Crofts MAJ. Use of amphotericin B in mucocutaneous leishmaniasis. J Trop Med. 1976;79:111–113. [PubMed] [Google Scholar]
- 29.Di Lella F, Vincenti V, Zennaro D, Afeltra A, Baldi A, Giordano D, Pasanisi E, Bacciu A, Bacciu S, Di Lella G. Mucocutaneous leishmaniasis: report of a case with massive involvement of nasal, pharyngeal and laryngeal mucosa. Int J Oral Maxillofac Surg. 2006;35:870–872. doi: 10.1016/j.ijom.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Soto J, Rea J, Balderrama M, Toledo J, Soto P, Valda L, Berman JD. Efficacy of miltefosine for Bolivian cutaneous leishmaniasis. Am J Trop Med Hyg. 2008;78:210–211. [PubMed] [Google Scholar]
- 31.Wöhrl S, Schnedl J, Auer H, Walochnik J, Stingl G, Geusau A. Successful treatment of a married couple for American leishmaniasis with miltefosine. J Eur Acad Dermatol Venereol. 2008;22:258–259. doi: 10.1111/j.1468-3083.2007.02311.x. [DOI] [PubMed] [Google Scholar]
- 32.Urcuyo FG, Zaias N. Oral ketoconazole in the treatment of leishmaniasis. Int J Dermatol. 1982;21:414–416. doi: 10.1111/j.1365-4362.1982.tb03163.x. [DOI] [PubMed] [Google Scholar]
- 33.Weinrauch L, Livshin R, El-On J. Cutaneous leishmaniasis: treatment with ketoconazole. Cutis. 1983;32:288–294. [PubMed] [Google Scholar]
- 34.Weinrauch L, Livshin R, El-On J. Ketoconazole in cutaneous leishmaniasis. Br J Dermatol. 1987;117:666–667. doi: 10.1111/j.1365-2133.1987.tb07504.x. [DOI] [PubMed] [Google Scholar]
- 35.Bellazoug S, Ammar-Khodja A, Belkaid M, Tabett-Derraz O. La leishmanisose cutanee du nord de L'Algeria. Bull Soc Pathol Exot. 1985;78:615–622. [PubMed] [Google Scholar]
- 36.Viallet J, MacLean JD, Robson H. Response to ketoconazole in two cases of longstanding cutaneous leishmaniasis. Am J Trop Med Hyg. 1986;35:491–495. doi: 10.4269/ajtmh.1986.35.491. [DOI] [PubMed] [Google Scholar]
- 37.Leitner V, Weingast J, Harmankaya K, Walochnik J, Pehamberger H, Petzelbauer P, Auer H, Binder M. Leishmaniasis in the tongue of an immunocompetent man. Am J Trop Med Hyg. 2010;82:597–599. doi: 10.4269/ajtmh.2010.09-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rongioletti F, Cannata GE, Parodi A. Leishmaniasis due to L. infantum presenting as macrocheilitis and responding to liposomal amphotericin B. Eur J Dermatol. 2009;19:281–282. doi: 10.1684/ejd.2009.0652. [DOI] [PubMed] [Google Scholar]
- 39.Paradisi A, Capizzi R, Zampetti A, Proietti I, De Simone C, Feliciani C, Amerio PL. Atypical multifocal cutaneous leishmaniasis in an immunocompetent patient treated by liposomal amphotericin B. J Infect. 2005;51:e261–e264. doi: 10.1016/j.jinf.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Davidson RN, Di Martino L, Gradoni L, Giacchino R, Russo R, Gaeta GB, Pempinello R, Scott S, Raimondi F, Cascio A, Prestileo T, Caldiera L, Wilkinson RJ, Bryceson ADM. Liposomal amphotericin B (AmBisome) in Mediterranean visceral leishmaniasis: a multi-centre trial. Q J Med. 1994;87:75–81. [PubMed] [Google Scholar]
- 41.Davidson RN, di Martino L, Gradoni L, Giacchino R, Gaeta GB, Pempinello R, Scotti S, Cascio A, Castagnola E, Maisto A, Gramiccia M, di Caprio D, Wilkinson RJ, Bryceson AD. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome) Clin Infect Dis. 1996;22:938–943. doi: 10.1093/clinids/22.6.938. [DOI] [PubMed] [Google Scholar]
- 42.Seaman J, Boer C, Wilkinson R, de Jong J, de Wilde E, Sondorp E, Davidson R. Liposomal amphotericin B (AmBisome) in the treatment of complicated kala-azar under field conditions. Clin Infect Dis. 1995;21:188–193. doi: 10.1093/clinids/21.1.188. [DOI] [PubMed] [Google Scholar]
- 43.Russo R, Nigro LC, Minniti S, Montineri A, Gradoni L, Caldeira L, Davidson RN. Visceral leishmaniasis in HIV infected patients: treatment with high dose liposomal amphotericin B (AmBisome) J Infect. 1996;32:133–137. doi: 10.1016/s0163-4453(96)91343-2. [DOI] [PubMed] [Google Scholar]
- 44.Meyerhoff A. U.S. Food and Drug Administration approval of liposomal amphotericin B for treatment of visceral leishmaniasis. Clin Infect Dis. 1999;28:42–48. doi: 10.1086/515085. [DOI] [PubMed] [Google Scholar]
- 45.Neub A, Krahl D, Stich A, Amon U. Cutaneous infection with Leishmania infantum in an infant successfully treated with miltefosine. J Dtsch Dermatol Ges. 2008;6:1061–1064. doi: 10.1111/j.1610-0387.2008.06779.x. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Villaverde R, Sanchez-Cano D, Villaverde-Gutierrez C. Chronic cutaneous leishmaniasis in an immunocompetent patient: response to miltefosine. J Eur Acad Dermatol Venereol. 2007;21:695–696. doi: 10.1111/j.1468-3083.2006.01990.x. [DOI] [PubMed] [Google Scholar]
- 47.Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, Junge K, Bryceson A, Berman J. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med. 2002;347:1739–1746. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- 48.Ritmeijer JK, Dejenie A, Assefa Y, Hundie T, Mesure J, Boots G, den Boer M, Davidson R. A comparison of miltefosine and sodium stibogluconate for treatment of visceral leishmaniasis in an Ethiopian population with high prevalence of HIV infection. Clin Infect Dis. 2006;43:357–364. doi: 10.1086/505217. [DOI] [PubMed] [Google Scholar]
- 49.Safaei A, Motazedian MH, Vasei M. Polymerase chain reaction for diagnosis of cutaneous leishmaniasis in histologically positive, suspicious and negative skin biopsies. Dermatology. 2002;205:18–24. doi: 10.1159/000063150. [DOI] [PubMed] [Google Scholar]
- 50.Bensoussan E, Nasereddin A, Jonas F, Schnur LF, Jaffe CL. Comparison of PCR assays for diagnosis of cutaneous leishmaniasis. J Clin Microbiol. 2006;44:1435–1439. doi: 10.1128/JCM.44.4.1435-1439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahbazi F, Shahabi S, Kazemi B, Mohebali M, Abadi AR, Zare Z. Evaluation of PCR assay in diagnosis and identification of cutaneous leishmaniasis: a comparison with the parasitological methods. Parasitol Res. 2008;103:1159–1162. doi: 10.1007/s00436-008-1111-4. [DOI] [PubMed] [Google Scholar]
- 52.Singh S, Sivakumar R. Challenges and new discoveries in the treatment of leishmaniasis. J Infect Chemother. 2004;10:307–315. doi: 10.1007/s10156-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 53.Jolliffe DS. Cutaneous leishmaniasis from Belize—treatment with ketoconazole. Clin Exp Dermatol. 1986;11:62–68. doi: 10.1111/j.1365-2230.1986.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 54.Navin TR, Arana BA, Arana FE, Berman JD, Chajón JF. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J Infect Dis. 1992;165:528–534. doi: 10.1093/infdis/165.3.528. [DOI] [PubMed] [Google Scholar]
- 55.Dan M, Verner E, el-On J, Zuckerman F, Michaeli D. Failure of oral ketoconazole to cure cutaneous ulcers caused by Leishmania braziliensis. Cutis. 1986;38:198–199. [PubMed] [Google Scholar]
- 56.Dedet JP, Jamet P, Esterre P, Ghipponi PM, Genin C, Lalande G. Failure to cure Leishmania braziliensis guyanensis cutaneous leishmaniasis with oral ketoconazole. Trans R Soc Trop Med Hyg. 1986;80:176. doi: 10.1016/0035-9203(86)90239-7. [DOI] [PubMed] [Google Scholar]
- 57.Ziaei H, Sadeghian G, Hejazi SH. Distribution frequency of pathogenic bacteria isolated from cutaneous leishmaniasis lesions. Korean J Parasitol. 2008;46:191–193. doi: 10.3347/kjp.2008.46.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.el-On J, Sneier R, Elias E. Leishmania major: bacterial contamination of cutaneous lesions in experimental animals. Isr J Med Sci. 1992;28:847–851. [PubMed] [Google Scholar]
- 59.Amato VS, Rabello A, Rotondo-Silva A, Kono A, Maldonado TP, Alves IC, Floeter-Winter LM, Neto VA, Shakanai-Yasuda MA. Successful treatment of cutaneous leishmaniasis with lipid formulations of amphotericin B in two immunocompromised patients. Acta Trop. 2004;92:127–132. doi: 10.1016/j.actatropica.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Gregoriadis G. Overview of liposomes. J Antimicrob Chemother. 1991;28((Suppl B)):39–48. doi: 10.1093/jac/28.suppl_b.39. [DOI] [PubMed] [Google Scholar]
- 61.Yardley V, Croft S. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob Agents Chemother. 1997;41:752–756. doi: 10.1128/aac.41.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wortmann GW, Fraser SL, Aronson NE, Davis C, Miller RS, Jackson JD, Oster CN. Failure of amphotericin B lipid complex in the treatment of cutaneous leishmaniasis. Clin Infect Dis. 1998;26:1006–1007. doi: 10.1086/517634. [DOI] [PubMed] [Google Scholar]
- 63.Yardley V, Croft SL. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int J Antimicrob Agents. 2000;13:243–248. doi: 10.1016/s0924-8579(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 64.Shani-Adir A, Kamil S, Rozenman D, Schwartz E, Ramon M, Zalman L, Nasereddin A, Jaffe CL, Ephros M. Leishmania tropica in northern Israel: a clinical overview of an emerging focus. J Am Acad Dermatol. 2005;53:810–815. doi: 10.1016/j.jaad.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 65.Escobar P, Yardley V, Croft SL. Activities of hexadecylophosphocholine (miltefosine), liposomal amphotericin B, and sodium stibogluconate (Pentostam) against Leishmania donovani in immunodeficient scid mice. Antimicrob Agents Chemother. 2001;45:1872–1875. doi: 10.1128/AAC.45.6.1872-1875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soto J, Toledo J, Gutierrez P, Nicholls RS, Padilla J, Engel J, Fischer C, Voss A, Berman J. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin Infect Dis. 2001;33:E57–E61. doi: 10.1086/322689. [DOI] [PubMed] [Google Scholar]
- 67.Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy. Analysis of 33 cases. Gastroenterology. 1984;86:503–513. [PubMed] [Google Scholar]
- 68.Heiberg JK, Svejgaard E. Toxic hepatitis during ketoconazole treatment. Br Med J (Clin Res Ed) 1981;283:825–826. doi: 10.1136/bmj.283.6295.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duarte PA, Chow CC, Simmons F, Ruskin J. Fatal hepatitis associated with ketoconazole therapy. Arch Intern Med. 1984;144:1069–1070. [PubMed] [Google Scholar]
- 70.Knight TE, Shikuma CY, Knight J. Ketoconazole-induced fulminant hepatitis necessitating liver transplantation. J Am Acad Dermatol. 1991;25:398–400. doi: 10.1016/0190-9622(91)70214-m. [DOI] [PubMed] [Google Scholar]
- 71.Lake-Bakaar G, Scheuer PJ, Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J (Clin Res Ed) 1987;294:419–422. doi: 10.1136/bmj.294.6569.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moen MD, Lyseng-Williamson KA, Scott LJ. Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs. 2009;69:361–392. doi: 10.2165/00003495-200969030-00010. [DOI] [PubMed] [Google Scholar]
- 73.Johnson MD, Drew RH, Perfect JR. Chest discomfort associated with liposomal amphotericin B: report of three cases and review of the literature. Pharmacotherapy. 1998;18:1053–1061. [PubMed] [Google Scholar]
- 74.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg RN, Dummer S, Schuster M, Holcenberg JS. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999;340:764–771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 75.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362:504–512. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 76.Soto J, Berman J. Treatment of New World cutaneous leishmaniasis with miltefosine. Trans R Soc Trop Med Hyg. 2006;100((Suppl 1)):S34–S40. doi: 10.1016/j.trstmh.2006.02.022. [DOI] [PubMed] [Google Scholar]


