Abstract
A new genotype of spotted fever group Rickettsia (SFGR) was identified in Rhipicephalus turanicus from eastern Sicily. On the basis of current molecular criteria, the genetic characteristics obtained from multiple locus sequence typing satisfy the requirements for Candidatus status of this SFGR. Further detection and identification of this SFGR during entomological and clinical surveys will be required to establish the prevalence of this Rickettsia and its potential pathogenicity for humans.
Sicily, the largest island in the Mediterranean Sea, is central to the Mediterranean endemic area of boutonnese fever, also called Mediterranean spotted fever (MSF), which is transmitted by the brown dog tick, Rhipicephalus sanguineus. The number of reported clinical cases of rickettsioses in Sicily is 9.3 for every 100,000 inhabitants compared with the overall Italian average of 1.6.1 Almost all cases of rickettsioses reported in Italy are diagnosed clinically and serologically and are assumed to be cases of MSF. However, molecular studies of tick and clinical samples in 1989 established that multiple genetic types of spotted fever group Rickettsia (SFGR) exist in Sicily.2 More recent reports indicate that a diversity of rickettsial agents are also present in ticks from other parts of Italy. In particular, besides Rickettsia conorii, the classic agent of MSF, R. conorii subsp. israelensis was detected in Rh. sanguineus and Rickettsia massiliae was retrospectively identified as the cause of a human infection that occurred in 1986 in Palermo Province of Sicily.3,4 Subsequent entomological surveys provided further evidence that Rickettsia slovaca, Rickettsia aeschlimannii, and Rickettsia africae or related potentially pathogenic Rickettsia also occur with high prevalence in a variety of ticks from the southern region of the island and might contribute to the rickettsial cases diagnosed in Sicily.5 A number of different species of ixodid ticks from the region feed on dogs, domestic and peridomestic and wild animals but can also bite people and thus transmit any rickettsial agents they carry. Here, we report the detection of a novel SFGR in a Rhipicephalus turanicus tick collected in eastern Sicily.
The Tick-Borne Disease Laboratory of the Entomological Sciences Program of the U.S. Army Public Health Command operates the Department of Defense (DOD) Human Tick Test Kit Program (HTTK), the identification and testing service for ticks removed from military personnel, dependents, and DOD civilian employees. Participation in the HTTK is restricted to ticks submitted from the continental United States; ticks removed from United States personnel in Europe are generally sent to the Department of Medical and Veterinary Entomology, U.S. Army Public Health Region—Europe, Landstuhl, Germany under a similar program. However, in June 2009, a tick was removed from an asymptomatic 22-month-old female Navy dependent at Naval Hospital Sigonella, Sicily, Italy and sent to the HTTK; testing proceeded at this laboratory to expedite reporting results to the patient's health care provider.
The tick was identified as a male of the Rh. sanguineus group using standard taxonomic keys.6 The DNA isolation was performed using the Zymo Genomic DNA II Kit (Orange, CA) according to the manufacturer's instructions. Purified DNA was eluted with 35 μL of highly purified water and kept refrigerated. The DNA from this tick sample was then tested in a quantitative real-time polymerase chain reaction (qPCR) assay in a Roche LightCycler (Roche Applied Science, Indianapolis, IN) using primers and probe targeting a conserved fragment of ompB of the SFGR.7 Two µL of tick DNA was used as template; cycling conditions consisted of an initial 3 min denaturation at 94°C, followed by 50 amplification cycles of 94°C for 5 s (20°C/s slope), and 60°C for 30 s (20°C/s slope, single acquisition mode).
The tick sample DNA (T090960) tested positive in the ompB qPCR assay (Ct 25.85) for SFGR and was sent to the Rickettsial Zoonoses Branch, Centers for Disease Control and Prevention (CDC), Atlanta, GA, for further identification.
Molecular identification of the tick species was performed by sequencing of a fragment of its 12S mitochondrial ribosomal RNA (rRNA) gene as described previously.8 This nucleotide sequence (NCBI GenBank accession no. HM014442) had 99% BLAST sequence similarity with a homologous fragment from Sicilian Rh. turanicus (AM410571), phylogenetically in the same group as Rh. turanicus ticks from Zimbabwe, Israel, and other countries. Multiple locus typing of Rickettsia gene fragments included 381-bp of gltA, 532-bp of ompA, 749-bp of ompB, and 928-bp of sca4, as described previously.9 The amplicons were purified using the Wizard SV gel and PCR clean-up system (Promega, Madison, WI) and sequenced using the BigDye Terminator v3.1 cycle sequencing kit (ABI, Foster City, CA) according to the manufacturer's recommendations on an ABI 3130xl genetic analyzer. Sequencing reads were assembled using Sequencher 4.8 (Gene Codes, Ann Arbor, MI). Primer sequences were removed and homologous sequences were detected using the National Center for Biotechnology Information (NCBI, Bethesda, MD) Basic Local Alignment Sequence Tool (BLAST) search engine. Homologous sequences of formally named SFGR species and Candidatus species that were downloaded from NCBI GenBank, sequences were concatenated, aligned using ClustalW, and a phylogenetic tree was drawn using MEGA4.0 software.10
The fragment of gltA (HM014438) was the most conserved sequence with 99% BLAST similarity to all the core SFGR (> 50 sequences) with at least 1 to 3 single-nucleotide polymorphisms (SNPs) within 323 nucleotides sequenced. Its ompA (479 nt, HM014439) shared no more than 95–96% BLAST sequence similarity with its nearest SFGR (≥ 15 SNPs), ompB (779 nt, HM014441) had 94–95% similarity (≥ 36 SNPs including 10 INDELs), and sca4 (711 nt, HM014440) had 97–98% sequence similarity (≥ 10 SNPs). Individual phylogenetic trees (not shown) constructed for each gene had a similar branch structure to the concatenated tree (Figure 1); the Sicilian SFGR had a separate lineage between the Rickettsia rickettsii and Rickettsia japonica clusters.
Figure 1.
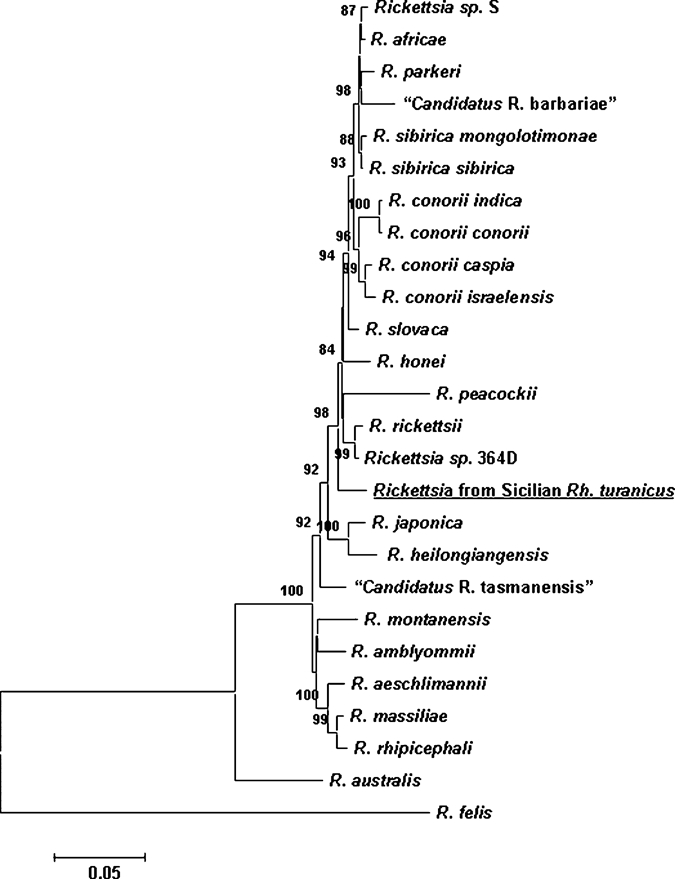
The phylogenetic position of T090960 spotted fever group Rickettsia (SFGR) from Rhipicephalus turanicus. The phylogenetic position was inferred using the neighbor-joining method. The optimal tree with the sum of branch length = 0.76774834 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches; only bootstrap values of 80 ≥ are shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method as base substitutions per site. All positions containing gaps, missing data, and primer sequences were eliminated from the dataset. Four genes were concatenated (gltA-ompA-sca4-ompB), and a total of 2,044 positions were analyzed. Phylogenetic analyses were conducted with MEGA4.10
The SFGR detected in this Sicilian Rh. turanicus exhibited the minimal current requirement for identification as a new species: ≤ 99.9% for gltA, ≤ 98.8%, ≤ 99.2%, and ≤ 99.3% for the ompA and ompB genes and gene D, respectively, to its closest SFGR relative.11 However, at this point we cannot propose a formal species description because only a single specimen was analyzed. The organism also needs to be isolated, grown, and deposited in several reference collections before naming, and until this has occurred, it can only be assigned a Candidatus status, namely “Candidatus R. siciliensis.” This previously uncharacterized Rickettsia belongs to the core group of SFGR, which is largely composed of human pathogens. Mura and others12 reported detection of “Candidatus R. barbariae” in Rh. turanicus from Sardinia where it was found in 4 out of 24 ticks tested. The later SFGR belongs to the R. africae–Rickettsia parkeri lineage, and is significantly different from the genotype of SFGR reported here.
Although Rh. turanicus is present on both Sicily and Sardinia, it is not known whether their tick populations are significantly divergent because of geographic separation or there are periodic exchanges between them related to human traffic and transportation of livestock and dogs and cats between the islands and the mainland. It is possible that completely unique populations of SFGR and ticks may be present on each island. Although known as a sheep tick, Rh. turanicus can be often found on dogs and cats, and on people.6 Furthermore, in a suitable climate this tick can establish suburban biotopes, hence increasing its opportunity to feed on people and companion animals.13 Rhipicephalus turanicus has been implicated as a vector of several human and veterinarian pathogens, including Babesia, Theileria, Anaplasma phagocytophilum, and R. massiliae.4,12 Rickettsia massiliae exhibits efficient transstadial and transovarial transmission in Rh. turanicus14; however, because other SFGR of undetermined human pathogenicity are also present in the area, it is unknown if several SFGR may co-circulate in the same area or there is a preferential association with Rh. turanicus or interference between them similarly as for R. rickettsii and Rickettsia peacockii in Dermacentor andersoni.15 Consequently, the disease ecology of spotted fever rickettsioses in different parts of Italy may be quite complex.
ACKNOWLEDGMENTS
We thank Ariana Salazaar and Kathryn Dirks for assistance with PCR and sequence reactions, and Gregory A. Dasch for review of the manuscript and helpful discussion.
Disclaimer: The findings and conclusions are those of the authors and do not necessarily reflect the views of the U.S. Department of Health and Human Services (MEE). The views expressed in this article are those of the author and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government (EYS).
Footnotes
Authors' addresses: Marina E. Eremeeva, Rickettsial Zoonoses Branch, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: MEremeeva@cdc.gov. Ellen Y. Stromdahl, Entomological Sciences Program, U.S. Army Public Health Command, Aberdeen Proving Ground, MD, E-mail: Ellen.Stromdahl@us.army.mil.
References
- 1.Ciceroni L, Pinto A, Ciarrocchi S, Ciervo A. Current knowledge of rickettsial diseases in Italy. Ann NY Acad Sci. 2006;1078:143–149. doi: 10.1196/annals.1374.024. [DOI] [PubMed] [Google Scholar]
- 2.Tringali G, Regnery R, Sferlazzo A, Intonazzo V, Spruill C, Perna AM, La Rosa G. Epidemiology of boutonneuse fever in western Sicily: demonstration of multiple spotted fever group-Rickettsiae subtypes. Microbiologica. 1989;12:189–194. [PubMed] [Google Scholar]
- 3.Giammanco G, Mansueto S, Ammatuna P, Vitale G. Israeli spotted fever Rickettsia in Sicilian Rhipicephalus sanguineus ticks. Emerg Infect Dis. 2003;9:892–893. doi: 10.3201/eid0907.030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale G, Mansuelo S, Rolain J-M, Raoult D. Rickettsia massiliae human isolation. Emerg Infect Dis. 2006;12:174–175. doi: 10.3201/eid1201.050850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beninati T, Genchi C, Torina A, Caracappa S, Bandi C, Lo N. Rickettsiae in ixodid ticks, Sicily. Emerg Infect Dis. 2005;11:509–511. doi: 10.3201/eid1103.040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker JB, Keirans JE, Horak IG. A Guide to the Brown Ticks of the World. Cambridge, UK: Cambridge University Press; 2000. (The genus Rhipicephalus (Acari, Ixodidae)). [Google Scholar]
- 7.Blair P, Jiang J, Schoeler G, Moron C, Anaya E, Cespedes M, Cruz C, Felices V, Guevara C, Mendoza L, Villaseca P, Sumner J, Richards A, Olson J. Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J Clin Microbiol. 2004;42:4961–4967. doi: 10.1128/JCM.42.11.4961-4967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beati L, Keirans JE. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol. 2001;87:32–48. doi: 10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Eremeeva M, Bosserman E, Demma L, Zambrano M, Blau D, Dasch G. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl Environ Microbiol. 2006;72:5569–5577. doi: 10.1128/AEM.00122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 11.Fournier P-E, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–5465. doi: 10.1128/JCM.41.12.5456-5465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mura A, Masala G, Tola S, Satta G, Fois F, Piras P, Rolain JM, Raoult D, Parola P. First direct detection of rickettsial pathogens and a new Rickettsia, ‘Candidatus Rickettsia barbariae', in ticks from Sardinia, Italy. Clin Microbiol Infect. 2008;14:1028–1033. doi: 10.1111/j.1469-0691.2008.02082.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilot B, Jarry D, Pautou G, Moncada E. Biotopes suburbains à Rhipicephalus turanicus (Pomerancev, Matikasvili, Lototzki, 1940). (Acarina, Ixodoidea): étude préliminaire. Ann Parasitol Hum Comp. 1977;52:353–362. doi: 10.1051/parasite/1977523353. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto K, Ogawa M, Brouqui P, Raoult D, Parola P. Transmission of Rickettsia massiliae in the tick, Rhipicephalus turanicus. Med Vet Entomol. 2005;19:263–270. doi: 10.1111/j.1365-2915.2005.00569.x. [DOI] [PubMed] [Google Scholar]
- 15.Burgdorfer W, Hayes SF, Mavros AJ. In: Rickettsiae and Rickettsial Diseases. Burgdorfer W, Anacker RL, editors. New York: Academic Press, Inc; 1981. pp. 585–594. (Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii). [Google Scholar]


