Abstract
In the past decades, cases of canine ocular onchocercosis have been reported worldwide, particularly in the United States and Europe. Onchocerca lupi, originally described from a wolf, has been implicated in some of these cases, and its zoonotic role has been hypothesized on the basis of the reexamination of two cases of human ocular onchocerciasis. In the present study, we describe, for the first time, the occurrence of O. lupi in the subconjunctival region of the human eye in a patient from Turkey. The nematode was identified as O. lupi based on its morphology and molecular phylogenetic analysis of partial cox1 and 12S ribosomal DNA genes. The results suggest that O. lupi should be considered in the differential diagnosis of other eye parasitic infections in humans. The role of dogs as natural hosts of O. lupi and the vectors of this zoonotic parasite need to be investigated.
Introduction
Human ocular parasitic infections may be caused by a range of nematodes, including ascarids and strongylids (ocular larva migrans), thelaziids (eye worm infection), and filarioids.1–3 These parasitic nematodes may infect, at their adult and/or larval stages, the human eyelids, conjunctival sacs, lachrymal glands, and in some cases, the ocular globe. Human zoonotic filariases are mostly caused by species belonging to the genera Dirofilaria, Onchocerca, and Brugia, which are transmitted by blood-feeding insects.1 For example, blackflies (Simulium spp.) and/or biting midges (Culicoides spp.) serve as intermediate hosts of Onchocerca spp. nematodes.4 Among the filarioid Onchocercidae, O. volvulus causes the so-called river blindness, a parasitic disease affecting about 37 million people globally.5 This human parasitosis induces visual impairment and blindness as an effect of the host immune response to microfilariae released by female adult worms in the subcutaneous tissues; it is endemic in East and West Africa as well as Central and South America.6 In addition, 15 clinical cases of zoonotic onchocerciasis have been reported worldwide,7,8 and they have been attributed to four nematode species: O. gutturosa1 and O. cervicalis (from cattle and horses, respectively),9 O. jakutensis (from the European deer in Austria),10 and O. dewittei japonica (from wild boar in Japan).8 The above-mentioned zoonotic Onchocerca spp. were found mostly in the subcutaneous tissues, and only O. gutturosa and O. cervicalis presented an ocular localization.
In the past decade, cases of canine ocular onchocercosis have been increasingly reported worldwide, particularly in the United States11–13 and Southern (Greece and Portugal) and Central (Germany, Hungary, and Switzerland) Europe.14–17 Although the identity of the parasite in most cases remained obscure, the disease is often characterized by acute or chronic ocular disease characterized by conjunctivitis, photophobia, lacrimation, ocular discharge, and exophthalmia.7 Incidentally, O. lupi, originally described from a wolf (Canis lupus), has been implicated in some cases of canine ocular onchocercosis in Europe.14,17,18 The zoonotic role of this parasitic species has only been hypothesized in two cases of human ocular onchocerciasis from the Crimean region19 and Albania.20 In the two aforementioned cases, O. lupi was suggested as the etiological agent on the basis of the similarities with the clinical presentation of canine ocular onchocercosis (e.g., target tissues, lesion presentation, and area of provenience of the patients). However, to date, there is neither morphological nor molecular evidence supporting the zoonotic role of O. lupi. The present study reports a case of ocular onchocerciasis in a human patient from an area of Turkey where cases of canine onchocercosis have never been reported. Detailed morphological and molecular analyses lead us to diagnose the first zoonotic human case of O. lupi ocular infection.
Case Report
An 18-year-old girl was presented at the Department of Ophthalmology of the Medical Faculty of Trakya University with a 6-day history of painless redness in the left eye. The patient lived in Edirne (41°40′N, 26°34′E; about 50 m above sea level) and Istanbul (Turkey) and had never traveled abroad or in other areas of Turkey. She referenced a painful fly-bite history on her left upper lid about 30 days before onset of symptoms during the evening (around 5:00 pm) while she was in Edirne. On the ophthalmologic examination, an approximately 3.5 × 5 × 1.5-mm subconjunctival mass was detected on the superonasal quadrant of bulbar conjunctiva. The patient had corrected visual acuity of 10/10 in both eyes, with normal anterior segments and intraocular pressure of 12 mmHg in both eyes. The patient did not show changes in the right fundus, and the left fundus revealed a convex retinal appearance in the superonasal quadrant. Magnetic resonance imaging was required for differential diagnosis.
Twenty-eight days after the first visit, the patient complained of pain in the left eye. Biomicroscopic examination revealed the presence of a foreign body in the subconjunctival mass (Figure 1). After topical anesthesia, the eye was opened with a blefarosta, and the nematode was extracted with a forceps. A nematode was detected and removed from the subconjunctival mass, but the anterior end of the parasite was accidentally cut during its removal (Figure 2A). The patient was examined 5 months after the last visit. Biomicroscopic and ophthalmoscopic examinations were normal, and no nematode was seen in the anterior chamber or vitreous cavity.
Figure 1.
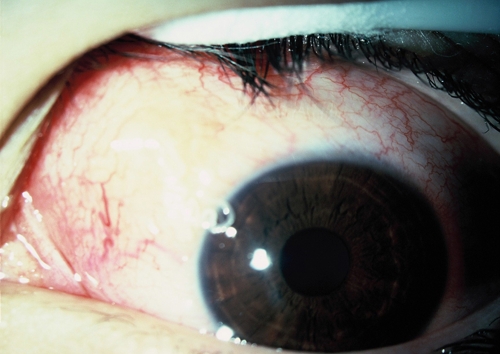
Ocular onchocerciasis. Episcleral hyperemia and subconjunctival mass on the superonasal quadrant of bulbar conjunctiva. This figure appears in color at www.ajtmh.org.
Figure 2.
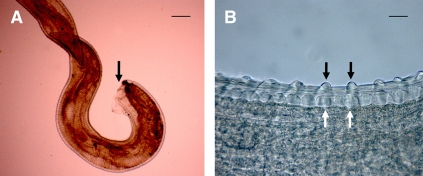
Female O. lupi. (A) Macroscopic view of the nematode removed from the subconjunctival mass, with an arrow pointing to the damaged end (Scale bar = 1,000 µm). (B) Thick and multilayered cuticle bearing prominent annular ridges (white arrow) on the external surface and typical transverse striae (black arrow) in the internal layer (Scale bar = 60 µm). This figure appears in color at www.ajtmh.org.
The nematode was stored in 70% ethanol and sent to the Unit of Parasitology at the Faculty of Veterinary Medicine, University of Bari, Italy, for species identification. The nematode species was identified according to morphological keys.21,22 Because the nematode was cut and internal organs collapsed, some distinctive characters (e.g., length of esophagus and distances of the nerve ring and the vulva from the anterior end) were not available. Thus, a molecular diagnosis was performed to confirm the morphological identification. A small piece of the nematode (about 3 mm) was used for genomic DNA extraction, and partial cox1 and 12S ribosomal DNA (rDNA) gene fragments were amplified as described elsewhere.23,24 Sequences were compared with those available in the GenBank database by Basic Local Alignment Search Tool (BLAST) analysis. A phylogenetic analysis of cox1 and 12S sequences was conducted by MEGA 4.025 under minimum evolution methods using 2,000 replicates bootstrap values, and Thelazia spp. was used as the out-group. Sequences were also conceptually translated into amino acid sequences using the invertebrate mitochondrial genetic code (MEGA 4.0), and variable, informative, and singleton sites occurring in the amino acid sequences and all DNA regions herein examined were also evaluated. A small portion of the parasite was deposited at the Muséum National d'Histoire Naturelle, Parasitologie Comparée, Paris, France (MNHN; accession number 184 YU).
The cut parasite measured 4.8 mm in length and 340 µm in width. The nematode presented a thick cuticle (20–80 µm) composed of an external layer bearing prominent, undulated annular ridges (distance from 30 to 70 µm) and an internal layer with transverse striae (Figure 2B). These characteristics are typical of female filarioids belonging to the Onchocerca genus. In addition, the presence of two transverse striae per each outer ridge interval, the prominent shape of ridges, and the ratio of the body diameter to the distances between ridges (i.e., 7–10:1) were strongly suggestive of O. lupi.21,22
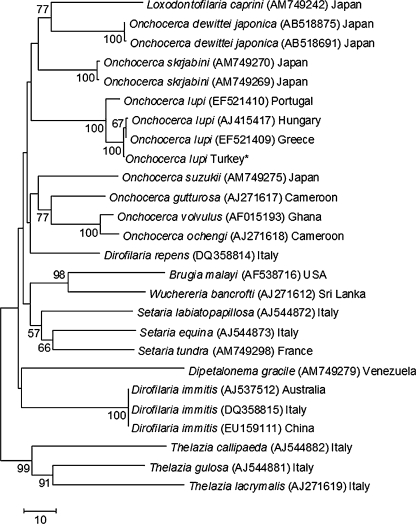
In accordance with the morphological identification, the BLAST analysis of cox1 and 12S rDNA genes showed a 99% nucleotide homology with sequences of O. lupi available in GenBank (12S rDNA: GU365879; cox1: AJ415417, EF521409, and EF521410). In addition, the phylogenetic analysis using cox1 sequences confirmed that the specimen here examined clustered with O. lupi from Hungary (AJ415417), Greece (EF521409), and Portugal (EF521410) with high bootstrap values (Figure 3). Similarly, in the phylogenetic analysis using 12S rDNA sequences (data not shown), our sequence clustered with O. lupi from Portugal (GU365879).
Figure 3.
Phylogeny of filarioid onchocercidae based on cox1 gene sequences. Numbers in parentheses are Genbank accession numbers.
The comparisons among cox1 sequences revealed 13 nucleotide variations, most of them (N = 12) being singletons occurring at the third (N = 11) and first (N = 1) codon position and only one parsimony site at the third codon position. The cox1 sequences of O. lupi had an A + T content of ~64.1%. The pair-wise comparisons over a total of 649 common sites of all O. lupi sequences available in GenBank showed differences between 0.2% (EF521409) and 1.7% (EF521410), with an overall difference of 1%. The translation at the second codon position of the cox1 sequence led to 216 amino acids without stop codons. The 12S rDNA sequences of O. lupi had an A + T content of ~79.5%. The comparison between sequences generated here and those available in GenBank (GU365879) showed only two T insertions occurring at 21 and 67 position sites.
Discussion
This study represents the first evidence of human zoonotic infection by O. lupi based on both morphological and molecular identification. In a previous study, the zoonotic role of this parasite was hypothesized,19 but a definitive etiological diagnosis was not provided. In the present report, the measurements of the body fragment and the morphological characteristics of the cuticle showed that the worm was a female of O. lupi. This indicates that O. lupi may develop in humans and play a role as a zoonotic parasite. The host range of Onchocerca species is narrow,4 and patent Onchocerca infections are usually seen in hosts closely related to the natural host (e.g., O. volvulus, which parasitizes humans, may also infect chimpanzees).1,26 However, the evidence of some parasites infecting eyes of both humans and dogs (e.g., Thelazia callipaeda and Dirofilaria immitis)27 also suggests that these zoonotic infections occur when zoologically distant hosts share the same environment.
Interestingly, canine ocular onchocercosis by O. lupi has never been diagnosed in Turkey, and thus, this case represents not only the first report of this species in a human patient but also the first report of this parasite in this country. Indeed, a single case of subcutaneous onchocerciasis by O. volvulus was recorded in Turkey in a 64-year-old man who traveled to Saudi Arabia for pilgrimage.28
O. lupi is unique among the 34 species of the genus29 in that it is a parasite of canids, whereas ungulates are hosts for the other species, except O. volvulus, the agent of the river blindness in humans. Our results suggest that O. lupi should be considered in the differential diagnosis of other eye parasitic infections of humans. Because little is known about the natural history of O. lupi, further studies are needed to clarify the suitability of dogs as natural hosts and spreaders of this potentially zoonotic parasite. Again, additional molecular analyses of Onchocerca worms from dogs are fundamental to determine the etiology of canine ocular onchocercosis in the United States and elsewhere in Europe.
ACKNOWLEDGMENTS
The American Committee on Clinical Tropical Medicine and Travelers' Health (ACCTMTH) assisted with publication expenses.
Footnotes
Authors' addresses: Domenico Otranto, Gabriella Testini, Riccardo P. Lia, and Filipe Dantas-Torres, Dipartimento di Sanità Pubblica e Zootecnia, Università Degli Studi di Bari, Valenzano (Bari), Italy, E-mails: d.otranto@veterinaria.uniba.it, g.testini@veterinaria.uniba.it, r.p.lia@veterinaria.uniba.it, and f.dantastorres@veterinaria.uniba.it. Nermin Sakru, Department of Microbiology and Clinical Microbiology, Trakya University, Edirne, Turkey, E-mail: nsakru@yahoo.com. Vuslat P. Gürlü and Konuralp Yakar, Department of Ophtalmology, Trakya University, Edirne, Turkey, E-mails: gurluvuslat@yahoo.com and alpyakar@yahoo.com. Odile Bain, Muséum National d'Histoire Naturelle, UMR 7205, Parasitologie Comparée, Paris, France, E-mail: bain@mnhn.fr.
References
- 1.Orihel TC, Eberhard ML. Zoonotic filariasis. Clin Microbiol Rev. 1998;11:366–381. doi: 10.1128/cmr.11.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisella PJ, Assaraf E, Rossaza C, Limon S, Baudouin C, Richard-Lenoble D. Conjunctivitis and ocular parasitic diseases. J Fr Ophtalmol. 1999;22:585–588. [PubMed] [Google Scholar]
- 3.Shen J, Gasser RB, Chu D, Wang Z, Yuan X, Cantacessi C, Otranto D. Human thelaziosis—a neglected parasitic disease of the eye. J Parasitol. 2006;92:872–875. doi: 10.1645/GE-823R.1. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RC. Nematode Parasites of Vertebrates—Their Development and Transmission. New York: CAB International; 2000. pp. 417–422. [Google Scholar]
- 5.Basáñez MG, Pion SD, Churcher TS, Breitling LP, Little MP, Boussinesq M. River blindness: a success story under threat? PLoS Med. 2006;3:e371. doi: 10.1371/journal.pmed.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards FO, Boatin B, Sauerbrey M, Seketeli A. Control of onchocerciasis today: status and challenges. Trends Parasitol. 2001;17:558–563. doi: 10.1016/s1471-4922(01)02112-2. [DOI] [PubMed] [Google Scholar]
- 7.Sréter T, Széll Z. Onchocercosis: a newly recognized disease in dogs. Vet Parasitol. 2008;151:1–13. doi: 10.1016/j.vetpar.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Uni S, Boda T, Daisaku K, Ikura Y, Maruyama H, Hasegawa H, Fukuda M, Takaoka H, Bain O. Zoonotic filariasis caused by Onchocerca dewittei japonica in a resident of Hiroshima Prefecture, Honshu, Japan. Parasitol Int. 2010;59:477–480. doi: 10.1016/j.parint.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Burr WE, Jr, Brown MF, Eberhard ML. Zoonotic Onchocerca (Nematoda:Filarioidea) in the cornea of a Colorado resident. Ophthalmology. 1998;105:1494–1497. doi: 10.1016/S0161-6420(98)98035-6. [DOI] [PubMed] [Google Scholar]
- 10.Koehsler M, Soleiman A, Aspöck H, Auer H, Walochnik J. Onchocerca jakutensis filariasis in humans. Emerg Infect Dis. 2007;13:1749–1752. doi: 10.3201/eid1311.070017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orihel TC, Ash LR, Holshuh HJ, Santenelli S. Onchocerciasis in a California dog. Am J Trop Med Hyg. 1991;44:513–517. doi: 10.4269/ajtmh.1991.44.513. [DOI] [PubMed] [Google Scholar]
- 12.Eberhard ML, Ortega Y, Dial S, Schiller CA, Sears W, Greiner E. Ocular Onchocerca infections in western United States. Vet Parasitol. 2000;90:333–338. doi: 10.1016/s0304-4017(00)00252-1. [DOI] [PubMed] [Google Scholar]
- 13.Zarfoss MK, Dubielzig RR, Eberhard ML, Schmidt KS. Canine ocular onchocerciasis in the United States: two new cases and a review of the literature. Vet Ophthalmol. 2005;8:51–57. doi: 10.1111/j.1463-5224.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- 14.Széll Z, Erdélyi I, Sréter T, Albert M, Varga I. Canine ocular onchocercosis in Hungary. Vet Parasitol. 2001;97:245–251. doi: 10.1016/s0304-4017(01)00397-1. [DOI] [PubMed] [Google Scholar]
- 15.Komnenou A, Eberhard ML, Kaldrymidou E, Tsalie E, Dessiris A. Subconjunctival filariasis due to Onchocerca sp. in dogs: report of 23 cases in Greece. Vet Ophthalmol. 2002;5:119–126. doi: 10.1046/j.1463-5224.2002.00235.x. [DOI] [PubMed] [Google Scholar]
- 16.Hermosilla A, Hetzel U, Bausch M, Grübl J, Bauer C. First autochthonous case of canine ocular onchocercosis in Germany. Vet Rec. 2005;154:450–452. doi: 10.1136/vr.156.14.450. [DOI] [PubMed] [Google Scholar]
- 17.Sréter-Lancz Z, Széll Z, Sréter T. Molecular genetic comparison of Onchocerca sp. infecting dogs in Europe with other spirurid nematodes including Onchocerca lienalis. Vet Parasitol. 2007;148:365–370. doi: 10.1016/j.vetpar.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Faísca P, Morales-Hojas R, Alves M, Gomes J, Botelho M, Melo M, Xufre A. A case of canine ocular onchocercosis in Portugal. Vet Ophthalmol. 2010;13:117–121. doi: 10.1111/j.1463-5224.2010.00763.x. [DOI] [PubMed] [Google Scholar]
- 19.Sréter T, Széll Z, Egyed Z, Varga I. Subconjunctival zoonotic onchocercosis in man: aberrant infection with Onchocerca lupi? Ann Trop Med Parasitol. 2002;96:497–502. doi: 10.1179/000349802125001267. [DOI] [PubMed] [Google Scholar]
- 20.Pampiglione S, Vakalis N, Lyssimachou A, Kouppari G, Orihel TC. Subconjunctival zoonotic Onchocerca in an Albanian man. Ann Trop Med Parasitol. 2001;95:827–832. doi: 10.1080/00034980120111163. [DOI] [PubMed] [Google Scholar]
- 21.Demiaszkiewicz AW, Matsaberidze GV, Kvavadze ES. The female of Onchocerca lupi Rodonaja, 1967 under a scanning electron microscope. Acta Parasitol. 1991;36:183–186. [Google Scholar]
- 22.Egyed Z, Sréter T, Széll Z, Beszteri B, Oravecz O, Màrialigeti K, Varga I. Morphologic and genetic characterization of Onchocerca lupi infecting dogs. Vet Parasitol. 2001;102:309–319. doi: 10.1016/s0304-4017(01)00541-6. [DOI] [PubMed] [Google Scholar]
- 23.Casiraghi M, Bain O, Guerrero R, Martin C, Pocacqua V, Gardner SL, Franceschi A, Bandi C. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int J Parasitol. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Casiraghi M, Bazzocchi C, Mortarino M, Ottina E, Genchi C. A simple molecular method for discriminating common filarial nematodes of dogs (Canis familiaris) Vet Parasitol. 2006;141:368–372. doi: 10.1016/j.vetpar.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Tamura K, Dudley J, Nei M, Kumar S. Mega4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 26.Eberhard ML, Dickerson JW, Tsang VC, Walker EM, Ottesen EA, Chandrashekar R, Weil GJ, Trips M, Strobert E, Constantinidis I. Onchocerca volvulus: parasitologic and serologic responses in experimentally infected chimpanzees and mangabey monkeys. Exp Parasitol. 1995;80:454–462. doi: 10.1006/expr.1995.1057. [DOI] [PubMed] [Google Scholar]
- 27.Otranto D, Traversa D. Thelazia eyeworm: an original endo- and ecto-parasitic nematode. Trends Parasitol. 2005;21:1–4. doi: 10.1016/j.pt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Özkara S, Isiksel S, Kilic G. A rare case of filarial infection in Turkey: Onchocerca volvulus infection. Turk J Infect. 2003;17:107–109. [Google Scholar]
- 29.Uni S, Bain O, Agatsuma T, Harada M, Torii H, Fukuda M, Takaoka H. Onchocerca eberhardi n. sp. (Nematoda: Filarioidea) from sika deer in Japan; relationships between species parasitic in cervids and bovids in the Holarctic region. Parasite. 2007;14:199–211. doi: 10.1051/parasite2007143199. [DOI] [PubMed] [Google Scholar]