Abstract
The Moroccan Health Ministry launched a Process of Eliminating Schistosomiasis in 1994. During 2005–2009, the epidemiologic status showed a clear interruption of disease transmission at the national level; only a few residual cases were recorded. Our present study is the first systematic serologic survey to evaluate the transmission status in remaining disease-endemic foci. A study population of 2,382 children born after the date of the last autochthonous cases were selected from provinces with histories of high schistosomiasis transmission (Tata, Chtouka Ait Baha, Errachidia, El Kelaa Des Sraghna, and Beni Mellal). To identify the presence of disease, specific antibodies directed against Schistosoma haematobium adult worm microsomal antigens were detected by using an enzyme-linked immunoelectrotransfer blot assay. The results showed an absence of antibodies in all serum samples. Consequently, our findings confirm either a low transmission status or an interruption of schistosomiasis transmission within the last disease endemic foci.
Introduction
Schistosomiasis is a worldwide public health problem affecting 200 million persons in third-world countries.1 The only form of schistosomiasis in Morocco is urinary schistosomiasis caused by Schistosoma haematobium.2 This disease has been endemic to rural areas in southern Morocco for decades, and the first cases were detected in 1914. The construction of large hydroagriculture projects beginning in 1967 resulted in the disease's spreading to new foci in the central and northern Morocco. The spread of schistosomiasis led to the implementation of an integrated control program (NPSC) in the early 1970s, which included a preparatory phase during 1976–1979, a pilot phase in three provinces during 1979–1981, and an operational phase in all areas beginning in 1982. The control strategy was based on four fundamental techniques: 1) disease control by diagnosis and treatment because screening and treatment were free; 2) transmission control and reduction of snail infestation by environmental modification and/or chemical mollusciciding, depending on the ecologic setting; 3) health education of the population to ensure cooperation and involvement in the control program; and 4) involvement of non-health sectors.
Diagnosis was based on the examination of sedimented urine, and a variety of screening methods were used: 1) mass detection by annual screening in schools and villages in all known transmission foci; 2) passive detection in health centers; 3) active detection by mobile teams in villages far removed from health centers; and 4) detection among household members of positive patients (contact tracking).
Treatment was mostly selective, although mass treatment was used in a few situations. Treatment options progressed from niridazole to metrifonate, but the introduction of praziquantel in 1987 was a major boost to the program. Used as a single dose (40 mg/kg), it reduced treatment failure rates, side effects, and cost.
Malacologic surveys to determine the level of snail infestation in various foci were carried out by traveling nurses and/or mobile teams once a month or once every two months. The preferred method of snail control was environmental modification, but chemical mollusciciding with niclosamide was also extensively used in certain areas.
Regular evaluation of the program was carried out by quarterly and/or annual reviews and was based on indicators such as the total number of cases, the percentage of positive cases, annual incidence rates, and the coverage rate. The number of parasitologically confirmed cases in 1982 was 6,582, representing a positivity rate of 6.2%. The exposed population by then was deemed to be 799,284 and the annual incidence rate was 8.2/1,000 population. These levels of infection and risk decreased each year, decreasing to 1,108 cases in 1994 (with a 2.1/1,000 incidence rate). Consequently, the number of affected localities decreased from 477 to 210. There was also a shift in age-specific infection patterns especially that school age children (7–14 years of age) accounted for 67% of cases in 1983 but only for 38% in 1996.3
Encouraged by these results, the NPSC upgraded its objectives in 1994 to the elimination of schistosomiasis infection and interruption of transmission by the year 2004, with different target dates set for each province.4 By 1999, elimination was achieved in 17 provinces. Only 231 cases were detected and 83% of them were from Tata, Chtouka Ait Baha, Taroudant, and Errachidia.5 Case detection and treatment remained the major control strategies, but, increasingly, these efforts were complemented by environmental snail control, community-based actions, and intersectoral collaboration.4 During 2005–2009, epidemiologic observations suggested that there was an interruption of transmission at the national level. No active focus of transmission was observed, despite intensified surveys within the at-risk provinces. Only 13 and 4 sporadic cases were detected in 2005 and 2006, respectively. Epidemiologic investigations conducted around these cases confirmed that nine cases were imported, and eight cases were residual cases that had not been detected during mass-survey operations.6
Diagnosis of Haematobium schistosomiasis is dependent on the detection of Schistosoma eggs in urine. However, because of low and sporadic egg production, the risk of not detecting infected persons is great. In 2008, many diagnostic techniques were developed to identify cases of schistosomiasis, including the detection of specific antibodies toward S. haematobium in different host bodily fluids. The adult worm microsomal antigens of Schistosoma haematobium (HAMA) have been identified as species-specific diagnostic antigens when used in the immunoblot assay for diagnosis of schistosomiasis.7 In our study, we examined the prevalence of human Haematobium schistosomiasis in a large cohort of persons less than 16 years old, who resided in previously disease-endemic areas to determine a possible recent interruption in transmission.
Materials and Methods
Study areas.
The following provinces in Morocco with histories of recent transmission of schistosomiasis were chosen: Tata, Chtouka Ait Baha, Errachidia, El Kelaa Des Sraghna, and Beni Mellal (Figure 1). In each province, we selected sectors and localities that represented foci of schistosomiasis transmission and where the last known cases of schistosomiasis were detected.
Figure 1.
Map of Morocco showing the selected study areas.
Tata.
This province is one of the oldest and largest foci of urinary schistosomiasis in Morocco. In 1983, 3,371 cases were detected in this province, and the incidence rate was 34.39/1,000 persons. In 2003, the prevalence was half of that detected in 1997 and accounted for 60% (75 cases) of the total number of cases detected at the national level.8 In this province, a prevalence of 1.5% and incidence of 8.5% were reported. Transmission occurred until 2004, when only 2 autochthonous cases were reported (Figure 2).6,9 One residual case was detected among 4,578 persons examined in 2005, and one residual case was detected among 8,623 people examined in 2006.6 For our study, we selected two sectors within the Tata province that had a history of high schistosomiasis incidence: the sector of Tata (locality of Agoujgal) and the sector of Akka (two localities, Rahala and Taourirt). The sector of Akka, first described by Connet in 1937, is situated in the lower Draa basin where agriculture depends almost entirely on a traditional irrigation scheme covering fields of approximately 1,530 hectares.10 The irrigation system consists of 10 springs with varying discharges emerging in the Akka River bed. The population in this palm oasis was estimated to be 13,000 persons. The region has a Saharan climate with cold, dry winters and hot, dry summers.11,12
Figure 2.
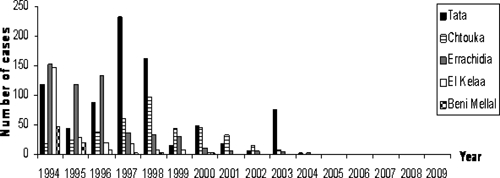
Annual distribution of urinary schistosomiasis cases in the past five disease- endemic areas in Morocco.
Chtouka Ait Baha.
This province is another old, endemic focus of urinary schistosomiasis where in 2001, 17% of the total number of cases of schistosomiasis detected nationally were found (32 cases), with a prevalence of 60% among persons more than 60 years old, 40% among persons 30–59 years old, and 10% among persons 16–29 years old.13 Transmission occurred until 2003, when 8 cases were identified (Figure 2).6
This province is located in the Anti-Atlas Mountains and has an area of 3,523 km2 and a population of 320,000. In our study, we selected the sector of Targa (localities of Laazite, Imzilene, Lmouda, Ait Abdelhak, and Tagadirt) because it was the most important focus of schistosomiasis in this province. This area is an oasis of mountains traversed by many water sources. The population in the oasis of Targa is estimated to be 6,552 persons. Agriculture depends almost entirely on traditional irrigation practices.
Errachidia.
This province was a large focus of schistosomiasis, where the prevalence of schistosomiasis was 50–60% in 1980–1981 among 2,000 persons. Eighty percent of cases were from the locality of Meski in the sector of Mdaghra and the locality of Azrou in the sector of Kheng. These localities are situated on Oued Ziz and were selected for our survey. Transmission was documented until 2004, when 1 case was recorded (Figure 2).6 In 2005, 2 cases were detected among 23,125 persons examined (one imported case and one residual case).6 The province of Errachidia is located in eastern Morocco and has an area of 59,585 km2 and a population of approximately 600,000 persons. The region has a desert climate, and agriculture depends almost entirely on traditional irrigation scheme. Water sources come from Oued Ziz, Oued Ghris, Oued Todra, and Oued Ferkla.2,14,15
El Kelaa Des Sraghna.
The schistosomiasis prevalence in this region was 16.2% in 1981 and 5% in 1982, and transmission continued until 2000, when the last case was identified (Figure 2).2–6 In 2006, one residual case was detected in 11,726 persons examined.6 This province is situated in the center of Morocco, within Wilaya of Marrakech.16 Its population is estimated to be approximately 775,000 persons in an area of 10,070 km2. It is traversed by Oued Lakhdar, Oued Tensift, and Oued Chdat. We selected two sectors with histories of high incidence: Zemrane (localities of Hachadiya and Charkiya), which has a population of 27,415 persons, and Laattaouiya (locality of Oued Marrek), which has a population of 20,237 persons and where schistosomiasis appeared after the construction of the Moulay Youssef Dam in 1970.17
Beni Mellal.
Beni Mellal is an old focus of schistosomiasis where the introduction of extensive irrigation into arid and semi-arid areas and the upgrading of older irrigation schemes accelerated the spread of the disease. Transmission occurred until the diagnosis of the last 2 cases in 2000 (Figure 2).6,18,19 Beni Mellal is a province with a population of 960,000 persons along the foot of the Middle Atlas Mountains. In our study, we selected two localities: Plan Agricol in the sector of Kourifat and Laasara in the sector of Bouaker, where urinary schistosomiasis was endemic with high incidence rates, especially in the irrigated agriculture areas.19
Study population.
The study population was composed of children 1–16 years old. A total of 2,382 persons were randomly selected from each selected locality. Primary school pupils were considered for this study because they were born after the date of the latest cases. Thus, if they were seropositive, they would serve as indicators of recent transmission. In addition, participation of school children and their parents has been known to be good.20–22 Data such as age, sex, address, urinary symptoms, and swimming practices were obtained from each participant or their parent by interview.
Blood collection.
Fingerstick blood samples were collected during May–June 2009. Blood spots were collected onto Nobuto filter paper, immediately transferred into the storage/extraction buffer StabilZyme (SurModics, Inc., Edina, MN) at a dilution of 1:10, and stored at 4°C as described by Handali and others.23 Blood spots were collected in each selected locality in Tata, Chtouka Ait Baha, Errachidia, El Kelaa Des Sraghna, and Beni Mellal.
Ethical considerations.
The study received institutional review board approval from the Moroccan Ministry of Health. The study team was taught to respect the community's willingness to participate, and consent was required for participation in the study. The privacy and safety of the participants were ensured, and informed consent was obtained from parents/guardians of the survey participants. Biological samples were collected under the norms and standards established by the Moroccan Ministry of Health.
Serologic detection of S. haematobium infections by using enzyme-linked immunoelectrotransfer blot.
Serologic analysis of samples was carried out in the Laboratory of Immuno-parasitology at the National Institute of Hygiene in Rabat, Morocco. Antibodies against Haematobium schistosomiasis were detected by an enzyme-linked immunoelectrotransfer blot (EITB) by using S. haematobium HAMA. The EITB strips were produced at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia.24,25 This assay has been shown to have a specificity of 99% and sensitivity of 99% for detection of S. haematobium-specific antibodies when confirmed S. haematobium cases from Malawi, Angola, Ghana, Kenya, Liberia, Madagascar, Mali, Namibia, Nigeria, South Africa, Saudi Arabia, and Sierra Leone were tested.26 The HAMA-EITB was conducted as described24,25,27 except that the blood sample diluted 1:20 in StabilZyme was further diluted to 1:5 in phosphate-buffered saline/0.3% Tween 20, 5% dried skim milk for evaluation, resulting in a final serum dilution of 1:100. Results were determined by visually observing reactions at the place of the diagnostic HAMA antigen (23 kD). Four positive serum samples were used as controls. Two were obtained from CDC and two were obtained from persons in Morocco previously infected with urinary schistosomiasis. The negative control, which had no antibodies against Schistosoma, was provided by CDC. To maximize the use of the available strips, serum samples were tested in batches, and the number of serum samples tested together was based on the expected prevalence in each age group. Serum samples were divided into three groups for batch testing: children less than 5 years old, 4 samples tested against 1 strip; children 5–12 years old, 2 samples tested against 1 strip; and persons more than 12 years old, 1 sample tested against 1 strip.
Results
Study characteristics.
A total of 2,382 children were surveyed; 52.6% (n = 1,253) were boys and 47.4% were girls. In the study, 23.1% (N = 551) of children were less than 5 years old, 42.4% (1,010) were 6–10 years old and 34% (810) were more than 11 years old. Most of the study population (99.9%) did not have clinical manifestations of schistosomiasis; only two children had hematuria. Fifty-one (2.14%) children reported a history of swimming in an irrigation channel.
Serologic analysis.
Of the 2,382 serum samples collected, none had antibodies against S. haematobium as measured by the HAMA-EITB (Table 1).
Table 1.
Distribution of children by age, sector, and serologic results in five study provinces in Morroco*
| Province | Sector | Locality | No. serum samples | Age group, years | EITB results | |||
|---|---|---|---|---|---|---|---|---|
| 0–5 | 6–10 | > 11 | No data | |||||
| Tata | Akka | Rahala | 210 | 36 | 91 | 83 | Negative | |
| Akka | Taourirt | 203 | 37 | 107 | 59 | Negative | ||
| Tata | Agoujgal | 216 | 56 | 95 | 63 | 2 | Negative | |
| Chtouka Ait Baha | Targa | Laazite, Imzilene, Lmoudaa | 211 | 2 | 124 | 84 | 1 | Negative |
| Targa | Aitabdelhak, Tagadirt | 208 | 24 | 102 | 82 | Negative | ||
| El Kelaa Des Sraghna | Zemrane | Hachadiya and Charkiya | 201 | 36 | 85 | 80 | Negative | |
| Diar | 61 | 4 | 24 | 33 | Negative | |||
| Attaouiya | Oued Marrek | 135 | 20 | 64 | 51 | Negative | ||
| Beni Mellal | Kourifat | Plan Agricol | 209 | 85 | 72 | 52 | Negative | |
| Bouaker | Laasara | 200 | 39 | 74 | 86 | 1 | Negative | |
| Errachidia | Kheng | Azrou | 210 | 97 | 64 | 48 | 1 | Negative |
| Mdaghra | Meski | 318 | 115 | 108 | 89 | 6 | Negative | |
| Total (%) | 2,382 (100) | 551 (23) | 1,010 (42) | 810 (34) | 11 (0.5) | (100) | ||
EITB = enzyme-linked immunoelectrotransfer blot.
Discussion
Measuring human infection rates is the current method for epidemiologic studies of schistosomiasis.28 To estimate the level of schistosomiasis transmission in Morocco, serologic detection was preferred for three reasons: current available serologic methods are exquisitely sensitive and specific; large numbers of samples can be obtained and tested quickly and inexpensively; and, the direct examination of urine for S. haematobium eggs is specific, but its sensitivity is highly limited by the sporadic fecundity of the adult worms and the low rate of recovery of parasitic ova from samples. Thus, patients with low egg counts can be misdiagnosed and can continue to transmit the disease.29 Urine examination is also slow, labor-intensive, and esthetically unpleasant.30 Moreover, a serologic test with a sensitivity of 95% and a specificity of 96–100% is a better predictor of prevalence than a single direct examination using 10 mL of urine. These arguments support the use of serologic detection to monitor the epidemiologic status of schistosomiasis in our study areas.27,31
There are two primary methods used for the serologic surveillance of schistosomiasis, the ELISA and the EITB. Typically, ELISAs use a crude antigen mixture, binding antibodies to any component, whether specific or non-specific, to produce a reaction. In contrast, the EITB uses components of the antigen mixture that are separated from each other by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and reactions to specific and non-specific antigen bands can be distinguished from each other. In some studies, screening using Falcon assay screening test–ELISA (FAST-ELISA) followed by MAMA-EITB has been used.7,32 In our study, EITB alone was selected for three reasons. The first reason is its high sensitivity of 99% and specificity of 99%. Because the FAST-ELISA uses S. mansoni MAMA antigens, it is only 90% sensitive for detection of S. haematobium, compared with its 99% sensitivity for detection of S. mansoni infections.32,33 No advantage in sensitivity or specificity was observed by using the FAST-ELISA in this setting. The second reason is that the EITB alone conserved HAMA antigen; the EITB required 5 ng of antigen per strip, and one strip was used to test up to four samples. In contrast, the FAST-ELISA would have used 200 ng of antigen/well, and only one sample could have been be tested per well. Finally, because the HAMA-EITB was used alone, only one test was performed. If the FAST-ELISA had been used as a screening assay followed by immunoblot confirmation, two tests would have to be performed. Thus, time saving was also realized.
In the Tata, Chtouka Ait Baha, Errachidia, El Kelaa des Sraghna, and Beni Mellal provinces, specific antibodies against S. haematobium were not detected in any cases in the study population. This study is our second in a series of studies to examine the current status of human Haematobium schistosomiasis in Morocco. The first study was carried out in 2001 in collaboration with the CDC in Atlanta, the diagnostics reference center at Cairo University, and our study team. Serum samples from approximately 1,500 persons of different ages were collected from three disease-endemic areas with low incidence: Targa Ntouchka, Oudaya, and Akka from Ait Baha, Marrakech, and Tata provinces, respectively. Samples were screened by using the FAST- ELISA and confirmed by using the S. haematobium EITB. In this previous study, a seroprevalence rate of 10% was found only in the Tata province where cases were limited to children less than 14 years of age.13 The present study showed the reduction to zero of the prevalence of schistosomiasis in all defined geographic areas that represents the last foci of the disease in Morocco.
The data presented are evidence that transmission of schistosomiasis has been interrupted in Morocco. These findings are the result of the effective and deliberate efforts of NPSC during which annual control activities were regularly performed during 1983–2007, and interventions were initiated in cases of reactivation of infection. These efforts included active detection in schools and localities; mass drug administration in disease-endemic localities in 1987, in Akka in 2004, and in Chtouka Ait Baha, Tata, and Larache in 2005 and 20066; annual surveillance of breeding sites and physical and chemical control of snails by using molluscocides; implementation of environmental controls with irrigation systems; intersectoral collaboration between the Ministries of Agriculture and the Interior; and education to prevent fecal contamination of affected and potential transmission sites.6 Another factor that impacted the schistosomiasis incidence rate was the rainfall deficit (> 20%), which occurred during 1990–2000 in Morocco and resulted in the natural drying of irrigation canals and a decrease in snail habitats. Furthermore, infrastructure developments, such as safe water supplies as a means of excreta disposal and an advanced primary health care system in rural zones, played crucial roles in schistosomiasis elimination.34,35 All of these factors contributed to reducing the prevalence and intensity of infection to a level of zero.
In conclusion, among 74 countries with schistosomiasis, only Japan, Puerto Rico, Tunisia, Sulawesi, and parts of China have eliminated schistosomiasis.36 During the past decade, the NPSC of Morocco made the deliberate decision to shift programmatic efforts from controlling the morbidity of infection to the ultimate interruption of transmission. The results of this study suggest that those efforts have been successful and that the transmission of S. haematobium from snails to human hosts has been interrupted.
ACKNOWLEDGMENTS
We thank the medical and technical personnel of Beni Mellal, Tata, Errachidia, El Kelaa Des Sraghna, and Chtouka Ait Baha provinces; Dr. Bouchra Delouane, Diouane Mohammed, Hraoui Abdelhak (Parasitology Department, National Institute of Hygiene, Rabat); and Haddou Nhammi (Direction of Epidemiology, Rabat) for assistance.
Footnotes
Financial support: This study was supported by the World Health Organization (Reference no. 2 008/237-0).
Authors' addresses: Mohamed Rhajaoui, Fatima Amarir, Bouchra El Mansouri, Hajiba Fellah, Faiza Sebti, and Lakranbi Mohammed, Department of Parasitology, National Institute of Hygiene, Avenue Ibn Battouta, Rabat, Morocco, E-mails: rhajaouimed@yahoo.fr, fatima_inh@yahoo.fr, mansouri.inh@hotmail.com, hajibafel@yahoo.fr, sfaiza3@yahoo.fr, and lakranbi@gmail.com. Sukwan Handali and Patricia Wilkins, Division of Parasitic Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: ahi0@cdc.gov and pma1@cdc.gov. Abderrahman Laamrani El Idrissi, Direction of Epidemiology and Control of Diseases, Rabat, Morocco, E-mail: laamrani55@gmail.com. Abderrahim Sadak, Department of Biology, University of Mohammed V, Rabat, Morocco, E-mail: sadakabderrahim@yahoo.fr.
References
- 1.Pontes LA, Dias-Neto E, Rabello A. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am J Trop Med Hyg. 2002;66:157–162. doi: 10.4269/ajtmh.2002.66.157. [DOI] [PubMed] [Google Scholar]
- 2.Doumenge JP, Mott KE. Global distribution of schistosomiasis: CEGET/WHO atlas. World Health Stat Q. 1984;37:186–199. [PubMed] [Google Scholar]
- 3.World Health Organization . Atlas of the Global Distribution of Schistosomiasis. Rabat, Morocco: CEGET-CNRS/OMS-WHO; 1987. 1987. [Google Scholar]
- 4.Laamrani H, Mahjour J, Madsen H, Khallaayoune K, Gryseels B. Schistosoma haematobium in Morocco: moving from control to elimination. Parasitol Today. 2000;16:257–260. doi: 10.1016/s0169-4758(00)01665-3. [DOI] [PubMed] [Google Scholar]
- 5.DELM Données épidémiologiques des maladies sous surveillance, Bilan année 1999. Bull Epidemiol Hebd. 1999:8. [Google Scholar]
- 6.DELM . Etat d'Avancement des Programmes de Lutte Contre les Maladies Parasitaires. Rapport Annuel d'Activités; 2005–2006. pp. 39–51. [Google Scholar]
- 7.Al-Sherbiny MM, Osman AM, Hancock K, Deelder AM, Tsang VC. Application of immunodiagnostic assays: detection of antibodies and circulating antigens in human schistosomiasis and correlation with clinical findings. Am J Trop Med Hyg. 1999;60:960–966. doi: 10.4269/ajtmh.1999.60.960. [DOI] [PubMed] [Google Scholar]
- 8.DELM Données épidémiologiques des maladies sous surveillance, Bilan année 2003. Bull Epidemiol. 2003:9. [Google Scholar]
- 9.DELM Données épidémiologiques des maladies sous surveillance, Bilan année 2004. Bull Epidemiol Hebd. 2004;57–60:30–31. [Google Scholar]
- 10.Connet M. Existence d'un foyer de bilharziose vesicale Akka (decouvert pour la premiere fois) Maroc Med. 1937;63:365–366. [Google Scholar]
- 11.Anonymous . Second Project de Developpement de la Petite et Moyenne Hydraulique (4-eme Tranche). Perimetre d'Akka. Rabat, Morocco: Ministry of Agriculture Direction de Amenagements Hydro Agricoles; 1996. [Google Scholar]
- 12.Boelee E, Laamrani H. Environmental control of schistosomiasis through community participation in a Moroccan oasis. Trop Med Int Health. 2004;9:997–1004. doi: 10.1111/j.1365-3156.2004.01301.x. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous . Report Inter Country Meeting on Strategies to Eliminate Schistosomiasis from the Eastern Mediterranean Region. Musat: Oman; 2007. [Google Scholar]
- 14.Brahim O. La Bilharziose Uro Genitale dans la Province d'Errachidia; Actualites et Perspective d'Avenir (1997 a 2006) Rabat, Morocco: Faculte de Pharmacie et Medicine de Rabat; 2008. [Google Scholar]
- 15.Laaziri M, Benouna M. Guide de la Lutte Contre la Bilharziose. Rabat, Morocco: Direction des Affaires Techniques, Ministere de la Sante Publique; 1982. [Google Scholar]
- 16.Nhammi H. Evaluation des Activities de Depistage des Cas de Bilharziose dans la Province de El Kelaa Des Sraghna. Rabat, Morocco: Institut National de l Administration de la Sante; Ministere de la Sante Publique; 1997. [Google Scholar]
- 17.Lahlou M, Berrada R. Etude comparative de l'infestation experimentale de deux souches de bulin schistosome au Maroc Tome. Bull Soc Fr Parasitol. 2001;25:19. [Google Scholar]
- 18.Camerlynck P, Alaoui A, Benmansour N. Epidemiologic study of Schistosoma haematobium (Bilharz 1852) schistosomiasis in Morocco. Maroc Med. 1974;54:641–649. [PubMed] [Google Scholar]
- 19.International Association for Medical Assistance to Travelers World Schistosomiasis Risk Chart. 2009. http://www.iamat.org/pdf/World_Schistosomiasis_Risk_Chart.pdf Available at. Accessed March 7, 2010.
- 20.Bundy DA, Hall A, Medley GF, Savioli L. Evaluating measures to control intestinal parasitic infections. World Health Stat Q. 1992;45:168–179. [PubMed] [Google Scholar]
- 21.Montresor A, Crompton DW, Hall A, Bundy DA, Savioli L. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level. Geneva: World Health Organization; 1998. [Google Scholar]
- 22.Uneke CJ, Oyibo PG, Ugwuoru CD, Nwanokwai AP, Iloegbunam RO. Urinary schistosomiasis among school age children in Ebonyi State, Nigeria. The Internet Journal of Laboratory Medicine 2. 2007. http://www.ispub.com/journal/the_internet_journal_of_laboratory_medicine/volume_2_number_1_31/article/urinary_schistosomiasis_among_school_age_children_in_ebonyi_state_nigeria.html Available at. Accessed October 8, 2010.
- 23.Handali S, Rodriguez S, Noh J, Gonzalez AE, Garcia HH, Gilman RH, Roberts JM, Hancock K, Tsang VC. A simple method for collecting measured whole blood with quantitative recovery of antibody activities for serological surveys. J Immunol Methods. 2007;320:164–171. doi: 10.1016/j.jim.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Tsang VC, Hancock K, Maddison SE, Beatty AL, Moss DM. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA) J Immunol. 1984;132:2607–2613. [PubMed] [Google Scholar]
- 25.Tsang VC, Tsang KR, Hancock K, Kelly MA, Wilson BC, Maddison SE. Schistosoma mansoni adult microsomal antigens, a serologic reagent. I. Systematic fractionation, quantitation, and characterization of antigenic components. J Immunol. 1983;130:1359–1365. [PubMed] [Google Scholar]
- 26.Tsang VC, Wilkins PP. Immunodiagnosis of schistosomiasis. Immunol Invest. 1997;26:175–188. doi: 10.3109/08820139709048925. [DOI] [PubMed] [Google Scholar]
- 27.Cetron MS, Chitsulo L, Sullivan JJ, Pilcher J, Wilson M, Noh J, Tsang VC, Hightower AW, Addiss DG. Schistosomiasis in Lake Malawi. Lancet. 1996;348:1274–1278. doi: 10.1016/S0140-6736(96)01511-5. [DOI] [PubMed] [Google Scholar]
- 28.Hamburger J, Hoffman O, Kariuki HC, Muchiri EM, Ouma JH, Koech DK, Sturrock RF, King CH. Large-scale, polymerase chain reaction-based surveillance of Schistosoma haematobium DNA in snails from transmission sites in coastal Kenya: a new tool for studying the dynamics of snail infection. Am J Trop Med Hyg. 2004;71:765–773. [PubMed] [Google Scholar]
- 29.Sturrock RF. Schistosomiasis epidemiology and control: how did we get here and where should we go? Mem Inst Oswaldo Cruz. 2001;96((Suppl)):17–27. doi: 10.1590/s0074-02762001000900003. [DOI] [PubMed] [Google Scholar]
- 30.Hancock K, Tsang VC. Development and optimization of the FAST-ELISA for detecting antibodies to Schistosoma mansoni. J Immunol Methods. 1986;92:167–176. doi: 10.1016/0022-1759(86)90162-6. [DOI] [PubMed] [Google Scholar]
- 31.Ramzy RM, Hillyer GV. Evaluation of an ELISA for the diagnosis of human infection with S. haematobium. J Egypt Soc Parasitol. 1993;23:315–322. [PubMed] [Google Scholar]
- 32.Hillyer GV, Tsang VC, Vivas-Gonzalez BE, Noh J, Ahn LH, Vorndam V. Age-specific decrease in seroprevalence of schistosomiasis in Puerto Rico. Am J Trop Med. 1999;60:313–318. doi: 10.4269/ajtmh.1999.60.313. [DOI] [PubMed] [Google Scholar]
- 33.Al-Sherbiny MM, Osman AM, Hancock K, Deelder AM, VC Tsang. Application of immunodiagnostic assays: detection of antibodies and circulating antigens in human schistosomiasis and correlation with clinical findings. Am J Trop Med Hyg. 1999;60:960–966. doi: 10.4269/ajtmh.1999.60.960. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization The control of schistosomiasis. Second report of the WHO Expert Committee. World Health Organ Tech Rep Ser. 1993;830:1–86. [PubMed] [Google Scholar]
- 35.Laamrani H, Khallaayoune K, Madsen H, Mahjour J, Gryseels B. New challenges in schistosomiasis control in Morocco. Acta Trop. 2000;77:61–67. doi: 10.1016/s0001-706x(00)00114-5. [DOI] [PubMed] [Google Scholar]
- 36.EMRO Report of Schistosomiasis . Inter-Country Meeting on Strategies to Eliminate Schistosomiasis from the Eastern Mediterranean Region. Muscat, Oman: 2007. November 6–8, 2007. [Google Scholar]


