Abstract
Tuberculosis outbreaks originating in prisons, mines, or hospital wards can spread to the larger community. Recent proposals have targeted these high-transmission institutional amplifiers by improving case detection, treatment, or reducing the size of the exposed population. However, what effects these alternative proposals may have is unclear. We mathematically modeled these control strategies and found case detection and treatment methods insufficient in addressing epidemics involving common types of institutional amplifiers. Movement of persons in and out of amplifiers fundamentally altered the transmission dynamics of tuberculosis in a manner not effectively mitigated by detection or treatment alone. Policies increasing the population size exposed to amplifiers or the per-person duration of exposure within amplifiers potentially worsened incidence, even in settings with high rates of detection and treatment success. However, reducing the total population size entering institutional amplifiers significantly lowered tuberculosis incidence and the risk of propagating new drug-resistant tuberculosis strains.
Introduction
Exposure to tuberculosis (TB) continues to be common, with approximately one-third of the world's population infected with Mycobacterium tuberculosis although most experience only a latent form of infection and escape the active and potentially deadly form of disease. Although this disease has been treatable since the 1940s, and despite commitments by the global community to reduce TB prevalence and death by 50% during 1990–2015 (Millennium Development Goals) and to reduce TB incidence to less than 1 new case/million by 2050 (StopTB program),1 TB control is failing in many regions. The reasons for this failure include an increased prevalence of human immunodeficiency virus (HIV) infection (which increases the risk of active TB and subsequent death), and the continued emergence of drug-resistant TB strains that hinder TB treatment efforts.1,2
The stable or rising prevalence of TB in areas of sub-Saharan Africa and the former Soviet Union is especially alarming because it seems to be happening despite continued improvements in implementing recommended anti-TB control strategies, such as the World Health Organization (WHO)–sponsored directly observed therapy program. Thus, although South Africa reports 100% directly observed therapy program coverage, it was the setting for the world's largest extensively drug-resistant TB epidemic.1,3,4 Even in regions with low prevalence (< 4%) of HIV in southern Africa, high rates of TB persist despite improvements in traditional control efforts.5
It has long been recognized that patterns of communicable disease are influenced by the existence of pockets of high transmission.6 For example, an early study of measles showed a clear association between the risk of infection and family size.7 Heterogeneity of populations should be taken account of when devising control strategies.8 Thus, research on the epidemiology of gonorrhea suggested that if a core of persons at high risk of transmitting the infection could be identified and treated, the disease might die out as the reproductive rate in the general population was less than one.9
Much of this research has focused on disparate groups within the general population. However, it is known that outbreaks of TB are often initiated among persons exposed to poor living conditions, characterized by poor ventilation, crowding, migration, and inconsistent healthcare access.5,10,11 Recent outbreaks of TB have been observed in resource-poor hospitals in which dozens of patients share poorly ventilated communal rooms, and in crowded prison cell blocks or mining barracks.3,4,12–19
Institutional amplifiers, such as prisons, hospital wards and mines, are characterized by a combination of multiple and interacting social determinants of infectious disease spread. In the past several years, the WHO and other major public health bodies have recognized that not only do medical treatment standards and access to medical technologies and healthcare determine key TB outcomes, but also that there are critical social factors influencing TB transmission risks and the risks of recurrent or resistant disease.20 A conceptual framework for analyzing these risks has been developed by the WHO, which called for further analysis of each part of the TB transmission process: the risk of exposure, the risk of infection after exposure, the risk of reactivation disease, and the risks associated with poor treatment access or poor treatment completion.21 Institutional amplifiers act on this process in several ways including close and prolonged human contact, poor ventilation, containment of highly susceptible or immunocompromised groups, and significant flows of persons into and out of these institutions. Such amplifiers have been linked not only to outbreaks within them, but also to spread to communities beyond them (Figure 1); these high-risk settings are thought increasingly to be a major factor in sustaining TB epidemics in regions with otherwise extensive TB control systems,22–24 with new evidence in support of this proposition coming from molecular fingerprinting techniques.22,25
Figure 1.
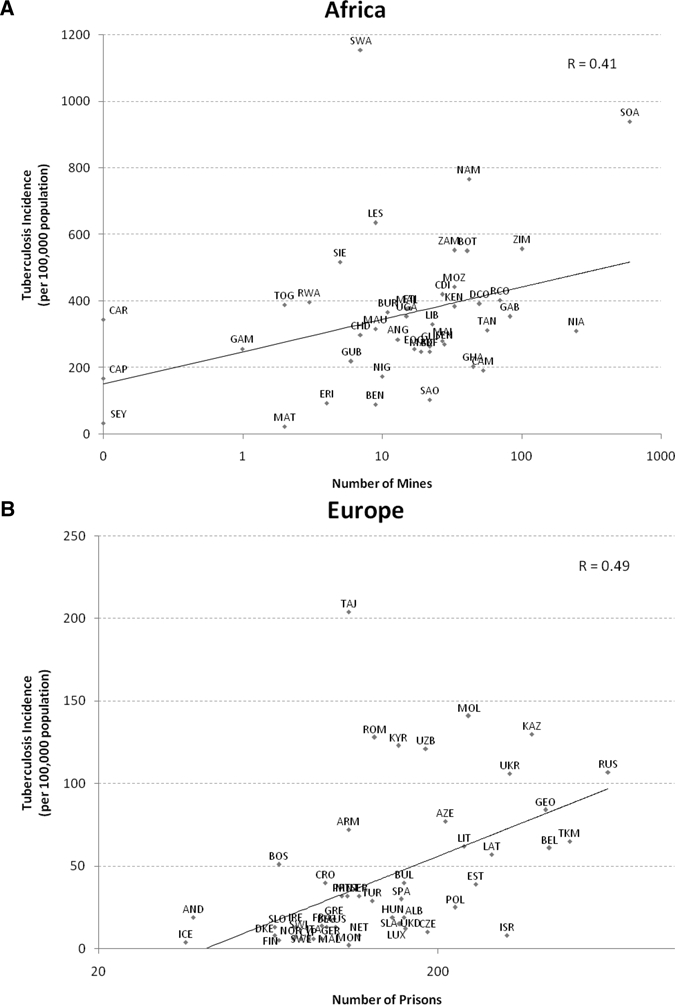
Associations between tuberculosis incidence and the number of mines (A) and prisons (B) in a country. Country name abbreviations are the standard three-letter abbreviations set by the United Nations,51 and the nations included in the African (A) and European (B) regions are from the World Health Organization regional designation.52
Similarly, the appearance of drug-resistant TB in labor-supplying rural regions of southern Africa has been linked to its occurrence in distant South African mines.24 As shown in Figure 1, there is a significant association between TB incidence in the overall community and the per capita frequency of prisons and mines, respectively (Pearson's rprisons = 0.41, P < 0.001; Pearson's rmines = 0.49, P < 0.001; although, at least for mines, the strength of this association may be underestimated because of movement between countries, which is likely to explain the outlying positions of countries that are exporters of labor, such as Lesotho and Swaziland). Communities affected by such institutional amplifiers, or confined institutions in which there is a high risk of transmission among persons who can ultimately interact with the broader community population, share striking commonalities (Supplemental Appendix, Table 1). Recognizing the importance of the issue, WHO has recently published an appeal to research the impact of alternative strategies to control these and related social determinants of TB transmission.21
How can we address the role of such institutional amplifiers when designing TB control programs? Three main strategies have been proposed or are being implemented by the WHO tuberculosis department and managers of high-risk institutions:21,22,26,27 1) to decrease the duration of per-person exposure within an amplifier; 2) primary risk reduction, in which the proportion of the population at risk of amplification is reduced; and 3) provision of extensive case detection and treatment services to amplified populations to enhance the likelihood that they will not be infectious for prolonged periods.
As an example of the first approach, prison managers in Russia have considered mass amnesty policies to maintain prison occupancy at official limits (avoiding overcrowding), arguing that this will reduce the risk of TB transmission per prisoner28. Some epidemiologists have argued that this policy may increase the likelihood of TB transmission to the community,28 but it is unclear under what circumstances the benefits may outweigh the risks. Analogously, it has been argued that TB epidemics in communal hospital wards may be averted by reducing the length of hospitalization of patients.29
As an example of the second approach, there is considerable debate about whether to decentralize care from central hospitals to smaller community clinics in light of the spread of drug-resistant TB strains throughout large hospital wards.4,26 One concern, however, is that strategies to decrease exposure of the first two proposals may conflict with the aims of penal institutions to exact punishment and to protect the public from potentially dangerous offenders.
Thus, a third approach involves addressing the conditions within the amplifier, ensuring that at-risk populations have access to appropriate care services while addressing the environmental aspects of the institutions that put persons at risk. This approach can be seen in current negotiations between the government, labor unions, and international companies in South Africa, which are constructing new standards for TB screening and treatment among workers used at major gold and diamond mines.24 Prisons can benefit from research from health care settings on new architectural designs to improve ventilation, maintain warm temperatures in the winter, and ultraviolet light.30,31
In this report, we present the results of a model designed to estimate the incidence of TB in populations where institutional amplifiers exist. Although we could focus on just one case of an amplifier and its dynamics, we choose to offer several alternative scenarios to derive general conclusions about the behavior of TB epidemics because they are affected by different types of amplifiers. Although we frame our findings in the context of mines, hospitals, and prisons (the three most commonly cited institutional amplifiers), the model is designed to illustrate general principles and will need to be refined further to take account of the specific dynamics of different institutional settings.
Methods
We simulated alternative strategies for communities affected by institutional amplifiers of TB, using mathematical models to vary assumptions about TB transmission and examine how these changes to our assumptions and policy parameters can alter the dynamics of TB transmission and the potential efficacy of epidemic control measures. We tested the hypothesis that institutional amplifiers increase the rate of transmission, which implies a need for enhancement of traditional TB control measures (increased case detection and treatment success rates32) and new methods of control that may not have been widely considered in the public health community to date.
Tuberculosis control measures are often examined using mathematical models that simulate how TB is acquired and spreads through a population. Most such models assume that every person has the same risk-profile (i.e., homogenous population risks); that is, pockets of high transmission are not accounted for, such that it is assumed that the same strategies will be effective in all groups within the population on average.32,33 We modified this standard approach in a meta-population model, which incorporates heterogeneity in transmission patterns, to explore the change in transmission risk generated by movement into and out of institutional amplifiers.
To identify what variables most impact the qualitative dynamics of institutional amplification, we extended a basic model of TB transmission into two population groups, the general population and its associated institutionally amplified population, and allowed for movement between these groups. Thus, some persons in the general population (an at risk group) could migrate to an amplifier (sometimes repeatedly), and stay there for various durations before migrating back to the community. They could be infected in the community (before or after their amplifier exposure period), or within the amplifier, and could be detected and treated for TB (or die of TB or another cause of death) in either environment. Each location carried independent risks for TB that we varied as described below.
As with standard models,33,34 we took account of how TB is acquired and develops by simulating how susceptible persons can experience infection, progressing to latency and potentially experiencing active disease (Figure 2). Some patients with active TB can be detected and be treated successfully, and others may remain undetected or fail therapy at rates that were varied based on the case detection and treatment programs simulated in different scenarios (Supplemental Appendix). A portion of active cases are infectious, as estimated from prior studies, and we ran our simulations with various HIV prevalences to simulate different environments of HIV exposure. HIV alters the natural history of TB among the portion of persons with HIV infection (Supplemental Appendix, parameter values taken from prior studies of TB risk and pathogenesis among HIV-infected persons).
Figure 2.
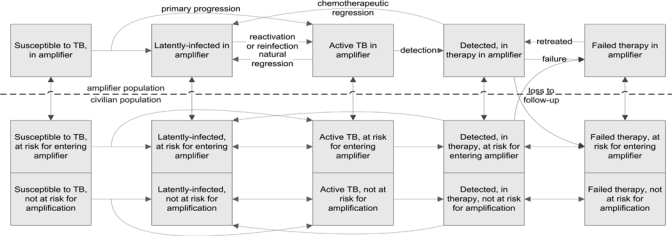
Flow diagram of amplifier model. Green arrows show migration to and from the amplifier, and gray arrows show rates of loss to follow-up from treatment during migration, where the probability of loss is varied in the model simulations. Drug resistance, human immunodeficiency virus (HIV), and mortality are included in the model (see Supplemental Appendix) but are not displayed in the diagram for simplicity. Mortality and HIV can affect persons in all compartments, and drug resistance can affect a portion of patients who are not successfully treated.
We varied the risks of transmission within the amplifier and civilian communities, and entry rates to and lengths of stay within the amplifier, to explore the impact of these factors on TB epidemics. In changing rates of case detection and treatment success, we compared the predictions of our model to traditional models of TB used to construct international standards for detection and treatment. We simulated the rate of transmission in the amplifier community using the dynamic formulation of the Wells-Riley equations describing airborne transmission,35,36 a set of equations describing TB transmission in close-quarters environments that fits well with data on confined transmission environments such as barracks and prison cells.37 To simulate TB transmission in the general population, we used typical rates of per-person contact in developing countries to model the civilian rate of airborne TB transmission in a general endemic community setting, taking this parameter and its range of values from prior WHO estimates.32,33 We then simulated alternative control measures discussed in the introduction: reducing the per-person duration of exposure to the amplifier, the number of persons exposed, and the rates of case detection and treatment in the at-risk group.
We next expanded the model to consider the effects of drug-resistance. We used the probability of acquired multi-drug resistant (MDR) TB from a previous study of resistance development rates in 47 countries,38 and tracked drug-susceptible and MDR TB transmission over time and simulated the alternative policy proposals. We varied the relative transmission fitness of drug-resistant strains across a broad range, starting from 50% transmissibility relative to drug-susceptible strains, and varied the value across the range of values observed in empirical studies (16–120%)38,39 (Supplemental Appendix).
Multivariate sensitivity and uncertainty analysis was performed across all parameters to examine how uncertainty in parameter values could alter observed model simulations,40 and to examine which parameters impacted most on the results. The parameters of the model, such as lengths of amplifier exposure, were taken from estimates from empirical studies (Supplemental Appendix). All model equations are detailed in the Supplemental Appendix to enable full independent replication of our results. The model was programmed in the software program MATLAB R2010a (The MathWorks, Inc., Natick, MA).
Results
In a simulation of TB epidemics with an institutional amplifier, such as a prison, mine, or communal hospital ward, even a large increase in case detection and treatment success rates in the population exposed to the institutional amplifier had little effect on the overall population incidence, and relatively modest improvements in these rates among the population unexposed to the amplifiers reduced the incidence considerably. This is illustrated in Supplemental Appendix, Table 1, in which case detection rates in the two populations are varied, holding other factors constant.
Several of the commonly proposed alternative strategies for addressing amplifier-exposed persons were also considered through this model. One strategy is to decrease the length of per-person exposure to amplifier environments through which persons cycle. For example, a proposed strategy to tackle TB in prisons is to release inmates early to reduce overcrowding41; hospitals with communal wards facing heavy demands have proposed similar policies of early discharge.42 Results of simulations to decrease the per-person length of exposure within the amplifier are shown in Figure 3. Rates of community-wide TB initially increased when persons were exposed to longer average stays in the amplifier, but these rates then declined as the duration of stay further lengthened beyond a peak. Essentially, the amplifier acted as a queuing system, where short stays in the amplifier increased potential flow into it (increasing the space available for new entrants, essentially increasing the opportunities for new susceptible hosts). In other words, simply releasing prison inmates early from prisons to accept new inmates and relieve overcrowding, if implemented without any other changes to the potential population that can enter the amplifier, could backfire and perversely contribute to an increase in TB incidence. Conversely, however, averting release was not a control strategy per se, but simply led to stagnation of incidence rates; even long periods of incarceration, up to 25 years, were insufficient to behave as an effective quarantine and eliminate the ultimate amplification of population-wide TB rates by the institution (Figure 3).
Figure 3.
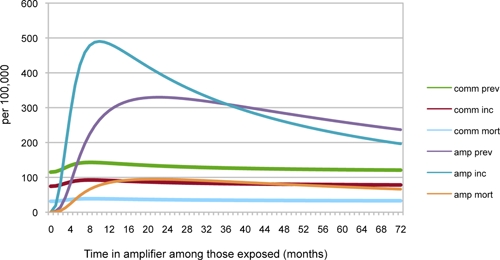
Tuberculosis rates/100,000 population. The length of exposure within the amplifier was varied. The simulation parameters are 1% of the community at risk for amplification, and World Health Organization standard model rates of 50% case detection and 70% treatment success.38 In southern African mines, the length of exposure is typically 9 months; in Russian prisons, the length of exposure is typically 62 months (5.2 years); see Supplemental Appendix, Table 1 for references. This figure appears in color at www.ajtmh.org.
A policy of primary risk reduction, in which the proportion of the population at risk of amplification was changed, had more beneficial impact. The proportion of the population at risk of entering the institutional amplifier was a critical determinant of the size of the overall population's TB epidemic (Figure 4). As the proportion of persons at risk of entering the amplifier was increased, the incidence, prevalence, and mortality from TB increased logistically in the amplifier and increased exponentially in the overall community.
Figure 4.
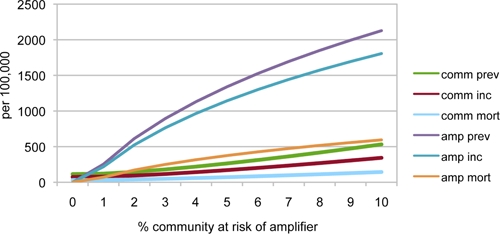
Steady state tuberculosis incidence, prevalence, and mortality/100,000 in overall community and in the amplifier when various portions of the general population are at risk for entering an amplifier. We use the prison parameter of 5.2 years average incarceration time,41 and World Health Organization parameters of 50% case detection and 70% treatment success.38 The amplifier community rates are in terms of 100,000 persons in the amplifier. This figure appears in color at www.ajtmh.org.
Another suggested policy is to provide follow-up care to amplified populations to enhance their likelihood of being treated successfully.24,43 During incarceration or release, or when miners travel to and from mines, TB treatment is usually discontinued and patients fail therapy.24 However, in this model, different degrees of loss to follow up when diagnosed patients migrate to or from an institutional amplifier were of limited impact. Varying the rate of loss to follow-up between 0% and 100% did not significantly change population-wide TB incidence; varying the loss to follow-up in the population exposed to amplifiers from 0% to 100% was associated with a change in incidence of less than 5%, which is consistent with our finding that these measures may work for the community in general but have limited impact on the very high-risk sub-population whose incidence was more sensitive to being exposed to an amplifier rather than the duration of infectiousness of peers. This finding is consistent with a situation in which transmission to persons who commonly associate with the infectious case takes place early in their infectious period, before TB treatment has been able to suppress their infectivity.
The proportion of the population at risk from amplification was also the key factor to affect the growth of MDR TB. Institutional amplifiers served as incubators of MDR TB, as with drug-susceptible TB, but because of the inherently lower rate of treatment success among MDR patients, the prevalence of MDR increased; subsequent incidence of MDR TB increased more rapidly than with drug-susceptible TB as the size of the population at risk for amplification was increased (Figure 5). In turn, the presence of an amplifier incubating many cases of MDR TB increased the mortality from MDR TB in the overall community, given the lower rate of treatment success among MDR cases (Figure 5). The rates of MDR TB predicted by the model were consistent with those seen in prison and mining hot zones, as shown in Figure 5 (prevalence rates of between 3% and 20%).44,45 Changing the rates of loss to follow-up, and introducing 100% sensitive screening to detect active TB upon entry to the amplifier, did not reduce MDR TB rates by more than 5% in this model.
Figure 5.
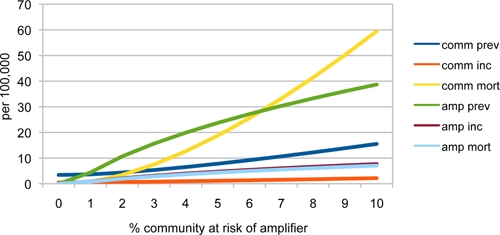
Effect of amplifier exposure on multidrug-resistant tuberculosis (MDR TB) rates and MDR TB mortality. This figure appears in color at www.ajtmh.org.
Resistant strains often emerge in amplifiers, posing a risk of transmission both within amplifiers and to the outside community, yet random processes can cause die-out of a new strain instead of an epidemic.46 A stochastic simulation algorithm (an algorithm that accounts for the likelihood that a new strain will die out instead of successfully propagating) was used to estimate the percentage of time that an emergent MDR TB strain would arise successfully in the community and generate sustained transmission.47 The proportion of the population at risk of amplification dramatically increased this risk (Figure 6), such that the probability that a new MDR TB strain would propagate increased by approximately 4% for each new emergent strain for each 1% increase in the population at risk of entering the amplifier; the probability of such propagation reached a plateau near 100% probability after 8% of the population was at risk for amplifier entry.
Figure 6.
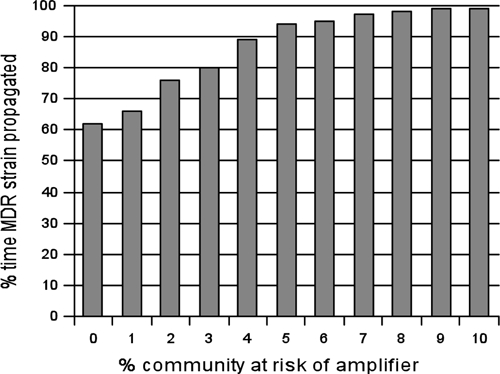
Proportion of time that a new multidrug-resistant tuberculosis strain successfully propagated in the community, when running the model stochastically and sampling from the uncertainty distributions of parameter values.
A series of sensitivity analyses was performed to ensure that our findings were not an artifact of severe modeling assumptions or improbable parameter values. We first examined how sensitive the model was to the prevalence of HIV in the different populations. In South Africa, migrant worker status is independently associated with HIV infection48 (although the role of migration in transmission is complex, with evidence that non-migrant wives also transmit infection to their husbands49). The limited evidence available in the former Soviet Union also suggests that HIV prevalence in prisons is substantially higher than in the general population.50 An increased prevalence of HIV in the model was associated with much greater amplification of TB rates when it occurred in the population exposed to amplifiers than in the unexposed population (Figure 7). Thus, institutional conditions can enhance the role of HIV, such that HIV and institutional amplifiers together can synergistically increase population-wide TB incidence.
Figure 7.
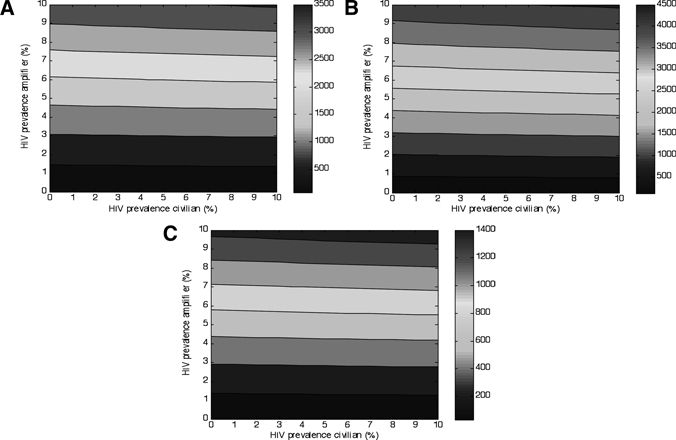
Effects of human immunodeficiency virus prevalence in amplifier and civilian community on tuberculosis (TB) incidence (A), prevalence (B), and mortality (C). The World Health Organization model 50% case detection rate and 70% treatment success rate,38 with 1% of the population at risk for amplification, and 9 months of exposure as with South African mines was used.24 The right-sided color bar indicates the TB rate/100,000 population.
In further sensitivity analyses, the incidence, prevalence, and mortality from TB were most sensitive to changes in the risk of primary progressive disease (how many persons develop active instead of latent disease after infection), per capita contact rates in the overall community, and the infectivity of active TB cases (Supplemental Appendix, Table 3). However, none of the findings regarding the risks associated with entering an institutional amplifier was qualitatively affected by uncertainty in these parameter values.
Discussion
All models are a simplified representation of reality and inevitably ours has certain limitations. There are still many uncertainties about the complex nature of transmission of co-existent HIV and TB and about the dynamics of movement between the institutional amplifiers we have examined. Thus, a future refinement of our model will take into account the complex demographic issues pertaining to who is at greatest risk of entering amplifiers (for example, working age men are at greatest risk of entry to mines and prisons and women are more likely to enter hospitals). Nonetheless, some general principles emerge from our simplified model, which are consistent with earlier research on the role of heterogeneity in the non-institutionalized population. Reducing the number of persons entering amplifiers was more effective than conventional TB control strategies to mitigate the amplifying effect of institutions such as prisons, mines, and communal hospital wards in reducing TB incidence in the general population. Existing strategies in settings with high-risk of transmission, such as increasing case detection and treatment completion among those who have already been exposed to high-transmission amplifiers, and reducing durations of stay in amplifiers, were less effective. We found that some approaches, such as mass amnesties, could perversely enhance TB transmission because they permit a larger number of exposures, which outweighed the benefits of increased rates of case detection and treatment success when the model simulation was run across a wide range of plausible parameter values.
As with any mathematical model, the model used here requires assumptions. We used the standard Wells-Riley equations to describe the close-quarter transmission dynamics of M. tuberculosis in amplifiers, which fits well with available data but assumes relatively even mixing of air within rooms in an amplifier.35,36 Thus, detailed architectural specifications of different amplifiers are not accounted for in this general model. Another limitation is that we only modeled the relationship between one amplifier and its associated wider community, though some communities contain many hot spot transmission areas and environments. The relationship between the number of prisoners and the prevalence of TB can vary widely, reflecting different penal policies. This is why we explored parameters related to overall community-wide TB prevalence, and the degree of amplification based on a simplified model of one amplifier setting, acknowledging that TB prevalence for instance is low in the United States (high incarceration rate) and high in Zimbabwe (low incarceration rate) because of a number of complex factors related to overall population dynamics and not just prisons alone. Our models enable us to identify the unique features of the institutional conditions that give rise to policy recommendations tailored to these settings. Our goal was to provide fundamental generalizable insights into the impact of institutional amplifiers on TB epidemics and standard TB models, rather than modeling only one specific community. Thus, our analysis included a broad range of parameter values to detect robust trends in the epidemiology of TB transmission. Future work could consider the independent effects of transition between numerous amplifiers, after studying what types of settings act as hubs for transmission in different regions.
These results have important implications for policy. Prison managers in Russia have considered mass amnesty policies to reduce overcrowding, in part to reduce the risk of TB transmission; others have argued that this policy may increase the likelihood of TB transmission to the community.28 Similarly, mining companies have been challenged by community organizations about their use of large numbers of migrant workers, and whether screening programs and treatment at mining sites is sufficient to curtail transmission.24 Such follow-up programs may have little impact if large numbers of persons were still exposed to the risks of infection seen in mining communities in the first place. Thus, although it is clearly necessary to improve medical programs for miners already infected, greater gains may come from addressing the role of migrant labor in the mining sector.
In summary, a key intervention to reduce TB incidence, prevalence, and mortality in a community is to limit the number of persons that enter institutional amplifiers. Thus, policies designed to achieve international goals for TB control require explicit consideration of the features of high-prevalence communities that are affected by such amplifiers, necessitating further study into the public health policies that may beneficially alter the current trajectory of community TB epidemics.
Supplementary Material
Note: Supplemental appendix is available at www.ajtmh.org.
Footnotes
Financial support: Sanjay Basu is supported by the U.S. Centers for Disease Control and Prevention (R36) and the National Institutes of Health (T32). Martin McKee is supported by the European Observatory on Health Systems and Policies.
Authors' addresses: Sanjay Basu, Department of Medicine, University of California, San Francisco, Division of General Internal Medicine, San Francisco General Hospital, San Francisco, CA, E-mail: sanjay.basu@ucsf.edu. David Stuckler, Department of Public Health and Policy, London School of Hygiene and Tropical Medicine, London, United Kingdom, and Department of Sociology, Oxford University, Meadow Flat, Christ Church, Oxford, United Kingdom, E-mail: david.stuckler@chch.ox.ac.uk. Martin McKee, European Centre on Health of Societies in Transition, London School of Hygiene and Tropical Medicine, London, United Kingdom, E-mail: martin.mckee@lshtm.ac.uk.
References
- 1.World Health Organization . Global Tuberculosis Control: Epidemiology, Strategy, Financing. Geneva: World Health Organization. WHO/HTM/TB/2009.411; 2009. [Google Scholar]
- 2.Dorman SE, Chaisson RE. From magic bullets back to the Magic Mountain: the rise of extensively drug-resistant tuberculosis. Nat Med. 2007;13:295–298. doi: 10.1038/nm0307-295. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 4.Basu S, Friedland GH, Medlock J, Andrews JR, Shah NS, Gandhi NR, Moll A, Moodley P, Sturm AW, Galvani AP. Averting epidemics of extensively drug-resistant tuberculosis. Proc Natl Acad Sci U S A. 2009;106:7672–7677. doi: 10.1073/pnas.0812472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva: World Health Organization. WHO/HTM/TB2008.393; 2008. [Google Scholar]
- 6.Post WM, DeAngelisa DL, Travis CC. Endemic disease in environments with spatially heterogeneous host populations. Math Biosci. 1983;63:289–302. [Google Scholar]
- 7.Black FL. Measles antibodies in the population of New Haven, Connecticut. J Immunol. 1959;83:74–82. [PubMed] [Google Scholar]
- 8.Hethcote HW. An immunization model for a heterogeneous population. Theor Popul Biol. 1978;14:338–349. doi: 10.1016/0040-5809(78)90011-4. [DOI] [PubMed] [Google Scholar]
- 9.Yorke JA, Hethcote HW, Nold A. Dynamics and control of the transmission of gonorrhea. Sex Transm Dis. 1978;5:51–56. doi: 10.1097/00007435-197804000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Dormandy T. The White Death: A History of Tuberculosis. London: Hambledon & London; 2001. [Google Scholar]
- 11.Iseman MD. A Clinician's Guide to Tuberculosis. Hagerstown, MD: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 12.Nolan CM. Nosocomial multidrug-resistant tuberculosis: global spread of the third epidemic. J Infect Dis. 1997;176:748–751. doi: 10.1086/514099. [DOI] [PubMed] [Google Scholar]
- 13.Lobacheva T, Sazhin V, Vdovichenko E, Giesecke J. Pulmonary tuberculosis in two remand prisons (SIZOs) in St Petersburg, Russia. Euro Surveill. 2005;10:93–96. [PubMed] [Google Scholar]
- 14.Godfrey-Faussett P, Sonnenberg P, Shearer SC, Bruce MC, Mee C, Morris L, Murray J. Tuberculosis control and molecular epidemiology in a South African gold-mining community. Lancet. 2000;356:1066–1071. doi: 10.1016/s0140-6736(00)02730-6. [DOI] [PubMed] [Google Scholar]
- 15.Di Perri G, Cruciani M, Danzi MC, Luzzati R, De Checchi G, Malena M, Pizzighella S, Mazzi R, Solbiati M, Concia E. Nosocomial epidemic of active tuberculosis among HIV-infected patients. Lancet. 1989;2:1502–1504. [PubMed] [Google Scholar]
- 16.Moro ML, Errante I, Infuso A, Sodano L, Gori A, Orcese CA, Salamina G, D'Amico C, Besozzi G, Caggese L. Effectiveness of infection control measures in controlling a nosocomial outbreak of multidrug-resistant tuberculosis among HIV patients in Italy. Int J Tuberc Lung Dis. 2000;4:61–68. [PubMed] [Google Scholar]
- 17.Moro ML, Gori A, Errante I, Infuso A, Franzetti F, Sodano L, Iemoli E. An outbreak of multidrug-resistant tuberculosis involving HIV-infected patients of two hospitals in Milan, Italy. Italian Multidrug-Resistant Tuberculosis Outbreak Study Group. AIDS. 1998;12:1095–1102. [PubMed] [Google Scholar]
- 18.Keshavjee S, Gelmanova IY, Farmer PE, Mishustin SP, Strelis AK, Andreev YG, Pasechnikov AD, Atwood S, Mukherjee JS, Rich ML, Furin JJ, Nardell EA, Kim JY, Shin SS. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–1409. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 19.Shilova MV, Dye C. The resurgence of tuberculosis in Russia. Philos Trans R Soc Lond B Biol Sci. 2001;356:1069–1075. doi: 10.1098/rstb.2001.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Health CoSDo . Closing the Gap in a Generation: Health Equity through Action on the Social Determinants of Health, Final Report. Geneva: World Health Organization; 2008. [Google Scholar]
- 21.Lonnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Stuckler D, Basu S, McKee M, King L. Mass incarceration can explain population increases in TB and multidrug-resistant TB in European and central Asian countries. Proc Natl Acad Sci U S A. 2008;105:13280–13285. doi: 10.1073/pnas.0801200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss AR, Alland D, Telzak E, Hewlett D, Jr, Sharp V, Chiliade P, LaBombardi V, Kabus D, Hanna B, Palumbo L, Brudney K, Weltman A, Stoeckle K, Chirgwin K, Simberkoff M, Moghazeh S, Eisner W, Lutfey M, Kreiswirth B. A city-wide outbreak of a multiple-drug-resistant strain of Mycobacterium tuberculosis in New York. Int J Tuberc Lung Dis. 1997;1:115–121. [PubMed] [Google Scholar]
- 24.AIDS and Rights Alliance for Southern Africa . The Mining Sector, Tuberculosis and Migrant Labour in Southern Africa. Johannesburg, South Africa: AIDS and Rights Alliance for Southern Africa; 2008. [Google Scholar]
- 25.Jones TF, Woodley CL, Fountain FF, Schaffner W. Increased incidence of the outbreak strain of Mycobacterium tuberculosis in the surrounding community after an outbreak in a jail. South Med J. 2003;96:155–157. doi: 10.1097/01.SMJ.0000053678.62096.6F. [DOI] [PubMed] [Google Scholar]
- 26.Basu S, Andrews J, Poolman E, Gandhi N, Shah NS, Moll A, Moodley P, Galvani AP, Friedland G. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu S, Galvani AP. Extensively drug-resistant tuberculosis in South Africa. Lancet. 2007;369:272–273. doi: 10.1016/S0140-6736(07)60143-3. [DOI] [PubMed] [Google Scholar]
- 28.Pesaran M, Shin Y, Smith RP. Pooled mean group estimation of dynamic heterogeneous panels. J Am Stat Assoc. 1999;94:621–634. [Google Scholar]
- 29.Moll A. Extensively Drug-Resistant (XDR) TB in KwaZulu-Natal. 38th International Conference on Tuberculosis and Lung Disease; Cape Town: 2007. pp. S240–1. [Google Scholar]
- 30.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 31.Escombe AR, Moore DA, Gilman RH, Navincopa M, Ticona E, Mitchell B, Noakes C, Martinez C, Sheen P, Ramirez R, Quino W, Gonzalez A, Friedland JS, Evans CA. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009;6:e43. doi: 10.1371/journal.pmed.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dye C, Williams BG. Criteria for the control of drug-resistant tuberculosis. Proc Natl Acad Sci USA. 2000;97:8180–8185. doi: 10.1073/pnas.140102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet. 1998;352:1886–1891. doi: 10.1016/s0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 34.Blower SM, McLean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, Moss AR. The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med. 1995;1:815–821. doi: 10.1038/nm0895-815. [DOI] [PubMed] [Google Scholar]
- 35.Wells WF. Airborne Contagion and Air Hygiene: An Ecological Study of Droplet Infections. Cambridge, MA: Harvard University Press; 1955. [Google Scholar]
- 36.Riley RL, Mills CC, O'Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 37.Noakes CJ, Beggs CB, Sleigh PA, Kerr KG. Modelling the transmission of airborne infections in enclosed spaces. Epidemiol Infect. 2006;134:1082–1091. doi: 10.1017/S0950268806005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dye C, Espinal MA. Will tuberculosis become resistant to all antibiotics? Proc Biol Sci. 2001;268:45–52. doi: 10.1098/rspb.2000.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJ. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312:1944–1946. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 40.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission. Int Stat Rev. 1994;2:229–243. [Google Scholar]
- 41.Shin G, Khoshnood K. The impact of prison amnesties on tuberculosis control in Russia. Harvard Health Pol Rev. 2004;5:20–35. [Google Scholar]
- 42.Loveday M, Thomson L, Chopra M, Ndlela Z. A health systems assessment of the KwaZulu-Natal tuberculosis programme in the context of increasing drug resistance. Int J Tuberc Lung Dis. 2008;12:1042–1047. [PubMed] [Google Scholar]
- 43.Public Health Research Institute . PHRI/Soros Russian TB Program: An Initiative of the International Center for Public Health. New York: Public Health Research Institute; 2000. [Google Scholar]
- 44.Coker RJ, Dimitrova B, Drobniewski F, Samyshkin Y, Balabanova Y, Kuznetsov S, Fedorin I, Melentsiev A, Marchenko G, Zakharova S, Atun R. Tuberculosis control in Samara Oblast, Russia: institutional and regulatory environment. Int J Tuberc Lung Dis. 2003;7:920–932. [PubMed] [Google Scholar]
- 45.South Africa Department of Health . Tuberculosis Strategic Plan for South Africa, 2007–2011. Pretoria, South Africa: Department of Health; 2007. [Google Scholar]
- 46.Lipsitch M, Cohen T, Cooper B, Robins JM, Ma S, James L, Gopalakrishna G, Chew SK, Tan CC, Samore MH, Fisman D, Murray M. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gillespie DT. Approximate accelerated stochastic simulation of chemically reacting systems. J Chem Phys. 2001;115:1176–33. [Google Scholar]
- 48.Lurie MN, Williams BG, Zuma K, Mkaya-Mwamburi D, Garnett G, Sturm AW, Sweat MD, Gittelsohn J, Abdool Karim SS. The impact of migration on HIV-1 transmission in South Africa: a study of migrant and nonmigrant men and their partners. Sex Transm Dis. 2003;30:149–156. doi: 10.1097/00007435-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Lurie MN, Williams BG, Zuma K, Mkaya-Mwamburi D, Garnett GP, Sweat MD, Gittelsohn J, Karim SS. Who infects whom? HIV-1 concordance and discordance among migrant and non-migrant couples in South Africa. AIDS. 2003;17:2245–2252. doi: 10.1097/00002030-200310170-00013. [DOI] [PubMed] [Google Scholar]
- 50.Drobniewski FA, Balabanova YM, Ruddy MC, Graham C, Kuznetzov SI, Gusarova GI, Zakharova SM, Melentyev AS, Fedorin IM. Tuberculosis, HIV seroprevalence and intravenous drug abuse in prisoners. Eur Respir J. 2005;26:298–304. doi: 10.1183/09031936.05.00136004. [DOI] [PubMed] [Google Scholar]
- 51.International Organization on Standardization . International Standard ISO 3166-1, Codes for the Representation of Names of Countries and their Subdivisions–Part 1: Country Codes, ISO 3166-1. Geneva; United Nations: 2006. [Google Scholar]
- 52.World Health Organization . World Health Report. Geneva: World Health Organization; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


