Abstract
In Lahanam Village, Savannakhet Province, Laos, 125 of 253 villagers (49.4%) were found by fecal examination to harbor hookworm eggs. The eggs were heterogeneous in morphology and size, suggesting infections of mixed nematode species. To confirm the hookworm egg species, on a voluntary basis, 46 hookworm egg–positive participants were treated with albendazole, and post-treatment adult worms were collected from purged fecal samples. The common human hookworm was found in only 3 participants; 1 case of Necator americanus, and 2 cases of Ancylostoma duodenale. In contrast, adult Trichostrongylus worms were expelled from most participants (43 of 46, 93.5%). The Trichostrongylus species were confirmed by morphology and internal transcribed spacer 2 sequences; all worms were of the same species (T. colubriformis). In addition, some Trichostrongylus worms were obtained from a goat in the same village and identified as T. colubriformis. The results suggested that T. colubriformis was the main zoonotic species causing hookworm infections in the village.
Trichostrongylus spp. are primarily parasites of herbivorous animals. Human trichostrongylosis cases have been reported sporadically from many countries, including Laos, Thailand, South Korea, China, the United States, and Australia.1–4
Trichostrongylus infection is diagnosed in the laboratory by detection of eggs in fecal samples. However, the egg morphology of Trichostrongylus and hookworm species is similar, and it is difficult to differentiate them.5 Because animal hookworm infections in humans have been regarded as rare or abnormal, human trichostrongylosis was supposedly overlooked, and common human hookworm infections may have been over-estimated.
In Iran, seven species of Trichostrongylus have been reported.6 Among those species, T. orientalis and T. colubriformis were detected more frequently in humans. In regions where humans were infected with T. colubriformis, this parasite was also prevalent in animals.6 Thus, T. colubriformis was thought to be the main zoonotic species. Conversely, T. orientalis was thought to be a predominantly human parasite. Thus, correct confirmation of the species is important from a public health viewpoint to understand potential zoonoses and to control parasitic infections adequately. The purpose of this study was to identify nematodes, the eggs of which resembled hookworm eggs, infecting humans in a rural area of southern Laos.
A total of 253 human fecal examinations were conducted by using the Kato-Katz method in Lahanam Village, Sonkon District, Savannakhet Province, Laos (Figure 1). Oral and written informed consent was obtained and the study was reviewed and approved by the Lao Medical Ethical Committee (172/NECHR) and the Mahidol University Ethics Committee (MUTM 2009-043-01).7 Among villagers positive for hookworm eggs, 46 agreed to participate in adult worm collection after albendazole treatment (400 mg, single dose) and purgation (60 mL of saturated magnesium sulfate solution). To compare species, Trichostrongylus were obtained from the stomach of a goat. All parasites were kept in 20% ethanol. All worms obtained were examined under a stereomicroscope and measured and identified according to the report of Levine.8 Only male worms were used for species identification.
Figure 1.
Location of Lahanam Village, Savannakhet Province, Laos (star).
Three Trichostrongylus adult worms obtained from each human sample and a goat (total = 6) were randomly chosen and DNA was extracted by using a Genomic DNA Mini Kit (Geneaid, Sijhih City, Taiwan), according to the manufacturer's instructions. A polymerase chain reaction specific for the ribosomal DNA internal transcribed spacer 2 (ITS2) region was conducted with primer sets jhTsp: 5′-TTATGTGCCACAAATGAAGA-3′ and NC2: 5′-TTAGTTTCTTTTCCTCCGCT-3′.4,9 The Trichostrongylus spp. amplicon size was 482 basepairs. The PCR products were sequenced by using an ABI3730XL sequencer (Applied Biosystems, Foster City, CA) at Macrogen Inc. (Seoul, South Korea). The sequences were aligned and compared with the sequence data for Trichostrongylus spp. in GenBank by using BioEdit.10 A phylogenetic tree was reconstructed by using the neighbor-joining method with MEGA version 3.1.11 The tree was evaluated by a bootstrap test based on 1,000 re-samplings.12
The Kato-Katz method showed that nearly half of the villagers (125 of 253, 49.4%) harbored hookworm eggs. Forty-six hookworm–positive villagers volunteered for adult worm recovery; surprisingly, most (43 of 46, 93.5%) expelled Trichostrongylus worms. In contrast, hookworm was found in only three participants: one Necator americanus (female worm) and two Ancylostoma duodenale (one male worm and one female worm). These three hookworm-positive participants were co-infected with Trichostrongylus parasites. Praziquantel (40 mg/kg of body weight) was administered to all cestode-positive or trematode-positive participants.
The female worms recovered were 3.7 (range = 2.7–4.5) mm in length, and the males were 3.6 (range = 2.8–5.0) mm in length. All male worms had a bursa, which can be easily recognized, and showed spicules of unequal length and a boat-shaped gubernaculum (Figure 2). The mean length of the left spicule was 132 μm, and the mean length of the right spicule was 123.5 μm. The worms were smaller than those in a previous report (female = 5.0–8.6 mm, male = 4.3–7.7 mm)8; this is probably because the worms were preserved in ethanol and may have shrunk. However, on the basis of the morphologic features observed, they were identified as T. colubriformis. Trichostrongylus egg found in a fecal sample preserved in 10% formalin is shown in Figure 3. It had a length of 83.3 µm and a width of 49.6 µm; one end was tapered slightly.
Figure 2.

Adult male Trichostrongylus colubriformis worm obtained from a human in Lahanam Village, Savannakhet Province, Laos. A pair of spicule (s) and a gubernaculum (g) are shown. Bar = 10 μm.
Figure 3.
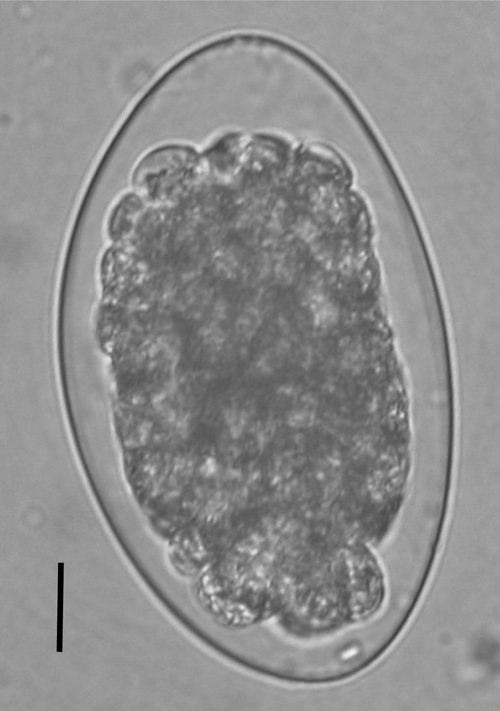
Trichostrongylus egg found in a human fecal sample and preserved with 10% formalin. Egg size was 83.3 × 49.6 µm, and the upper end tapered slightly. Bar = 10 μm.
Six ITS2 sequence samples were successfully amplified and sequenced; all were identical. A phylogenetic tree was reconstructed (Figure 4). The sequences in this study were identical to those of the T. colubriformis sequence in GenBank. Thus, we confirmed by morphology and ITS2 sequencing that human trichostrongylosis caused by T. colubriformis was highly prevalent in this village. The worms recovered from the goat were also identified as T. colubriformis.
Figure 4.
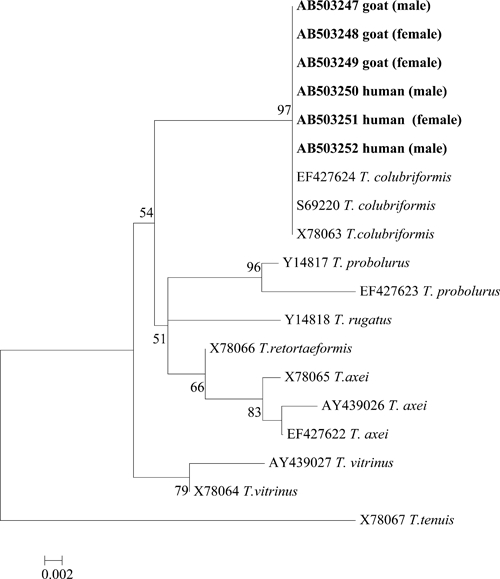
Phylogenetic tree constructed by the neighbor-joining method and based on the internal transcribed spacer 2 region sequencing of adult Trichostrongylus spp. worms recovered from humans and from a goat (bold letters). Sequence was deposited in GenBank. Values at nodes represent a bootstrap confidence level > 50% based on 1,000 re-samplings. The sexes of the worms are indicated in brackets.
A high prevalence of human trichostrongylosis was reported in Japan (44.4%) in the 1950s and among nomads in Iran (87%) in the 1970s.5,6 However, for a long period, such a high prevalence of human trichostrongylosis was not reported elsewhere. The present study found a high prevalence of human trichostrongylosis in village in Laos by recovering adult worms.
The worms from humans and a goat were identified as T. colubriformis by morphology and ITS2 sequencing. The worm sequences obtained from humans and the goat were identical. In areas where humans are infected with T. colubriformis, human-to-human, animal-to-animal, animal-to-human, and human-to-animal transmission is possible.6 Examination of one goat suggested that zoonosis caused by T. colubriformis may be prevalent at the study site.
Humans become infected with Trichostrongylus parasites by ingesting infective-stage larvae. The study village had many semi-domesticated goats, cows, fowl, and dogs, which wandered almost freely; animals were everywhere. Thus, the risk of vegetables in the fields and water resources being contaminated with animal feces was high.
Laos is an agricultural country and this high prevalence and risk factors may not be limited to the study village; zoonotic parasites may be prevalent in many other areas of the country. In 2003, a nationwide survey of intestinal parasitic infections was conducted in schoolchildren; it found that 19.1% of the children surveyed were hook worm egg positive.13 Previously, in other areas of Laos, adult T. colubriformis were accidentally found during fecal examination.4 However, the high rate of adult worm recovery in this study suggested that Trichostrongylus infections might be more common in Laos. Therefore, it is crucial to expand animal research and epidemiologic studies to prevent and control zoonotic parasites.
ACKNOWLEDGMENTS
We thank Nirandon Homsuwan (Department of Helminthology, Faculty of Tropical Medicine, Mahidol University), Sichanh Pansansy, and Vongphaka Boutsyhalath (Health Center, Lahanam Village, Sonkon District, Savannakhet Province, Laos) for generous help in the field.
Footnotes
Financial support: This study was supported by the Research Institute for Humanity and Nature research project Environmental Changes and Infectious Diseases in Tropical Asia (Kazuhiko Moji) and by the Faculty of Tropical Medicine, Mahidol University (Jitra Waikagul).
Authors' addresses: Megumi Sato, Laboratório de Parasitologia, Escola de Medicina Veterinária e Zootecnia, Universidade Federal do Tocantins, Araguaína, Tocantins, Brazil, E-mail: mi_197439@hotmail.com. Tippayarat Yoonuan, Surapol Sanguankiat, Supaporn Nuamtanong, and Jitra Waikagul, Department of Helminthology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mails: teesuphanat@yahoo.com, tmsurapon@mahidol.ac.th, tmsnt@mahidol.ac.th, and tmjwk@mahidol.ac.th. Tiengkham Pongvongsa, Inthava Phimmayoi, and Vilayphone Phanhanan, Station of Malariology, Parasitology and Entomology, North Phonesavang Village, Kaysone District, Savannakhet Province, Laos, E-mail: tpongvongsa@yahoo.com. Boungnong Boupha, National Institute of Public Health, Ministry of Health, Vientiane, Laos, E-mail: laoniph@laotel.com. Kazuhiko Moji, Research Institute for Humanity and Nature, 457-4 Motoyama, Kamigamo, Kita-ku, Kyoto, Japan, E-mail: moji-k@chikyu.ac.jp.
References
- 1.Beaver PC, Jung RC, Cupp EW. Clinical Parasitology. Philadelphia, PA: Lea and Febiger; 1984. pp. 289–291. [Google Scholar]
- 2.Boreham RE, McCowan MJ, Ryan AE, Allworth AM, Robson JM. Human trichostrongyliasis in Queensland. Pathology. 1995;27:182–185. doi: 10.1080/00313029500169842. [DOI] [PubMed] [Google Scholar]
- 3.Panasoponkul C, Radomyos P, Singhasivanon V. Trichostrongylus infection in a Thai boy. Southeast Asian J Trop Med Public Health. 1985;16:513–514. [PubMed] [Google Scholar]
- 4.Yong TS, Lee JH, Sim S, Lee J, Min DY, Chai JY, Eom KS, Sohn WM, Lee SH, Rim HJ. Differential diagnosis of Trichostrongylus and hookworm eggs via PCR using ITS-1 sequence. Korean J Parasitol. 2007;45:69–74. doi: 10.3347/kjp.2007.45.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter GW, 3rd, Ritchie LS, Kaufman EH, Pan C, Yokogawa M, Ishii N, Szewczka JT, Weber RE, Asakura S, Hishinuma Y, Williams F., Jr Parasitological studies in the Far East. IV. An epidemiologic survey in Yamanashi Prefecture, Honshu, Japan. Jpn J Med. 1951;4:113–124. doi: 10.7883/yoken1948.4.113. [DOI] [PubMed] [Google Scholar]
- 6.Ghadirian E, Arfaa F. Present status of trichostrongyliasis in Iran. Am J Trop Med Hyg. 1975;24:935–941. doi: 10.4269/ajtmh.1975.24.935. [DOI] [PubMed] [Google Scholar]
- 7.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 8.Levine ND. Nematode Parasites of Domestic Animals and of Man. Second edition. Minneapolis, MN: Burgess Publishing Company; 1980. pp. 135–140. [Google Scholar]
- 9.Gasser RB, Stewart LE, Speare R. Genetic markers in ribosomal DNA for hookworm identification. Acta Trop. 1996;62:15–21. doi: 10.1016/s0001-706x(96)00015-0. [DOI] [PubMed] [Google Scholar]
- 10.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 11.Kumar S, Tamura K, Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 13.Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, Sohn WM, Yong TS, Deodato G, Standgaard H, Phommasack B, Yun CH, Hoang EH. Prevalence of intestinal parasites infections on a national scale among primary schoolchildren in Laos. Parasitol Res. 2003;91:267–272. doi: 10.1007/s00436-003-0963-x. [DOI] [PubMed] [Google Scholar]


