Abstract
World-wide dengue vector control is hampered by the spread of insecticide resistance in Aedes aegypti. We report the resistance status of a wild Ae. aegypti population from Martinique (Vauclin) to conventional larvicides (Bacillus thuringiensis var israeliensis [Bti] and temephos) and potential alternatives (spinosad, diflubenzuron, and pyriproxyfen). The efficacy and residual activity of these insecticides were evaluated under simulated and field conditions. The Vauclin strain exhibited a high level of resistance to temephos, a tolerance to insect growth regulators, and full susceptibility to spinosad and Bti. In simulated trials, pyriproxyfen and Bti showed long residual activities in permanent breeding containers (28 and 37 weeks), whereas under field conditions they failed to curtail Ae. aegypti populations after four weeks. Conversely, diflubenzuron and spinosad showed a residual efficacy of 16 weeks, suggesting that these chemicals may be promising alternatives to Bti and temephos for controlling insecticide-resistant Ae. aegypti populations.
Introduction
Dengue virus exists as four antigenically distinct serotypes (DEN-1, DEN-2, DEN-3, and DEN-4) and causes 50–100 million cases of dengue and thousands of deaths every year.1 Because of global changes (environment, climate) and increasing transportation, recent decades have seen a dramatic resurgence of dengue throughout regions where dengue vectors are present and this has lead to major public health problems.2 Because there is still no vaccine or specific treatment available against the virus, vector control remains the only strategy for reducing dengue transmission. Effective vector control measures rely on active community participation, health education programs, and environmental management that include improvement of water supplies and storage, solid waste management, and modification of human-made larval habitats.3 During inter-epidemic periods or when the elimination of breeding habitats of the mosquito is not easily achievable, insecticide application in larval habitats is routinely conducted by public health services.4–6
Unfortunately, many dengue vector control programs are threatened by development of insecticide resistance in Aedes aegypti (L.), the main vector of dengue virus.7,8 Recent field trials carried out in Martinique (French West Indies) showed that the level of pyrethroid resistance in adult mosquitoes is high9 and can drastically reduce the efficacy of space spray applications.10 The organophosphate temephos was widely used as larvicide for the control of dengue vectors in Martinique. Strong levels of resistance to organophosphates have also been detected in Southeast Asia,11 South America,12 and the Caribbean.9 In Martinique, temephos was replaced in 2009 by Bacillus thuringiensis var israeliensis (Bti) after European Biocide legislation (European Directive 98/8EC, February 1998). Bti has desirable properties for mosquito control because of its fast killing effect, a good toxicologic profile,13 and the absence of cross-resistance with conventionally used pesticides. Unfortunately, Bti shows low residual activity in natural breeding sites14 and needs frequent re-application, especially in polluted habitats.15,16
There is now a strong consensus among scientists and public health workers that alternative tools (insecticides) and strategies are urgently needed to ensure effective and sustainable control of dengue in the tropics.17 The new tools need to be specific, cost-effective, and safe for the environment and non-target fauna. Unfortunately, the research and development of new chemical classes for use in public health control has been rather limited in the past 20 years because it involves a long, expensive, and non-profitable process for private companies.
The rare new chemicals available for dengue vector control at the larval stage (e.g., diflubenzuron, spinosad, pyriproxyfen) have all arisen from the agricultural market.18 Diflubenzuron is an insect growth regulator (IGR) that belongs to the family of benzoylurea and acts by disrupting chitin synthesis and deposition.19 Several studies reported the potent insecticidal activity of diflubenzuron against several mosquito species, especially Ae. aegypti.20 Spinosad belongs to the class of naturalytes that are based on natural metabolites (spinosine A and D) derived from the actinomycetale Saccharopolyspora spinosa and, as such, may be considered as a bioinsecticide.21 Because of its unique mode of action involving the post-synaptic nicotinic acetylcholine and γ-aminobutyric acid receptors,22 spinosad shows promising potential for the control of dengue vectors.23 Pyriproxyfen is an IGR that mimics the action of juvenile hormones and is a potent inhibitor of embryogenesis, metamorphosis, and adult formation.24 Pyriproxyfen is safe for non-target organisms and effective at low doses against Ae. aegypti.25 All of the above insecticides are recommended by the World Health Organization (WHO) for use for vector control in drinking water sources and containers and may be used routinely by mosquito control services.26–28
The purpose of this study was to characterize the resistance status of Ae. aegypti larvae from Martinique to conventional (Bti and temephos) and alternative insecticides (pyriproxyfen, diflubenzuron, and spinosad) and to assess their efficacy and residual activity under simulated and field conditions. The value of these tools for vector control is discussed.
Materials and Methods
Mosquito strains.
Two strains of Ae. aegypti were used in trials conducted in the laboratory and in semi-field conditions. The susceptible reference Bora strain, which originated in Bora-Bora (French Polynesia), has been colonized for many years and is checked regularly as part of our laboratory routine for resistance mechanisms (e.g., knockdown resistance mutation, detoxification enzyme activity).9 The Vauclin strain was established from larvae caught in natural breeding sites of the community of Vauclin in Martinique (14°54′N, 60°84′W). Larvae of the first generation (F1) were used for the laboratory and semi-field assays. This population showed strong resistance to commonly used pesticides because of the V1016I knockdown resistance mutation and increased metabolic detoxification.9,29
Insecticides.
For larval bioassays, technical grades of temephos (97.3%, Pestanal®; Sigma-Aldrich, Riedel-de Haën, Germany), pyriproxyfen (98.7%; Sumitomo Chemical Co., Ltd. First, Tokyo, Japan), diflubenzuron (99.5%, Pestanal®; Sigma-Aldrich), spinosad (90.4%; Dow AgroScience, Indianapolis, IN), and Bti Vectobac®12AS (Abbott Laboratories, Abbott Park, IL) (1.2%, 1,200 international toxic units/mg) were used. For the semi-field trials, temephos (Abate®; 1% granules [GR]), Bti (Vectobac®, water dispersible granule (WG), 3,000 UTI/mg, 37.4%), spinosad (7.48%, dispersible tablets [DTs], 0.5% GR), and pyriproxyfen (Sumilarv®, 0.5% GR) were used. For field trials, Bti (Vectobac® DT, 3,400 international toxic units/mg), spinosad (0.5% GR), pyriproxyfen (Sumilarv®, 0.5% GR), and diflubenzuron (Dimilin® TB-2, 2%) were used.
Laboratory evaluation.
Larval bioassays were carried out using technical grades of temephos, Bti, spinosad, pyriproxyfen, and diflubenzuron according to WHO guidelines.30 Bioassays were performed using late third and early fourth-instar larvae of the Bora and Vauclin strains. For each bioassay, 20 larvae of each strain were transferred to cups containing 99 mL of distilled water and 1 mL of the insecticide tested at the desired concentration. Five cups per concentration (100 larvae) and 5–8 concentrations in the activity range of each insecticide were diluted in ethanol, except for Bti, which was diluted in water. Control treatments consisted of the addition of 1 mL of ethanol to 99 mL of water (distilled water for Bti assays). Larval mortality was recorded after an exposure of 24 hours to temephos, spinosad and Bti. Because of the delayed action of diflubenzuron and pyriproxyfen, larval, pupal, and adult mortality was assessed every day until emergence. In these cases, larvae were fed every day with dry cat food at a concentration of 100 mg/L. For each bioassay, temperature was maintained at 27°C with a 12-hour light:12-hour dark photoperiod.
Simulated field trial.
The trial was carried out in Fort de France, Martinique according to WHO procedures.30 The effects of spinosad, temephos, Bti, and pyriproxyfen were evaluated and compared until the efficacy, i.e., the emergence inhibition rates (EIs) decreased to < 80%. Blue plastic containers (drums) with a capacity of 175 liters were used because they are widely used for water storage in Martinique and have been shown to be the most productive breeding habitats for Ae. aegypti.31 These containers were filled with 145 liters of domestic water and covered with a mosquito net to prevent oviposition by wild female mosquitoes in the area and to prevent the deposition of debris. Containers were placed under a shelter to prevent direct exposure to rain and sunlight. All insecticides were tested at the WHO-recommended dosages for the control of mosquito larvae.32,33 A total of 24 containers (3 replicates per insecticide) were put in rows and allocated to insecticide treatments at random: 6 drums were treated with spinosad GR at concentrations of 0.1 mg/L and 0.5 mg/L, 3 with spinosad DT (1 tablet/145 liters, equivalent to 0.67 mg/L active ingredient [AI]), 6 with pyriproxyfen at concentrations of 0.02 mg/L and 0.05 mg/L AI, 3 with Bti (5 mg/L AI), 3 with temephos (1 mg/L AI), and 3 were left untreated (controls). Groups of 100 third-instar larvae of the F1 generation of the Vauclin strain were added to each container with one gram of food (dry cat food) at time 0, and then every 10 days until the end of the experiment. The containers were replenished every 10 days to maintain the initial level of water.
Emerging adults were collected in each container by using electric aspirators. In each container, the temperature and pH were checked every 10 days with a portable tester (HI98128; Hanna Instruments, Ontario, NY) to detect any discrepancies between replicates and/or treatments. External temperature and hygrometry were recorded by using a meteorologic unit (Auria 4®; Degreane Horizon, Cuers, France) provided by the General Council of Martinique (Château Paille). Temephos, Bti, and pyriproxyfen were evaluated during February–December 2007. Spinosad GR and DTs were evaluated in September 2007–May 2008 because we received these formulations later in the year.
Large-scale field trials.
The efficacy and the residual activity of the larvicides against natural populations of Ae. aegypti were evaluated in three sites in the commune of Vauclin, which is located in southeastern Martinique. This commune has a dry tropical climate with a rainy season during May–November and an annual precipitation of 2,000 mm. The three study sites were selected because they were isolated locations at least 2 km from each another (to avoid possible migration of Ae. aegypti between locations). The first site was Anse Maroquet, which is a group of houses located near a creek on the coast of the Atlantic Ocean, the second site was Château Paille, a classical housing estate next to the ocean, and the third site was Cadette, a hamlet situated approximately 4 km from the ocean in a more humid area (Figure 1). The areas of the treated areas were 0.02 km2, 0.12 km2, and 0.11 km2 for Anse Maroquet, Château Paille, and Cadette, respectively.
Figure 1.
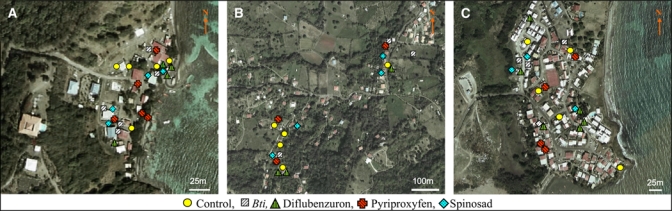
Satellite picture of the three field studies chosen for the evaluation of the insecticide formulations, Martinique. A, Anse Maroquet (14°33′N, 60°49′W). B, Cadette (14°33′N, 60°52′W). C, Château Paille (14°33′N, 60°50′W). The breeding habitats of Aedes aegypti were plotted by using a global positioning system. Different treatments of the containers are represented by symbols. This figure appears in color at www.ajtmh.org.
Formulation and dosages used in the field trial were Vectobac® DT (1 tablet/50 liters, 5 mg/L), Sumilarv® 0.5% GR (0.05 mg/L), Dimilin® TB-2, 2% (1 tablet/200 liters, 0.2 mg/L), and Spinosad DT (1 tablet/200 liters, 0.5 mg/L). The DT formulations were preferred when possible because they were more convenient for field applications than WG formulations. In each location, 5 breeding sites of Ae. aegypti per insecticide were selected at random (i.e., 15 containers per insecticide in total and 15 untreated controls). The application rate of each larvicide was based on the capacity of the container and not the amount of water present at the time of application. Each container was positive for Ae. aegypti larvae before the trial began and their physical coordinates were recorded one week before treatments were applied (Figure 1).
Each container was sampled to determine the density of Ae. aegypti larvae and pupae prior to treatment (D0), two days post treatment (D2), and then every week thereafter (e.g., D7, D14, D21). The larval sampling method consisted of 3 dips using small fish nets (15 × 15 cm) that were conducted by the same operator in each location. Each fish net was allocated for use with one insecticide to avoid contamination among the containers. To compare the relative density (RD) of Ae. aegypti populations before and after treatment, third and fourth instars and pupae were counted per dip. The first, second, and third instars were replaced in their habitats whereas the fourth instars and pupae were brought back to the laboratory, put in 200 mL of water from their original container, and checked for adult emergence. Temperature and pH in each container were measured weekly. Meteorologic data were obtained by a meteorologic unit of the General Council of Martinique (Château Paille).
Statistical analysis.
For determination of intrinsic activity of each larvicide, three replicates with larvae from different rearing batches were made at different times and the results were pooled for analysis (n = 300 per dose). In case of mortality in the control treatment > 5%, larval mortality was corrected by using the formula of Abbott.34 Results were analyzed by using log-probit method of Finney35 and probit software of Raymond and others.36 This software estimates the slope of regression lines and 50% and 90% lethal concentrations (LC50 and LC90) with 95% confidence intervals (CIs). The Bora-Bora and Vauclin strains were considered as having different susceptibilities to a given insecticide when the ratio between their LC50/95 had CIs excluding the value of 1 (significant at P < 0.05).
Regarding the simulated field trial, emergence inhibition rates (% EI) and 95% CIs were calculated for the average of the 3 replicates per insecticide according to the formula % EI = ((C – T)/C) × 100, where C is the emergence in the control and T is the emergence in the treated container at the same time period. For each formulation, curves are presented until the % EI decreased to < 80%, which corresponds to the threshold generally considered for reapplication of the treatment.29
For large-scale field trials, the RD of Ae. aegypti larvae and pupae for the five insecticide treatments and their SEs over time were estimated on the logit scale by using a mixed model analysis of variance in a split-plot design (see below). The RD was estimated by using the formula of Mulla:30 RD = 100 – (C1/T1) × (T2/C2) × 100, where C1 is the average number of larvae in control containers prior to treatment (D0) calculated separately for each location, and C2 is the average number of larvae in control containers at each location and day of sampling. The log (number of larvae and pupae + 1) per container was used to calculate average values prior to back-transformation. T1 is the number of larvae and pupae on D0 in each container to be treated with insecticides, and T2 is the number of larvae and pupae in each treated container for each day of sampling. Estimates of RD with negative values were set to zero. Analysis of RD was conducted on a logit scale (log[RD/(1 – RD)]). To do so, values of RD were transformed by the equation 0.005 + 0.99 × (RD/100) to avoid values of either 0 or 1.
Analysis of variance used to analyze the data involved a split-plot design in which the three localities were treated as whole-plots (random, nominal) and the effect of insecticide treatments (fixed, nominal) was tested against the interaction between treatments and localities (random, nominal). The model also estimated variation among containers within each locality and treatment (random, nominal). The sub-plot level of the model involved the effects of day of sampling and the interaction between day of sampling and treatments. These fixed nominal effects were tested against the residual error variation. The restricted maximum likelihood method in JMP® version 7.1 (SAS Institute, Cary, NC) was used to fit the model.
Containers that disappeared during the experiment or that were emptied completely (e.g., because of domestic use) were considered missing data. Estimates of RD are shown until populations recovered to > 20% of their initial size.
Results
Insecticide resistance status of Ae. aegypti in Martinique.
Results of larval bioassays on the Bora and Vauclin strains are shown in Table 1. For each strain and each insecticide, the dose-mortality relationships were fitted by regression (P > 0.05). With the susceptible Bora strain, pyriproxyfen, diflubenzuron, and temephos showed the highest insecticidal activity, followed by Bti and spinosad. The Vauclin strain showed strong resistance to temephos (RR50 = 44 and RR95 = 175). The slope of the regression line for temephos was significantly lower in the Vauclin strain (2.1) than in the Bora strain (8.1), indicating a heterogeneous response of mosquitoes to this insecticide. A slight but significant reduction of pyriproxyfen and diflubenzuron insecticidal activity was observed in the Vauclin strain (RR50 = 2.2, RR95 = 1.9) than in the susceptible Bora strain (RR50 = 1.8). Conversely, the Vauclin strain was fully susceptible to Bti (RR50 = 1) and spinosad (RR50 = 0.6).
Table 1.
Resistance status of Aedes aegypti (Bora and Vauclin strains) against the five larvicides tested, Martinique*
| Larvicide | Strain | Slope (SE) | LC50 (95% CI) (µg/L) | LC95 (95% CI) (µg/L) | RR50 (95% CI) | RR95 (95% CI) |
|---|---|---|---|---|---|---|
| Temephos | Bora | 8.5 (0.4) | 3.7 (3.6–3.8) | 5.7 (5.5–6) | – | – |
| Vauclin | 2.1 (0.1) | 160 (150–180) | 1,000 (870–1,180) | 44 (40–48) | 175 (150–205) | |
| Bti | Bora | 5.4 (0.5) | 120 (110–130) | 250 (220–280) | – | – |
| Vauclin | 4.9 (0.6) | 130 (120–140) | 270 (220–330) | 1 (0.8–1.3) | 1.1 (0.7–1.7) | |
| Pyriproxyfen | Bora | 3.5 (0.2) | 0. 11 (0.10–0.11) | 0. 32 (0.29–0.36) | – | – |
| Vauclin | 3.9 (0.5) | 0.24 (0.21–0.27) | 0. 64 (0.48–0.84) | 2.2 (1.8–2.6) | 1.9 (1.3–3.0) | |
| Spinosad | Bora | 3.9 (0.6) | 350 (310–400) | 920 (640–1,340) | – | – |
| Vauclin | 4.2 (0.2) | 220 (210–230) | 550 (510–600) | 0.6 (0.5–0.8) | 0.6 (0.5–0.8) | |
| Diflubenzuron | Bora | 5.5 (1.2) | 1.7 (1.4–2.1) | 3.4 (2.4–4.9) | – | – |
| Vauclin | 6 (1.1) | 3.1. (2.8–3.5) | 5.9 (4.7–7.5) | 1.8 (1.1–3) | 1.7 (0.6–4.5) |
LC50 = 50% lethal concentration; CI = confidence interval; RR50 = 50% resistant ratio; Bti = Bacillus thuringiensis var israeliensis. Resistant ratios = LC50 of vauclin strain/LC95 of Bora strain; RRs in bold are significantly different from 1 (P < 0.05).
Residual activity of formulated insecticides in simulated field trial.
During the trial, outdoor temperatures recorded daily ranged from 19°C to 35°C. The temperatures recorded in the containers ranged from 24 to 28°C and the pH was 7.7 ± 0.22. Limited variation in temperature and pH was recorded between treatments throughout the trial, suggesting that the environmental conditions did not have an impact on the efficacy of the different formulated insecticides.
Data from the semi-field trial and the corresponding statistical analysis are shown in Figure 2 and Table 2, respectively. During the trial, the emergence inhibition rate of the control group was generally low (< 10%) except in cohorts 1, 5, and 11 in which it was > 20%. Analysis of variance showed that there was a significant effect of the treatments over time (effect TD, Table 2). The results showed that the EI for the temephos and Bti decreased to < 80% after 140 days and 200 days, respectively. With temephos, the EI varied between 90% and 100% until 130 days, and the efficacy of Bti remain maximal (100%) until 110 days. The unusually long persistence of Bti WG in drums (EI > 80% until 190 days) is unusual.
Figure 2.
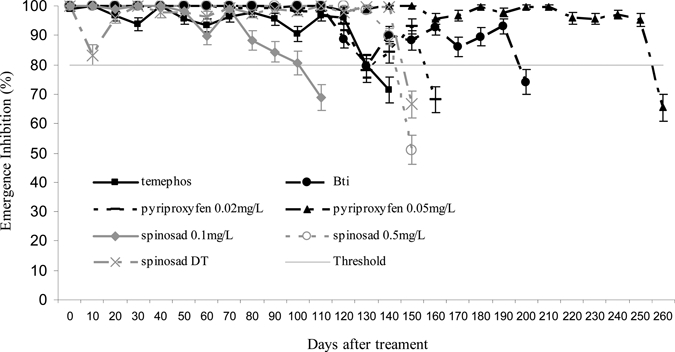
Emergence inhibition rates and their 95% confidence intervals in simulated field trials for the larvicide formulations tested against Aedes aegypti, Martinique.
Table 2.
Split-plot repeated measures analysis of variance for treatment effects on Aedes aegypti in semi-field trials and field experiments, Martinique*
| Source | No. | DFnum | DFden | F | P | CV |
|---|---|---|---|---|---|---|
| Emergence inhibition in semi-field assay | ||||||
| Treatment (T) | 4†/3‡ | 3†/2‡ | 8†/6‡ | 2.64†/6.47‡ | 0.12†/0.032‡ | – |
| Day (D) | 29†/18‡ | 28†/17‡ | 224†/102‡ | 29.73†/25.17‡ | < 0.001†‡ | – |
| TD | 116†/54‡ | 84†/34‡ | 224†/102‡ | 2.09†/2.79‡ | < 0.001†‡ | – |
| Container (C) treatment | 12†/9‡ | – | – | – | – | 48%†/10%‡ |
| Relative density | ||||||
| T | 5 | 4 | 6.806 | 8.75 | 0.008 | – |
| D | 22 | 21 | 792.9 | 17.36 | < 0.001 | – |
| DT | 110 | 84 | 791.8 | 2.04 | < 0.001 | – |
| Locality (L) | 3 | – | – | – | – | < 1% |
| LT | 15 | – | – | – | – | 2.3% |
| C (LT) | 62 | – | – | – | – | 24.9% |
| Emergence inhibition in field experiment | ||||||
| T | 5 | 4 | 6.99 | 7.55 | 0.011 | – |
| D | 20 | 19 | 824.5 | 15.31 | < 0.001 | – |
| DT | 80 | 76 | 822.7 | 1.44 | 0.009 | – |
| L | 3 | – | – | – | – | < 1% |
| LT | 15 | – | – | – | – | < 1% |
| C (LT) | 62 | – | – | – | – | 13.97 |
No. = number of parameters; DFnum = degrees of freedom numerator; DFden = degrees of freedom denominator; CV = coefficient of variance.
= first experiment.
= second experiment.
The residual activities of spinosad and pyriproxyfen were dosage-dependent. The EI of pyriproxyfen GR decreased to < 80% after 160 and 260 days (0.02 mg/L and 0.05 mg/L, respectively). Pyriproxyfen GR (0.05 mg/L) showed the longest residual activity in comparison to all other insecticides tested (P < 0.05). Spinosad GR showed far lower residual activity than pyriproxyfen GR, i.e., 110 days and 150 days were required to go below the cut-off point (EI80) at concentrations of 0.1 mg/L and 0.5 mg/L, respectively. No increase in residual activity was noted for the spinosad DT formulation compared with spinosad GR, despite similar application rates (0.67 mg/L versus 0.5 mg/L).
Large-scale field trials.
The field trial was conducted during February 2008–August 2008. During this period, the average temperature per month was 27°C and ranged from 21°C in February to 33°C in August at the three study sites. The average precipitation over the trial was 60 mm per month, with a maximum of 91 mm in August and a minimum of 32 mm in March. The rainiest day was in April (32 mm). The pH recorded in the containers remained constant and close to neutrality in each locality (range = 6.4–8.2), and temperatures within drums ranged from 25°C to 33°C throughout the trial.
The efficacy of the different formulated products was expressed in term of RD of Ae. aegypti over time (Figure 3). Analysis of variance (Table 2) showed that there was a strong and significant difference of RD among treatments over time (effect DT; P < 0.001). These results were not influenced by random variation among the different localities (effect L, coefficient of variance < 1%) or by an interaction between localities and treatments (effect LT coefficient of variance < 3%). However, the treatment effect was strongly significant (P = 0.008).
Figure 3.
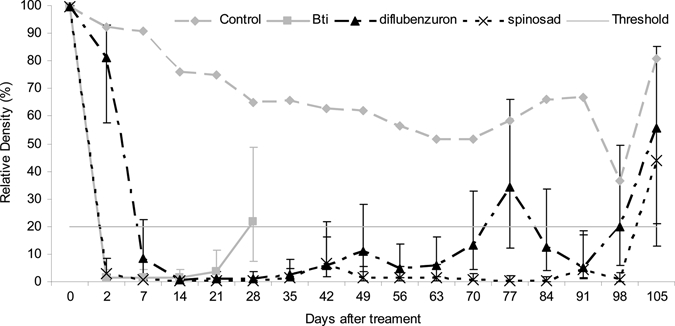
Relative densities and standard errors of Aedes aegypti in breeding habitats after treatments with Bacillus thuringiensis var israeliensis (Bti), diflubenzuron, and spinosad, Martinique.
In the control containers, there was a time-dependant decrease of mosquito density that reached a minimum level of 37% of the original abundance 98 days post-treatment (D98) (Figure 3). With Bti tablets, the RD decreased drastically from 100% to 1% (95% CI = 0.4–4%) at D2, but rapidly increased again to 22% (95% CI = 7–49%) at D28. This finding indicates a fast-killing effect but a low residual activity of this insecticide in plastic containers. The RD with diflubenzuron decreased to 8% one week after the treatment but increased beyond the threshold of 20% RD twice at D77 (34% RD) and D98. We suspect the first increase in RD was caused by natural fluctuation rather than a real loss of efficacy. The residual activity of spinosad was 100% until 98 days post-treatment and was comparable to that of diflubenzuron.
The relative density of Ae. aegypti exposed to pyriproxyfen is shown in Figure 4. Because the RD of mosquitoes strongly fluctuated over the time, it was difficult to estimate its residual efficacy by using this criteria. This finding is most likely explained by the specific mode of action of pyriproxyfen that acts only at the pupal stage. Thus, the data indicated the natural fluctuation of larval density in the containers.
Figure 4.
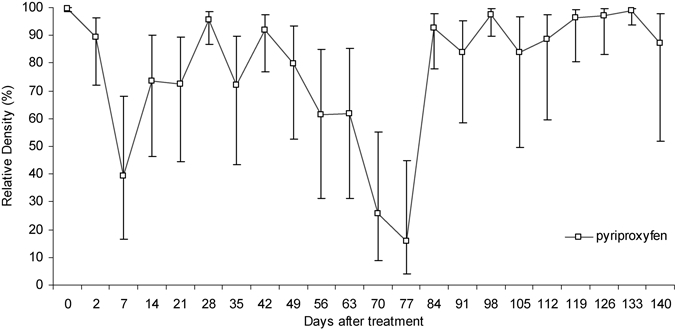
Relative densities and standard errors of Aedes aegypti in breeding habitats after treatments with pyriproxyfen, Martinique.
The emergence inhibition rates of the different formulations against fourth instar larvae and pupae of Ae. aegypti collected in the different containers and tested in the laboratory are shown in Figure 5. Statistical analysis (Table 2) showed that the EIs were not correlated with the study site effect (effect L coefficient of variance < 1%). However, the treatment effect was significant (P = 0.011). Of the variance explained, 13% could be attributed to the effect of random variation among containers (Table 2). The EI rates for Bti and pyriproxyfen were > 95% two days after the treatment, but rapidly decreased to 1.7% (SE = 0.6–5%) and 7.9% (SE = 3–21%) 28 days post-treatment. This period of 28 days corresponded to the duration required to obtain relative densities beyond 20% for Bti under field conditions (Figure 4). The EI with spinosad was strong (SE = 85–100%) and lasted for 98 days, before decreasing to 37% (SE = 10–76%) after 105 days (Figure 5). The EI for diflubenzuron decreased to < 80% after 126 days (21%, SE = 5–59%).
Figure 5.
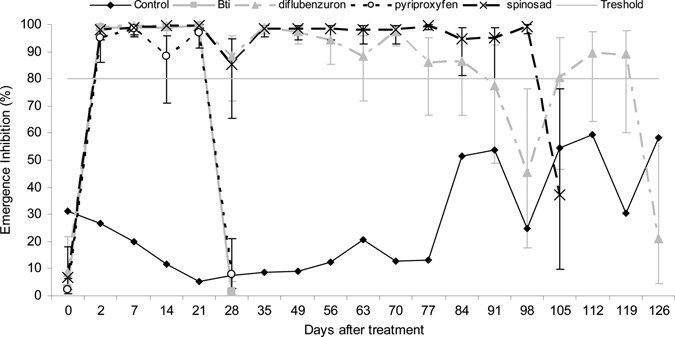
Emergence inhibition rates and standard errors of the fourth instar (L4) larvae and pupae collected in the breeding habitats treated against Aedes aegypti, Martinique.
Discussion
This study tested the field efficacy of conventionally used and alternative larvicides for the control of Ae. aegypti, the main dengue vector in Martinique. The simulated field trial conducted at Fort de France showed 28 weeks and 20 weeks residual activity (EI > 80%) for Bti and temephos, respectively, against the local Vauclin population. In a similar study conducted in Malaysia on Ae. aegypti and Ae. albopictus, the efficacy of temephos and Bti was much lower (efficacy = 7–9 weeks and 11 weeks, respectively).15 The rather long residual activity of Bti and temephos in this study could be questioned because it is known that Bti toxins sediment rapidly from the water surface37 and because of the high level of temephos resistance in the Vauclin population. Regarding temephos, this finding may be explained by an absence of degradation by ultraviolet light and by the fact that the dosage (1 mg/L) applied in drums corresponded to a concentration that killed 95% of the resistant mosquitoes in laboratory conditions (Table 1). Regarding Bti, the high dose used (5 mg/L) and the absence of organic content may have extended the duration of activity, as reported.17
In the same conditions, spinosad GR at a concentration of 0.1 mg/L showed the lowest residual activity (EI > 80% for 14 weeks). A similar performance of spinosad (16 weeks) was observed against Ae. aegypti in semi-field conditions.33 Spinosad GR and DT applied at a concentration of 0.5 mg/L showed longer and comparable residual activity (20 weeks), but the DT formulation is easier to use in the field because the insecticide immediately sank to the bottom of the container, unlike the granules, which floated on the surface of the water for several days.
The long efficacy of pyriproxyfen (EI > 80% for almost 9 months) has been reported,38,39 although its residual activity was much longer in our simulated conditions than in previous conditions. However, the performance of larvicides in well-controlled conditions has already been pointed out by several authors17,40 and should be interpreted with caution because of the absence of direct exposure to rain, sunlight, and organic matter could lead to an over-estimation of the residual activity of a insecticide. Simulated-field experiments represent a useful method for screening new insecticides and/or to select dosages for field application, but they cannot be used to predict the performance of formulated products under real conditions.
As expected, a lower residual activity was observed for all insecticides during the field trial compared with the simulated-field trial. Bti DT and pyriproxyfen GR lost their efficacy after 4 weeks (RD > 20% and EI < 80%), and diflubenzuron DT and spinosad DT provided much longer residual activity (14 weeks). As reported,17 the poor efficacy of Bti (1 tablet/50 L, equivalent a concentration of 5 mg/L) may render the control of Ae. aegypti populations in Martinique difficult in preventing dengue. No data could be generated for temephos because it was banned in 2009 from the insecticides available for use in the European Community. However, a previous study conducted in Martinique showed that 18% of the containers treated with the organophosphate were positive for Ae. aegypti 28 days post-treatment (Yp-Tcha MM and Etienne M, unpublished data). Organophosphate resistance is widespread in dengue vectors in the Caribbean and probably explains the poor performance of temephos against wild Ae. aegypti mosquitoes.
The short residual activity of pyriproxyfen contrasts with that obtained in the simulated field trial. Sihuincha and others41 demonstrated longer performance (i.e., mortality rate > 80% for 5 months) against Ae. aegypti in water tanks treated with the same dosages in Peru. Similar to Bti, pyriproxyfen may have degraded because of environmental conditions. As previously observed by Vythilingham and others,39 temperatures in the containers periodically increased to as high as 33°C and this increases might have contributed to reduced residual activity of pyriproxyfen. The low residual activity of pyriproxyfen GR in Martinique and its specific mode of action on pupae may render its future use and evaluation at an operational scale difficult.
In contrast, spinosad DT (0.5 mg/L) showed good residual efficacy and emergence inhibition rates against Ae. aegypti (15 weeks). The fast killing effect of spinosad DT (> 97% reduction of mosquitoes 2 days post-treatment) and a long-residual effect may be attributed to the specific composition of the DT tablet, which is made of two specific layers: an outer layer consisting of an effervescent system that provides fast release of the AI, and a second layer formulated to dissolve the insecticide gradually over time into the water.33 Similar to spinosad, diflubenzuron remained effective in drums for up to 15 weeks. This finding is consistent with the results of Thavara and others,20 who demonstrated that diflubenzuron can provide effective control of Aedes mosquitoes for 3–4 months in the field. The long residual activity of spinosad and diflubenzuron and their favorable environmental profile1 make them very attractive alternatives for dengue vector control in water tanks and water storage containers in the tropics.
During the field trial, a time-dependant reduction in the number of larvae and pupae was observed in control containers. This result is probably explained by the simultaneous presence of treated and control containers in the same localities in which the effect of insecticide treatments led to a global reduction in the numbers of mosquitoes emerging and ovipositing in the local Ae. aegypti population. This finding clearly suggests an impact of the treatments at a community level. The absence of locality-dependent effects on the effect of insecticide treatments (coefficient of variance < 1%) may be explained by the fact that the three localities were 4 km from each other and experienced similar environmental conditions. Whatever the treatment or locality, 25% of the variance in relative density that could be explained was caused by variation among containers. Variation of temperature in the larval habitats over time could be responsible for some of this change. However, a more likely explanation is that each container experienced its own particular environmental variation, e.g., rainfall, habitant use, sunlight exposure, organic content. This variation at the level of individual containers could influence the presence or absence of larvae beyond the presence of an insecticide. This explanation indicates the necessity of having replicate containers in field trials to avoid accurate estimates of mosquito density being obscured by random variation among individual containers.
Finally, our study showed that the field population of mosquitoes from Vauclin was highly resistant to temephos, tolerant to pyriproxyfen and diflubenzuron, but fully susceptible to Bti and spinosad. The absence of Bti resistance even after 12 years of use for larval control is encouraging if it is used for regularly treated larval habitats (< 4 weeks). As suggested, this finding is probably caused by the complex structure of the multiple Bti toxins, which may slow down the acquisition of resistance in natural populations.42 Similarly, the absence of spinosad resistance in the Vauclin population is promising and may be explained by the fact that this pesticide has never been used for mosquito control in the Caribbean. Moreover, because of its unique mode of action on post-synaptic receptors and the simultaneous action of two neurotoxins, one might expect a lower risk for the appearance of resistance compared with classical synthetic insecticides.43,44 Unfortunately, decreased susceptibility to pyriproxyfen and diflubenzuron was observed in the Vauclin population (significant resistant ratios).
Previous authors have reported similar findings in mosquito populations exhibiting high levels of resistance to temephos.45,46 In Martinique, the organophosphate temephos has been used since the 1970s but has recently been abandoned because of European Biocide legislation. Previous studies showed that temephos resistance is widespread in the Caribbean and South America, and this resistance is strongly associated with increased activity of glutathione S-transferases, α-esterases and β-esterases, and, to a lesser degree, mixed function oxidases.9,47 Because diflubenzuron and pyriproxyfen have never been used in public health programs in Martinique, it is possible that the cross-tolerance of mosquito larvae to IGR has arisen through the extensive use of temephos for vector control. This suggestion may have operational consequences for dengue vector control and the future use of IGRs if these products may contribute to selection for existing resistance mechanisms in natural populations of Ae. aegypti.
ACKNOWLEDGMENTS
We thank the staff of the Mosquito Control Centre of Martinique and the Entente Interdepartmental de la Démoustication, Méditerranée, France for technical support; and Dow AgroScience (Indianapolis, IN) and Sumitomo Chemical Co. Ltd. First (Tokyo, Japan) for providing technical grades and formulations of insecticides.
Footnotes
Financial support: This study was supported by the French Agency for Environmental Health and Safety and the Institut de Recherche pour le Developpement.
Authors' addresses: Sébastien Marcombe and Frédéric Darriet, Laboratoire de Lutte Contre les Insectes Nuisibles, Institut de Recherche pour le Développement, Montpellier Cedex 5, France, E-mails: sebastien.marcombe@ird.fr and frederic.darriet@ird.fr. Philip Agnew, Génétique et Evolution des Maladies Infectieuses, Centre National de la Recherche Scientifique, Unité de Mixte Recherche, 2724, Institut de Recherche pour le Développement, Montpellier Cedex 5, France, E-mail: philip.agnew@ird.fr. Manuel Etienne, Marie-Michelle Yp-Tcha, and André Yébakima, Centre de la Démoustication, Fort de France, Martinique, E-mails: etienne.manuel@cg972.fr, yp-tcha@cg972.fr, and Yebakima@cg972.fr. Vincent Corbel, Institut de Recherche pour le Développement, Centre de Recherche Entomologique de Cotonou, Cotonou, Benin, E-mail: vincent.corbel@ird.fr.
References
- 1.World Health Organization . Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 2009. Document WHO/HTM/NTD/DEN/2009.1. [PubMed] [Google Scholar]
- 2.World Health Organization . Report of the Scientific Working Group on Dengue. Geneva: World Health Organization; 2006. Document WHO/TDR/SWG/08. [Google Scholar]
- 3.Erlanger TE, Keiser J, Utzinger J. Effect of dengue vector control interventions on entomological parameters in developing countries: a systematic review and meta-analysis. Med Vet Entomol. 2008;22:203–221. doi: 10.1111/j.1365-2915.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 4.Rawlins SC. Spatial distribution of insecticide resistance in Caribbean populations of Aedes aegypti and its significance. Rev Panam Salud Publica. 1998;4:243–251. doi: 10.1590/s1020-49891998001000004. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez MM, Bisset J, Ruiz M, Soca A. Cross-resistance to pyrethroid and organophosphorus insecticides induced by selection with temephos in Aedes aegypti (Diptera: Culicidae) from Cuba. J Med Entomol. 2002;39:882–888. doi: 10.1603/0022-2585-39.6.882. [DOI] [PubMed] [Google Scholar]
- 6.Thavara U, Tawatsin A, Kong-Ngamsuk W, Mulla MS. Efficacy and longevity of a new formulation of temephos larvicide tested in village-scale trials against larval Aedes aegypti in water-storage containers. J Am Mosq Control Assoc. 2004;20:176–182. [PubMed] [Google Scholar]
- 7.Brengues C, Hawkes NJ, Chandre F, McCarroll L, Duchon S, Guillet P, Manguin S, Morgan JC, Hemingway J. Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med Vet Entomol. 2003;17:87–94. doi: 10.1046/j.1365-2915.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- 8.Strode C, Wondji CS, David JP, Hawkes NJ, Lumjuan N, Nelson DR, Drane DR, Karunaratne SH, Hemingway J, Black WC IV, Ranson H. Genomic analysis of detoxification genes in the mosquito Aedes aegypti. Insect Biochem Mol Biol. 2008;38:113–123. doi: 10.1016/j.ibmb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Marcombe S, Poupardin R, Darriet F, Reynaud S, Bonnet J, Strode C, Brengues C, Yebakima A, Ranson H, Corbel V, David JP. Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: a case study in Martinique Island (French West Indies) BMC Genomics. 2009;10:494. doi: 10.1186/1471-2164-10-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcombe S, Carron A, Darriet F, Etienne M, Agnew P, Tolosa M, Yp-Tcha MM, Lagneau C, Yebakima A, Corbel V. Reduced efficacy of pyrethroid space sprays for dengue control in an area of Martinique with pyrethroid resistance. Am J Trop Med Hyg. 2009;80:745–751. [PubMed] [Google Scholar]
- 11.Jirakanjanakit N, Saengtharatip S, Rongnoparut P, Duchon S, Bellec C, Yoksan S. Trend of temephos resistance in Aedes (Stegomyia) mosquitoes in Thailand during 2003–2005. Environ Entomol. 2007;36:506–511. doi: 10.1603/0046-225x(2007)36[506:totria]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Martins AJ, Belinato TA, Lima JB, Valle D. Chitin synthesis inhibitor effect on Aedes aegypti populations susceptible and resistant to organophosphate temephos. Pest Manag Sci. 2008;64:676–680. doi: 10.1002/ps.1547. [DOI] [PubMed] [Google Scholar]
- 13.Lacey LA. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc. 2007;23:133–163. doi: 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Lima JB, de Melo NV, Valle D. Residual effect of two Bacillus thuringiensis var. israelensis products assayed against Aedes aegypti (Diptera: Culicidae) in laboratory and outdoors at Rio de Janeiro, Brazil. Rev Inst Med Trop Sao Paulo. 2005;47:125–130. doi: 10.1590/s0036-46652005000300002. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . Report of the Seventh WHOPES Working Goup Meeting. WHO/HQ, Geneva, December 2–4, 2003, Review of Vectobac WG, Permanet, Gokilath-S 5EC. Geneva: World Health Organization; 2004. Document WHO/CDS/WHOPES/2004.8. [Google Scholar]
- 16.World Health Organization . Report of the Ninth WHOPES Working Group Meeting. WHO/HQ, Geneva, December 5–9, 2005. Review of Dimilin GR and DT, Vectobac DT, Aqua K-Othrine, Aqua Reslin Super. Geneva: World Health Organization; 2006. Document WHO/CDS/NTD/WHOPES/2006.2. [Google Scholar]
- 17.Corriveau R, Philippon B, Yebakima A. La Dengue dans les Départements Français d'Amérique. Comment Optimiser la Lutte Contre Cette Maladie? IRD Édition. Paris: Expertise Collégiale; 2003. [Google Scholar]
- 18.Bill and Melinda Gates Foundation . Market Assessment for Public Health Pesticide Products: A Report by the Bill and Melinda Gates Foundation and the Boston Consulting Group. Seattle, WA: Bill and Melinda Gates Foundation; 2007. [Google Scholar]
- 19.Matsumura F. Studies on the action mechanism of benzoylurea insecticides to inhibit the process of chitin synthesis in insects: a review on the ststus of research activities in the past, the present and the future prospects. Pesticide Biochenistry and Physiology. 2010;97:133–139. [Google Scholar]
- 20.Thavara U, Tawatsin A, Chansang C, Asavadachanukorn P, Zaim M, Mulla MS. Simulated field evaluation of the efficacy of two formulations of diflubenzuron, a chitin synthesis inhibitor against larvae of Aedes aegypti (L.) (Diptera: Culicidae) in water-storage containers. Southeast Asian J Trop Med Public Health. 2007;38:269–275. [PubMed] [Google Scholar]
- 21.Copping LG, Menn JJ. Biopesticides: a review of their action, application and efficacy. Pest Manag Sci. 2000;56:651–676. [Google Scholar]
- 22.Salgado VL. The modes of action of spinosad and other insect control products. Down Earth. 1997;52:35–43. [Google Scholar]
- 23.Darriet F, Corbel V. Laboratory evaluation of pyriproxyfen and spinosad, alone and in combination, against Aedes aegypti larvae. J Med Entomol. 2006;43:1190–1194. doi: 10.1603/0022-2585(2006)43[1190:leopas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Dhadialla TS, Carlson GR, Le DP. New insecticides with ecdysteroidal and juvenile hormone activity. Annu Rev Entomol. 1998;43:545–569. doi: 10.1146/annurev.ento.43.1.545. [DOI] [PubMed] [Google Scholar]
- 25.Paul A, Harrington LC, Scott JG. Evaluation of novel insecticides for control of dengue vector Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2006;43:55–60. doi: 10.1603/0022-2585(2006)043[0055:EONIFC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization . Diflubenzuron in Drinking-Water: Use for Vector Control in Drinking-water Sources and Containers. Geneva: World Health Organization; 2008. Document WHO/HSE/AMR/08.03/6. [Google Scholar]
- 27.World Health Organization . Pyriproxyfen in Drinking-Water: Use for Vector Control in Drinking-water Sources and Containers. Geneva: World Health Organization; 2008. Document WHO/HSE/AMR/08.03/9. [Google Scholar]
- 28.World Health Organization . Spinosad DT in Drinking-Water: Use for Vector Control in Drinking-water Sources and Containers. Geneva: World Health Organization; 2010. Document WHO/HSE/WSH/10.01/12. [Google Scholar]
- 29.Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores AE, Fernandez-Salas I, Bisset J, Rodriguez M, McCall PJ, Donnelly MJ, Ranson H, Hemingway J, Black WC IV. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol Biol. 2007;16:785–798. doi: 10.1111/j.1365-2583.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Geneva: World Health Organization; 2005. Document WHO/CDS/WHOPES/GCDPP/13. [Google Scholar]
- 31.Yebakima A. Recherche sur Aedes aegypti et Culex pipiens en Martinique. Ecologie Larvaire, Résistance aux Insecticides, Application à la Lutte. Montpellier, France: Université Montpellier II; 1991. Thèse de Doctorat d'Etat es Sciences. [Google Scholar]
- 32.World Health Organization . Pesticides and their Application for the Control of Vectors and Pests of Public Health Importance. Sixth edition. Geneva: World Health Organization; 2006. WHO/CDS/NTD/WHOPES/GCDPP/2006.1. [Google Scholar]
- 33.World Health Organization . Report of the Eleventh WHOPES Working Group Meeting WHO/HQ. Geneva, December 10–13, 2007. Review of: Spinosad 7.48% DT, Netprotect, Duranet, Dawaplus, Icon Maxx. Geneva: World Health Organization; 2008. Document WHO/HTM/NTD/WHOPES/2008.1. [Google Scholar]
- 34.Abbott W. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. [Google Scholar]
- 35.Finney D. Probit Analysis. Cambridge, UK: Cambridge University Press; 1971. [Google Scholar]
- 36.Raymond M, Prato G, Ratsira D. Probit and Logit Analysis Program version 2.0. Montpellier, France: Praxème R&D; 1997. [Google Scholar]
- 37.World Health Organization . Bacillus thuringiensis israelensis (Bti) in Drinking-Water, Background Document for Development of WHO Guidelines for Drinking-Water Quality. Geneva: World Health Organization; 2009. Document WHO Revised Fourth Edition Bacillus thuringiensis_Bti_July272009_2. [Google Scholar]
- 38.World Health Organization . Report of the 4th WHOPES Working Group Meeting – IR3535, KBR3023, (RS)-Methoprene 20%EC, Pyriproxyfen 0.5%GR and Lambda-Cyhalothrin 2.5%CS. December 4–5, 2000, Geneva, 2001. Geneva: World Health Organization; 2001. Document WHO/CDS/WHOPES/2001.2. [Google Scholar]
- 39.Vythilingam I, Luz BM, Hanni R, Beng TS, Huat TC. Laboratory and field evaluation of the insect growth regulator pyriproxyfen (Sumilarv 0.5G) against dengue vectors. J Am Mosq Control Assoc. 2005;21:296–300. doi: 10.2987/8756-971X(2005)21[296:LAFEOT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Becker N, Petric D, Zgomba M, Boase C, Dahl C, Lane J, Kaiser A. Mosquitoes and Their Control. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 41.Sihuincha M, Zamora-Perea E, Orellana-Rios W, Stancil JD, Lopez-Sifuentes V, Vidal-Ore C, Devine GJ. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. J Med Entomol. 2005;42:620–630. doi: 10.1093/jmedent/42.4.620. [DOI] [PubMed] [Google Scholar]
- 42.Tilquin M, Paris M, Reynaud S, Despres L, Ravanel P, Geremia RA, Gury J. Long lasting persistence of Bacillus thuringiensis Subsp. israelensis (Bti) in mosquito natural habitats. PLoS ONE. 2008;3:e3432. doi: 10.1371/journal.pone.0003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bret BL, Larson LL, Schoonover JR, Sparks TC, Thompson GD. Biological properties of Spinosad. Down to Earth. 1997;52:6–13. [Google Scholar]
- 44.Darriet F, Duchon S, Hougard JM. Spinosad: a new larvicide against insecticide-resistant mosquito larvae. J Am Mosq Control Assoc. 2005;21:495–296. doi: 10.2987/8756-971X(2006)21[495:SANLAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 45.Macoris-Andrighetti MT, Cerone F, Rigueti M, Galvani KC, de Lourdes, da Graça M. Effect of pyriproxyfen in Aedes aegypti populations with different levels of susceptibility to the organophosphate temephos. Dengue Bull. 2008;32:186–198. [Google Scholar]
- 46.Kasai S, Shono T, Komagata O, Tsuda Y, Kobayashi M, Motoki M, Kashima I, Tanikawa T, Yoshida M, Tanaka I, Shinjo G, Hashimoto T, Ishikawa T, Takahashi T, Higa Y, Tomita T. Insecticide resistance in potential vector mosquitoes for West Nile virus in Japan. J Med Entomol. 2007;44:822–829. doi: 10.1603/0022-2585(2007)44[822:iripvm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Melo-Santos MA, Varjal-Melo JJ, Araujo AP, Gomes TC, Paiva MH, Regis LN, Furtado AF, Magalhaes T, Macoris ML, Andrighetti MT, Ayres CF. Resistance to the organophosphate temephos: mechanisms, evolution and reversion in an Aedes aegypti laboratory strain from Brazil. Acta Trop. 2010;113:180–189. doi: 10.1016/j.actatropica.2009.10.015. [DOI] [PubMed] [Google Scholar]


