Abstract
We investigated the type and strength of the immune response to schistosome antigens in a group of 20 Dutch travelers who had been infected with Schistosoma spp. during a group visit to Mali in 1991 and 8 non-infected controls. At the time, 9 had Katayama syndrome (KS), and 11 remained asymptomatic. All had been treated with praziquantel. Eight years later, serology remained positive in all 20 formerly infected travelers. The lymphocyte proliferative responses and cytokine responses (interleukin 13 [IL-13], IL-10, and interferon [IFN-γ] responses to soluble egg antigens and the IL-13, IL-10, and IL-5 response to adult worm antigen) were stronger in the travelers than in the controls and tended to be stronger in those with KS compared with those who had remained asymptomatic. In conclusion, Schistosoma infection induced a memory immune response, and people who experienced KS tended to have a stronger immune response to schistosome antigens than their asymptomatic counterparts.
Introduction
Most of the information on the immunopathology of schistosomiasis is derived from murine models.1–6 Studies in humans have mainly focused on chronic infections seen in endemic areas.7 The acute response known as Katayama syndrome is thought to occur in non-immune hosts only.8 Prior exposure to antigens in utero9–12 or infection early as opposed to late in life is believed to account for this difference in symptoms between persons living in an endemic area and non-immune hosts.
Several studies have analyzed the acute response after a primary Schistosoma infection.13–16 The symptoms in non-immune hosts vary widely.17,18 Some non-immune subjects develop Katayama syndrome, whereas others remain (virtually) asymptomatic. The reason for this difference remains unknown.19–21 Immunologically, eosinophilia and circulating immune complexes have been associated with acute schistosomiasis,14,17,19 and it has been suggested that the cause of Katayama syndrome is a systemic hyperreactive immune response to migrating schistosomula.21
Schistosomiasis in travelers can be considered an experiment of nature with a defined exposure in time, a non-immune host, low infection intensity, and lack of coinfection or reinfection. The aim of the present study was to investigate the type and strength of the cellular immune response to schistosome antigens in a defined group of previously treated travelers. The secondary aim was to analyze the difference in the immune response between those who had and those who had not experienced Katayama syndrome.
Materials and Methods
Subjects.
Subjects were recruited from an single episode of schistosomiasis that occurred among 28 Dutch travelers who had been infected during a swim in fresh water pools in the Dogon area in Mali in 1991.17 At the time, 15 had developed Katayama syndrome, which was defined as occurrence of two or more of the following symptoms: fever, sweating, abdominal pain, myalgia, arthralgia, diarrhea, dry cough, weight loss, hepatomegaly, splenomegaly, urticaria, or swollen eyelids.
Treatment with praziquantel had resulted in parasitological cure in all travelers. In 1999, when this current study was performed, 21 of the initial 28 subjects could be contacted for collection of venous blood. To exclude actual Schistosoma infection, stool and urine samples of all 21 subjects were screened for schistosome eggs by sedimentation selective filtration methods.22 In short, washed stool samples were sifted first through a sieve with 106-µm pores and then through a sieve with 53-µm pores. Five wet smears of each sample were searched for schistosome eggs. Urine samples were centrifuged for 10 minutes at 2,500 rpm, and the entire sediment was examined. Stool and urine tests were performed two times on separate occasions before considered negative. As controls, eight Dutch individuals who had never traveled to Schistosoma-endemic regions provided venous blood.
Serology.
Antibodies to S. mansoni-derived somatic antigens (adult worm antigen [AWA]) were assessed by an indirect immunofluorescence assay (IFA) for the detection of immunoglobulin M (IgM) antibodies using paraffin sections of adult male Schistosoma mansoni with Rossmann fixative. IgG antibodies to egg antigens (soluble egg antigens [SEA]) were assessed by enzyme-linked immunosorbent assay (ELISA).23,24
Antigens.
AWA and SEA were prepared from 1.5 to 2 g S. mansoni adult worms and eggs, respectively. After homogenizing in an all-glass homogenizer in a 0.035 M phosphate buffered saline (PBS), pH 7.8, at 0°C, the homogenate was transferred to a glass tube and sonicated for 3 minutes at level 7 in a sonicator (Branson Sonic Power Company, Sonicator B-12 power supply and converter, Danbury, CT) at 0°C. Next, the homogenate was centrifuged for 20 minutes at 25,000 rpm at 4°C, and the supernatant was collected. The pellet was homogenized again, and the supernatant was collected for a second time. The first and second collected supernatants were pooled together and dialyzed against distilled water at 4°C. During this procedure, the water was changed two times. The dialyzed supernatant was lyophilized and stored at 4°C. The protein content of the antigen fractions in the dialyzed supernatants was determined by a bichronic acid method (BCA; Pierce III, Rockford, IL) against standard series from solution of bovine serum albumin. Finally, the antigens were dissolved in Iscoves medium at a protein concentration of 20 µg/mL.
Purified protein derivative (PPD) of Mycobacterium tuberculosis (Statens Serum Institute, Copenhagen, Denmark) was diluted in Iscoves medium (Gibco, Pailsey, Scotland) to a concentration of 20 µL derivative per 1 mL. Tetanus toxoid (TT; RIVM, Bilthoven, The Netherlands) was diluted to a concentration of 1.5 Lf (flocculation units) per 1 mL of Iscoves medium. Phytohaemaglutine (PHA; Murex Biotech Ltd., United Kingdom) was diluted to a concentration of 4 µg per 1 mL of Iscoves medium.
Cellular stimulation assay.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood by Ficoll-Hypaque density gradient centrifugation. Cells were frozen in Roswell Park Memorial Institute medium (RPMI; Gibco) supplemented with 2 mM/L glutamine, 1 mM/L pyruvate, 20% (vol/vol) pooled human serum, and 10% (vol/vol) dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany). Viability after thawing was determined by trypan blue dye exclusion. Only cell suspensions with at least 90% viability were used. For the proliferation assay, PBMC (105 cells per well) were incubated in flat-bottomed microtiter wells (NUNC maxisorb; Life Technologies, Breda, The Netherlands) in 100 µL of Iscoves medium (Gibco) supplemented with 10% (vol/vol) pooled human serum, 2 mM/L glutamine, 1 mM/L pyruvate, 100 U/mL penicillin, and 100 µg/mL streptomycin in triplicate at 37°C in humidified air containing 7.5% C02 in the presence or absence of antigen.
For the determination of cytokine production, PBMC (106 cells per well) were incubated in round-bottomed microtiter wells (NUNC maxisorb) in the presence or absence of antigen in 100 µL of Iscoves medium (Gibco) supplemented with 5% (vol/vol) fetal calf serum, 2 mM/L glutamine, 1 mM/L pyruvate, 100 U/mL penicillin, and 100 µg/mL streptomycin in triplicate at 37°C in humidified air containing 7.5% C02. After the indicated time, the supernatants were collected, and they were immediately frozen and stored at −20°C for subsequent determination of cytokine production. The final concentrations of antigens used were: AWA, 10 µg/mL; SEA, 10 µg/mL; PHA, 2 µg/mL; PPD, 10 µg/mL; TT, 0.75 Lf.
Proliferation assay.
The lymphoproliferative responses to antigen stimulation (AWA and SEA) and stimulation with the mitogenic stimulus PHA were determined by adding 1 µCu of [3H]-thymidine at day 5. After 15 hours of incubation with [3H]-thymidine, uptake was measured by a scintillation counter. Values were expressed as stimulation index (SI). SI equals the geometric mean of (mean counts per minute [cpm] of the stimulated culture)/(mean cpm of the unstimulated cultures).
Cytokine production.
Supernatants were collected for determination of interleukin 10 (IL-10) and IL-13 on day 3 and interferon (IFN-γ) and IL-5 responses on day 5. Cytokines were measured by use of ELISA using specific capture and detection monoclonal antibodies (IFN-γ, IL-13, and IL-10, Pelikine Compact ELISA kit; Central Laboratory of Bloodtransfusion, Leiden, The Netherlands and IL-5; BD; Pharmingen; Franklin Lakes, NJ). The detection limits of the assays were 3 pg of IFN-γ/mL, 3 pg of IL-10/mL, 3 pg of IL-5/mL, and 3 pg of IL-13/mL. The upper limit was 30.000 pg/mL, and any value above was defined as 30.000 pg/mL. Detectable values in unstimulated cultures were subtracted from the value in stimulated cultures. When this difference was negative, the value of produced cytokine after stimulation was defined as 1.5 pg/mL. Cytokine responses could not be determined in all subjects because of technical problems with the assay and the limited amount of blood.
Statistical analysis.
Differences between responses were tested with the non-parametric Mann–Whitney test. Statistical significance was defined as a P value < 0.05. No correction was made for multiple testing.
Results
Study subjects' infection and clinical status.
Twenty-one subjects who had been treated for schistosomiasis in 1991 volunteered to participate in the current study. At the time of diagnosis in 1991, all subjects had positive schistosome serology, and 15 subjects (71%) had eggs in the feces and/or urine. In 1999, renewed microscopic examination of stool and urine was performed two times on separate occasions in all subjects. Schistosome eggs were found in the stool of only 1 traveler. This patient was treated with praziquantel and excluded from further analysis. Twenty travelers were included in the present analysis, 9 who had suffered Katayama syndrome in 1991 and 11 who were asymptomatic. Of these 20 subjects, 14 (70%) had eggs in the feces and/or urine in 1991. Twelve of these subjects (12/14; 86%) had been infected with S. mansoni, often as part of a mixed infection with S. haematobium (Table 1).
Table 1.
Patient characteristics
| Katayama syndrome (N = 9) | No Katayama syndrome (N = 11) | Controls (no infection; N = 8) | |
|---|---|---|---|
| Male/female | 4/5 | 4/7 | 5/3 |
| Mean age in years | 52.0 | 52.1 | 22 |
| Number of subjects positive for Schistosoma spp. eggs in stool or urine in 1991 | 7 (78%) | 7 (64%) | – |
| S. mansoni | 3 (33%) | 1 (9%) | – |
| S. intercalatum | 1 (11%) | 0 | – |
| S. haematobium | 0 | 1 (9%) | – |
| S. mansoni and S. intercalatum | 2 (22%) | 2 (18%) | – |
| S. mansoni and S. haematobium | 0 | 2 (18%) | – |
| S. mansoni, S. intercalatum, andS. haematobium | 1 (11%) | 1 (9%) | – |
| No eggs found | 2 (22%) | 4 (36%) | – |
| Number of subjects positive for Schistosoma spp. eggs in stool or urine in 1999 | 0 | 0 | – |
Serologic response to AWA and SEA.
Serum antibodies to AWA and SEA were determined in all travelers at 12.6 (±2.5) weeks after fresh water exposure in 1991 and 55.7 (±15) weeks and 8 years after treatment. Eight years after treatment, none of the travelers had reverted to negative serology for both AWA and SEA, although IgM anti-AWA titers had decreased. Median IgM anti-AWA titers were: 1:1,024 (IQR = 1:1,024–1:2,048) at 12.6 weeks, 1:1,024 (IQR = 1:512–1:1,024) at 55.7 weeks, and 1:362 (IQR = 1:128–1:861) at 8 years. Median IgG anti-SEA titers were 1:128 (IQR = 1:64–1:256) at 12.6 weeks, 1:256 (IQR = 1:76–1:256) at 55.7 weeks, and 1:128 (IQR = 1:76–1:256) at 8 years. At all three time points, median antibody levels did not differ significantly between the group with Katayama syndrome and the group that had remained asymptomatic.
Lymphocyte proliferative response.
The lymphoproliferative response could be determined for 18 of 20 travelers. A lymphoproliferative response was seen to both AWA and SEA in the 18 formerly infected travelers but not in 8 non-infected controls: the median SI in travelers in response to AWA was 15 (mean = 25, standard error = 6) and in response to SEA was 10 (mean = 12, standard error = 3); the median SI in controls in response to both AWA and SEA was 1 (mean = 1, standard error = 1). Although the median responses were stronger in those who had experienced Katayama syndrome in the past (SI for AWA = 30, SI for SEA = 15) compared with those who had remained asymptomatic (SI for AWA = 9, SI for SEA = 7), these differences did not reach statistical significance (P values for the differences = 0.17 and 0.08, respectively) (Table 2).
Table 2.
Stimulation index to AWA, SEA, and PHA 8 years after treatment in 20 Dutch travelers*
| Antigen | Stimulation index median (mean ± standard error) | P value† | |
|---|---|---|---|
| Katayama syndrome (N = 8) | No Katayama syndrome (N = 10) | ||
| AWA | 30 (33 ± 10) | 9 (18 ± 7) | 0.17 |
| SEA | 15 (13 ± 2) | 7 (10 ± 5) | 0.08 |
| PHA | 408 | 568 | 0.48 |
SEA = soluble egg antigen; AWA = adult worm antigen; PHA = phytohaemaglutinin.
The stimulation index was not determined for 1 person in each group, because their sample had less than 90% viable peripheral blood mononuclear cells after thawing.
Mann–Whitney test.
Cytokine responses.
In comparison to non-infected controls, travelers had higher levels of IL-5, IL-10, and IL-13 in response to AWA. In response to SEA, travelers showed higher production of IFN-γ, IL-10, and IL-13 (Figure 1). Travelers who had experienced Katayama syndrome in the past showed higher production of IL-13 (P = 0.03) in response to AWA and higher production of IL-13 (P = 0.009), IFN-γ (P = 0.004), and IL-5 (P = 0.06) in response to SEA compared with those who had been infected but remained asymptomatic (Figure 1). The IL-10 responses were similar in those with and without Katayama syndrome. Ten samples were taken at random and stimulated with TT and PPD. No differences in production of IL-5, IL-10, IL-13, and IFN-γ were seen between four travelers who had Katayama syndrome and six travelers who did not.
Figure 1.
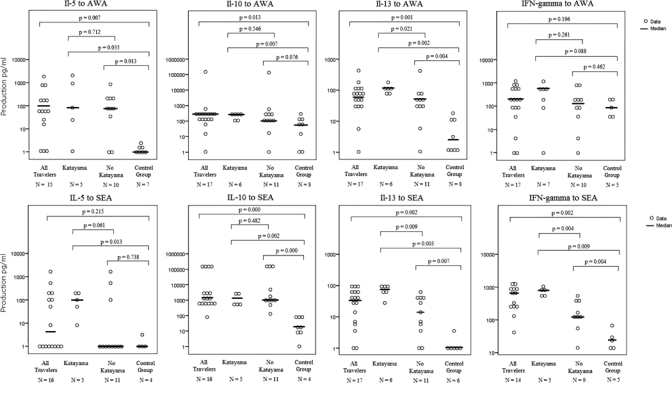
In vitro cytokine production in response to AWA and SEA 8 years after treatment of schistosomiasis; 20 formerly infected travelers, of which 9 had experienced Katayama syndrome and 11 had not, were compared with 8 non-infected controls. Cytokine responses could not be determined for all subjects because of technical problems with the assay and the limited amount of blood. Differences were tested using the non-parametric Mann–Whitney test.
Discussion
Eight years after treatment of schistosomiasis, positive serology persisted in all 20 travelers. There was also a specific lymphoproliferative response to schistosome antigens, which indicates that an acute schistosome infection in a naïve subject induces a memory response that lasts for at least 8 years. Long-lasting positive serology after treatment of schistosomiasis is consistent with previous reports.25,26 This may be caused by persisting egg antigens, providing a stimulus to the immune system even after worms are eliminated. However, worms have been known to survive in the human host for up to 31 years,27 and treatment has been known to fail. Egg secretion was the only method to establish whether an active infection was still present. Although we performed microscopic analysis two times, examining five wet smears per stool sample and the entire urine sediment on each occasion, we cannot exclude the possibility of persisting low-grade infection as a cause of long-lasting positive serology. Furthermore, the antibodies can be cross-reactive to antigens from other sources such as certain carbohydrates.28 Therefore, even without infection, stimulation of antibody production may occur from time to time.
It is surprising that AWA induced IFN-γ production in the controls but no lymphocyte proliferation in the controls. High IFN-γ levels in controls have been reported before in response to AWA and SEA.16 It is possible that components of these antigenic mixtures bear pathogen-associated molecular patterns and react with pattern recognition receptors on immune cells, such as monocytes, B-lymphocytes, or natural killer (NK) cells. The IFN-γ response to AWA in uninfected subjects might be produced by NK cells, which can readily release IFN-γ in response to stimulation by pathogen-associated molecular patterns (PAMPs).29 We do not know if these PAMPs are schistosome-specific or caused by endotoxin contamination.
The data suggest that 8 years after treatment of schistosomiasis, those who had Katayama syndrome in the past had stronger or less well-regulated lymphoproliferative and cytokine responses to schistosome antigens compared with those who were infected but remained asymptomatic. However, most differences did not meet conventional levels for statistical significance. In addition, all stimulation assays were done with S. mansoni-derived antigens. We can not fully rule out that S. haemaobium- or S. intercalatum-infected subjects would react less with S. mansoni antigens. Studies conducted on Senegalese patients living in an endemic area with single S. haemaobium, single S. mansoni, or mixed S. haemaobium and S. mansoni infections indicated that the antigens are cross-reactive when it comes to cytokine production. In other words, there was no consistent pattern showing that S. haemaobium-infected subjects respond better to S. haemaobium antigen than S. mansoni antigen or vice versa (unpublished data). Nevertheless, the percentage with proven S. mansoni infection was slightly higher in the group with Katayama syndrome than in the group without Katayama syndrome, and this may have influenced results.
We studied a limited number of cytokines that we believed to be important in the immune response in naive subjects. It has been argued that it makes biological sense for IL-13, IL-5, and IFN-γ to have a central role in the response to infection with Schistosoma spp.16,30 Because of IL-13's function in signaling B cells to switch to IgE production and IL5's and IL-13's principal role in recruiting and activating eosinophils, these cytokines are important in the initial response to invading cercaria and later on, to schistosomula.30 Eosinophilia is known to be associated with Katayama syndrome,17,19 and the stronger IL-5 and IL-13 responses that we found may reflect stronger activation of eosinophils at the time of infection in the travelers with Katayama syndrome. The higher IFN-γ levels in response to SEA in travelers with Katayama syndrome may reflect a stronger immune response to the infection in this group.
This study shows that acute schistosomiasis induces a memory response that can be detected 8 years after treatment. Furthermore, we found that formerly infected travelers who had Katayama syndrome had an overall stronger action of the immune system to schistosome antigens compared with their asymptomatic counterparts. This is in line with the idea that Katayama syndrome is caused by a hyperreactive immune response to migrating schistosomula or eggs. Why some do and others do not mount such a hyperreactive immune response remains unknown. Differences in the genetic background or the antigen load during the acute infection offer plausible but unproven explanations.
ACKNOWLEDGMENTS
The authors thank Yvonne C. M. Kruize for her assistance in performing the laboratory analysis. The study protocol was approved by the Medical Ethics Committee of the Leiden University Medical Center (Protocol P42/99). Informed consent was obtained from all participants.
Footnotes
Financial support: There was no dedicated funding for this project.
Authors' addresses: Darius Soonawala, Jan-Willem H. J. Geerts, and Leo G. Visser, Department of Infectious Diseases and Tropical Medicine, Leiden University Medical Center, Leiden, The Netherlands, E-mails: d.soonawala@lumc.nl, geerts.jw@gmail.com, and l.g.visser@lumc.nl. Marissa de Mos, Department of Medical Informatics, Erasmus Medical Center, Rotterdam, The Netherlands, E-mail: m.vrolijk-demos@erasmusmc.nl. Maria Yazdanbakhsh, Department of Parasitology, Leiden University Medical Center, Leiden, The Netherlands, E-mail: m.yazdanbakhsh@lumc.nl.
References
- 1.Souza SS, de Souza Neto JP, Andrade ZA. Pulmonary changes during acute experimental murine manson schistosomiasis. Rev Soc Bras Med Trop. 2009;42:5–8. doi: 10.1590/s0037-86822009000100002. [DOI] [PubMed] [Google Scholar]
- 2.Herbert DR, Orekov T, Perkins C, Finkelman FD. IL-10 and TGF-beta redundantly protect against severe liver injury and mortality during acute schistosomiasis. J Immunol. 2008;181:7214–7220. doi: 10.4049/jimmunol.181.10.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, Heiny S, Lewis F, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 4.Pearce EJ. Priming of the immune response by schistosome eggs. Parasite Immunol. 2005;27:265–270. doi: 10.1111/j.1365-3024.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 5.Fallon PG, Smith P, Dunne DW. Type 1 and type 2 cytokine-producing mouse CD4+ and CD8+ T cells in acute Schistosoma mansoni infection. Eur J Immunol. 1998;28:1408–1416. doi: 10.1002/(SICI)1521-4141(199804)28:04<1408::AID-IMMU1408>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 6.Fallon PG. Immunopathology of schistosomiasis: a cautionary tale of mice and men. Immunol Today. 2000;21:29–35. doi: 10.1016/s0167-5699(99)01551-0. [DOI] [PubMed] [Google Scholar]
- 7.Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 8.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 9.Eloi-Santos SM, Novato-Silva E, Maselli VM, Gazzinelli G, Colley DG, Correa-Oliveira R. Idiotypic sensitization in utero of children born to mothers with schistosomiasis or Chagas' disease. J Clin Invest. 1989;84:1028–1031. doi: 10.1172/JCI114225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novato-Silva E, Gazzinelli G, Colley DG. Immune responses during human schistosomiasis mansoni. XVIII. Immunologic status of pregnant women and their neonates. Scand J Immunol. 1992;35:429–437. doi: 10.1111/j.1365-3083.1992.tb02878.x. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra I, Ouma J, Wamachi A, Kioko J, Mungai P, Omollo A, Elson L, Koech D, Kazura JW, King CL. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J Clin Invest. 1997;99:1759–1766. doi: 10.1172/JCI119340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King CL, Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, Kazura JW. B cell sensitization to helminthic infection develops in utero in humans. J Immunol. 1998;160:3578–3584. [PubMed] [Google Scholar]
- 13.Caldas IR, Campi-Azevedo AC, Oliveira LF, Silveira AM, Oliveira RC, Gazzinelli G. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Trop. 2008;108:109–117. doi: 10.1016/j.actatropica.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 14.de Jesus AR, Silva A, Santana LB, Magalhaes A, de Jesus AA, de Almeida RP, Rego MA, Burattini MN, Pearce EJ, Carvalho EM. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J Infect Dis. 2002;185:98–105. doi: 10.1086/324668. [DOI] [PubMed] [Google Scholar]
- 15.Williams ME, Montenegro S, Domingues AL, Wynn TA, Teixeira K, Mahanty S, Coutinho A, Sher A. Leukocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th0 pattern to worm antigens. J Infect Dis. 1994;170:946–954. doi: 10.1093/infdis/170.4.946. [DOI] [PubMed] [Google Scholar]
- 16.Morais CN, Souza JR, Melo WG, Aroucha ML, Miranda P, Domingues AL, Abath FG, Montenegro SM. Cytokine profile associated with chronic and acute human schistosomiasis mansoni. Mem Inst Oswaldo Cruz. 2008;103:561–568. doi: 10.1590/s0074-02762008000600009. [DOI] [PubMed] [Google Scholar]
- 17.Visser LG, Polderman AM, Stuiver PC. Outbreak of schistosomiasis among travelers returning from Mali, West Africa. Clin Infect Dis. 1995;20:280–285. doi: 10.1093/clinids/20.2.280. [DOI] [PubMed] [Google Scholar]
- 18.Colebunders R, Verstraeten T, Van GA, Van den Ende J, De Roo A, Polderman A, Visser L. Acute schistosomiasis in travelers returning from Mali. J Travel Med. 1995;2:235–238. doi: 10.1111/j.1708-8305.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 19.Hiatt RA, Sotomayor ZR, Sanchez G, Zambrana M, Knight WB. Factors in the pathogenesis of acute schistosomiasis mansoni. J Infect Dis. 1979;139:659–666. doi: 10.1093/infdis/139.6.659. [DOI] [PubMed] [Google Scholar]
- 20.van Lieshout L, Polderman AM, Visser LG, Verwey JJ, Deelder AM. Detection of the circulating antigens CAA and CCA in a group of Dutch travelers with acute schistosomiasis. Trop Med Int Health. 1997;2:551–557. doi: 10.1046/j.1365-3156.1997.d01-324.x. [DOI] [PubMed] [Google Scholar]
- 21.Ross AG, Vickers D, Olds GR, Shah SM, McManus DP. Katayama syndrome. Lancet Infect Dis. 2007;7:218–224. doi: 10.1016/S1473-3099(07)70053-1. [DOI] [PubMed] [Google Scholar]
- 22.Visser PS, Pitchford RJ. A simple apparatus for rapid recovery of helminth eggs from excreta with special reference to Schistosoma mansoni. S Afr Med J. 1972;46:1344–1346. [PubMed] [Google Scholar]
- 23.Nash TE, Ottesen EA, Cheever AW. Antibody response to a polysaccharide antigen present in the schistosome gut. II. Modulation of antibody response. Am J Trop Med Hyg. 1978;27:944–950. doi: 10.4269/ajtmh.1978.27.944. [DOI] [PubMed] [Google Scholar]
- 24.Deelder AM, Kornelis D. Immunodiagnosis of recently acquired Schistosoma mansoni infection. A comparison of various immunological techniques. Trop Geogr Med. 1981;33:36–41. [PubMed] [Google Scholar]
- 25.Rabello AL, Garcia MM, Pinto da Silva RA, Rocha RS, Katz N. Humoral immune responses in patients with acute Schistosoma mansoni infection who were followed up for two years after treatment. Clin Infect Dis. 1997;24:304–308. doi: 10.1093/clinids/24.3.304. [DOI] [PubMed] [Google Scholar]
- 26.Whitty CJ, Mabey DC, Armstrong M, Wright SG, Chiodini PL. Presentation and outcome of 1107 cases of schistosomiasis from Africa diagnosed in a non-endemic country. Trans R Soc Trop Med Hyg. 2000;94:531–534. doi: 10.1016/s0035-9203(00)90077-4. [DOI] [PubMed] [Google Scholar]
- 27.Harris AR, Russell RJ, Charters AD. A review of schistosomiasis in immigrants in Western Australia, demonstrating the unusual longevity of Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1984;78:385–388. doi: 10.1016/0035-9203(84)90129-9. [DOI] [PubMed] [Google Scholar]
- 28.Van der Kleij D, Van Remoortere A, Schuitemaker JH, Kapsenberg ML, Deelder AM, Tielens AG, Hokke CH, Yazdanbakhsh M. Triggering of innate immune responses by schistosome egg glycolipids and their carbohydrate epitope GalNAc beta 1-4(Fuc alpha 1-2Fuc alpha 1-3)GlcNAc. J Infect Dis. 2002;185:531–539. doi: 10.1086/338574. [DOI] [PubMed] [Google Scholar]
- 29.Lauzon NM, Mian F, MacKenzie R, Ashkar AA. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell Immunol. 2006;241:102–112. doi: 10.1016/j.cellimm.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Dessein A, Kouriba B, Eboumbou C, Dessein H, Argiro L, Marquet S, Elwali NE, Rodrigues V, Li Y, Doumbo O, Chevillard C. Interleukin-13 in the skin and interferon-gamma in the liver are key players in immune protection in human schistosomiasis. Immunol Rev. 2004;201:180–190. doi: 10.1111/j.0105-2896.2004.00195.x. [DOI] [PubMed] [Google Scholar]


