Abstract
Methylene Blue (MB), following its introduction to biology in the 19th century by Ehrlich, has found uses in various areas of medicine and biology. At present, MB is the first line of treatment in methemoglobinemias, is used frequently in the treatment of ifosfamide-induced encephalopathy, and is routinely employed as a diagnostic tool in surgical procedures. Furthermore, recent studies suggest that MB has beneficial effects in Alzheimer's disease and memory improvement. Although the modulation of the cGMP pathway is considered the most significant effect of MB, mediating its pharmacological actions, recent studies indicate that it has multiple cellular and molecular targets. In the majority of cases, biological effects and clinical applications of MB are dictated by its unique physicochemical properties including its planar structure, redox chemistry, ionic charges, and light spectrum characteristics. In this review article, these physicochemical features and the actions of MB on multiple cellular and molecular targets are discussed with regard to their relevance to the nervous system.
1. Introduction
In the late nineteenth century, the increasing demand for dyes in the prospering textile industry brought rapid advancements in the research of synthetic dyes. One of the most obvious results of this progress was the introduction of a vast number of colors into the everyday life of European cities (Streba et al., 2007). The synthesis of the first aniline-based dyes, such as mauve by William Perkin in 1856, led to an increase in their popularity and encouraged research on the use of aniline derivatives as dye precursors. In 1876, Methylene Blue (MB) was synthesized by Heinrich Caro of Badische Anilin und Soda Fabrik (BASF) as an aniline-based dye for cotton staining. A year later, BASF was awarded Germany's first dye patent (Caro, 1877). Although MB (Swiss blue, aniline violet, methylthionine hydrochloride, tetramethylthione hydrochloride) failed to live up to the standards of the textile industry, scientists such as Robert Koch and Paul Ehrlich were quick to realize that it was not only possible to stain different cellular structures with different dyes, but also to stain and inactivate microbial species selectively. In turn, this discovery led to the testing of these dyes against tropical diseases, and the first candidate to be administered to humans was MB, which Ehrlich showed in 1891 to be effective in the treatment of malaria (Guttmann and Ehrlich, 1891). Thus, MB was the first synthetic compound ever used as an antiseptic in clinical therapy and the first antiseptic dye to be used therapeutically. In fact, the use of MB and its derivatives was widespread before the advent of sulfonamides and penicillin in chemotherapy (for a review Wainwright and Crossley, 2002). MB has also been a lead compound in drug research against various bacterial and viral infections (Wainwright and Crossley, 2002) and cancer (Wainwright and Crossley, 2002; Wainwright, 2003), not to mention its part in the development of the phenothiazine neuroleptic family (Kristiansen, 1989; Shen, 1999; Frankenburg and Baldessarini, 2008).
Over the years, MB has also been used in human and veterinary medicine for a number of therapeutic and diagnostic procedures including use as a stain in neuroanatomy and bacteriology, as a redox coloring agent in biochemical studies, as a targeting agent for various types of cancer such as melanoma and lung cancer, and as an antiseptic compound (for reviews, Barbosa and Peters, 1971; Wainwright and Crossley, 2002; Wainwright, 2003). One of the most common clinical applications is for treating methemoglobinemia, either inborn (Cawein et al., 1964) or induced by overexposure to drugs, to industrial chemicals such as nitrophenols, or to environmental poisons such as excessive nitrate in well water or cyanide compounds (Sills and Zinkkam, 1994). Presently, MB is used clinically in a wide range of indications, such as methemoglobinemia, ifosfamide-induced encephalopathy and thyroid surgery (Table 1). In June 2009, 14 clinical trials are registered in the United States (clinicaltrials.gov; NIH) to investigate the clinical utility of MB in areas ranging from anesthesiology to treatments of depression and psychosis (Table 2).
Table I.
| Clinical indications of methylene blue |
Dose | References |
|---|---|---|
| Methemoglobinemias | 1-2 mg/kg I.V. | Bradberry, 2003 |
| Ifosfamide-induced encephalopathy | 50 mg IV every four hour till symptoms resolve |
Küpfer et al., 1994; Alici-Evcimen and Breitbart, 2007 |
| Treatment of vasoplegic syndrome | 2 mg/kg, 20-min infusion time |
Shanmugam, 2005 |
| Parathyroid imaging | 3-7.5 mg/kg I.V. |
Dudley et al., 1971; Gordon et al., 1975; Rowley et al., 2009 |
| Sentinel lymph node biopsy | Local application of 1-5 ml of 1% MB |
Varghese et al., 2007 |
| Treatment of malaria | 10 mg/kg twice a day orally |
Coulibaly et al., 2009 |
Table II.
2. Pharmacokinetic Properties
In clinical use, MB is either dissolved in sterile water to a concentration of 10 mg/ml (1%) or administered orally in gelatin capsules to avoid staining of the oral mucous membranes and to ensure complete gastrointestinal delivery. The generally accepted therapeutic bolus dose of MB is 1–2 mg/kg body weight over 10–20 min (Harvey, 1980). In humans, mean plasma concentration of 5 μM MB was reported after intravenous bolus injection of 1.4 mg/kg MB (Aeschlimann et al., 1996). The clinically used oral dose of MB appears to be between 50-300 mg (Herman et al., 1999). In healthy individuals, whole blood concentrations of up to 25 ng/ml were reached after oral administration of 100 mg MB (Peter et al., 2000). A recent study comparing the administration of single doses of MB (50 mg intravenously versus 500 mg orally) indicated that the absolute bioavailability of MB after oral administration was 72.3% (Walter-Sack et al., 2009). However, while oral MB results in higher intestinal and liver concentrations, intravenous administration results in higher MB concentrations in the brain (Peter et al., 2000), MB has been shown to pass the blood-brain barrier, when administered intraperitoneally (O'Leary et al., 1968), intraduodenally, and intravenously (Peter et al., 2000) to rats. MB has also been demonstrated to penetrate selectively certain neuronal cell types after systemic administration (Müller, 1998).
It is important to note that MB concentrations in whole blood have been found to be 4 to 5-fold higher than in plasma, suggesting that MB binds to and is taken up by blood cells (Peter et al., 2000; Rengelshausen et al., 2004; Buchholz et al., 2008). Thus, whole blood measurements of MB may not reflect its bio-phase concentrations. In addition, MB binds to bovine serum albumin with a stoichiometry of 1:1 and with a dissociation constant of 2.90 μM (Buchholz et al., 2008). Thus, not surprisingly, MB has an exceedingly high volume distribution of 21.0 l/kg in rabbits (Kozaki and Watanabe, 1981). However, pharmacokinetic properties of MB vary significantly depending on the dose of MB and animal species involved (DiSanto and Wagner; 1972; Kozaki and Watanabe, 1981; Burrows, 1984).
Both intravenous and oral administrations of MB demonstrate multi-compartmental pharmacokinetics with a terminal plasma half-life of 5-7 h (Peter et al., 2000). The oral absorption of MB is reported to be 53–97% with plasma peak concentrations after 30–60 min (DiSanto and Wagner; 1972). About 65-85% of MB is reduced in erythrocytes and peripheral tissues to the leucoMB form (DiSanto and Wagner; 1972). MB and leucoMB are predominantly excreted in urine, but also in bile and feces (Peter et al., 2000; Clifton and Leikin, 2003). MB causes a green-blue discoloration of the urine (DiSanto and Wagner; 1972), which can also be observed in saliva and bile, and disappears within a few days after its discontinuation.
3. Physicochemical Properties and Mechanisms of Actions
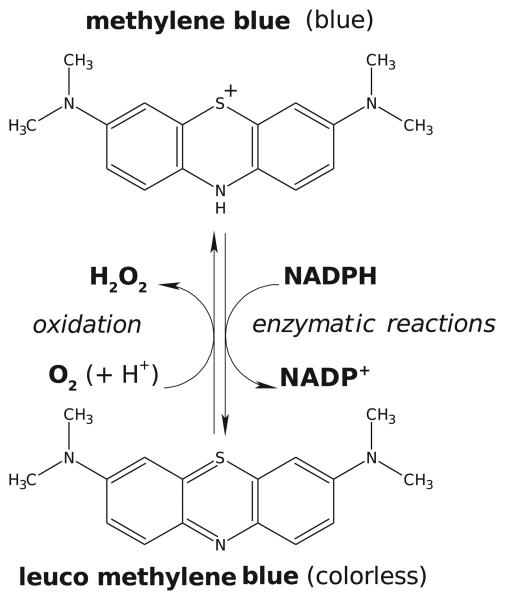
Methylene blue (tetramethylthionine chloride, C16H18ClN3S) is a heterocyclic aromatic dye, a member of thiazine dyes. It occurs as odorless dark blue crystals and is soluble in water and chloroform but only sparingly so in alcohol. In its oxidized state, the color of MB is blue due to the fact that the phenothiazinium molecule absorbs visible light strongly in the region of 600–700 nm, thus allowing the remainder of the visible spectrum (350–600 nm) to be transmitted. In its reduced form (leucoMB), it is colorless and does not absorb light in the visible region (Lee and Mills, 2003). LeucoMB is uncharged, lipophilic, and enters cells by diffusion across the plasma membrane where it can be re-oxidized and thus sequestered within the cells (May et al., 2003). Oxidized MB and leucoMB together form a reversible oxidation-reduction system or electron donor-acceptor couple (Figure 1). The redox-indicator properties of this couple have been used extensively in the quantitative analysis of a large number of reducing agents, such as glucose and ascorbic acid (Wainwright, 2003). In clinics, the redox properties of MB have been utilized in treatments of methemoglobinemias and ifosfamide-induced encephalopathy (see section 7). MB is the first-line treatment for congenital, occupational, and drug induced methemoglobinemias (Bradberry, 2003). MB acts as an electron donor in the non-enzymatic reduction of methemoglobin. An NADPH methemoglobin reductase catalyses the reduction of MB to leucoMB. This reaction transfers electrons to methemoglobin non-enzymatically, restoring functional hemoglobin and MB (Bradberry, 2003).
Figure 1.
Methylene blue (MB) as a redox-cycling substrate for various reducing and oxidizing substances. The reducing agents such as NADPH convert MB to leucoMB, which is then oxidized by O2. Each reaction cycle, catalyzed by the MB-enzyme ensemble, leads to the consumption of NADPH and O2 and to the production of reactive oxygen species, predominantly to H2O2 (modified from Buchholz et al., 2008).
MB absorbs energy directly from a light source and then transfers this energy to molecular oxygen creating singlet oxygen (1O2). Singlet oxygen is extremely electrophilic and can oxidize directly electron rich double bonds in biological molecules and macromolecules. For this reason, MB has been utilized as a photosensitizer in cancer treatment and protection of serum from viral agents (Wainwright 2000; Tardivo et al., 2005). The antifungal and antibacterial activity of MB can be increased significantly by light activation (Wainwright and Crossley 2002). In photosensitization, MB can have two different mechanisms of action. Excitation of MB by light can induce singlet and triplet stages of the molecule and transfer the energy through either electrons (Type I mechanism) or energy (Type II mechanism). The Type I mechanism can lead to the formation of hydroxyl radicals and lipid hydroperoxides. The Type II mechanism proceeds through singlet oxygen resulting mainly in breakages of nucleic acids, mostly at the guanosine site (Wainwright, 2000). Photosensitization reactions induced by MB excitation are known to cause damage to several biomolecules. Damage to nucleic acids, proteins and lipids have been described in the literature. Especially lipid peroxidation, induced by MB photosensitization, has detrimental effects on membrane integrity, leading to a loss of fluidity and alterations on the functions of several ion channels, receptors, and transporters (see section 8). This damage is thought to be triggered both by type I and type II mechanisms and has been comprehensively described in earlier reviews (Tuite and Kelly, 1993; Tardivo et al., 2005).
However, MB also exhibits antioxidant effects (McCord and Fridovich, 1970; Kelner et al., 1988; Salaris et al., 1991; Riedel et al., 2003). As mentioned earlier, MB can readily accept and donate electrons from and to a variety of compounds, allowing it to be either prooxidant or antioxidant under different conditions. For example, MB inhibits superoxide production by serving as an artificial electron acceptor by diverting electron flow away from the enzyme sites of various oxidases where molecular oxygen is converted to superoxide radicals (Salaris et al., 1991; Riedel et al., 2003). Furthermore, in HepG2 cells, MB induces phase-2 antioxidant defense enzymes such as thioredoxin reductase 1 and NADPH quinone oxidoreductase (Atamna et al., 2008).
In pharmacological studies, 1-10 μM MB is used routinely to inhibit soluble guanylate cyclase (sGC) for the analysis of cGMP-mediated processes (Moncada and Higgs, 2006). MB also directly inhibits both constitutive and inducible forms of nitric oxide synthase (NOS) by oxidation of ferrous iron bound to the enzyme (Mayer et al., 1993; Volke et al., 1999). It inactivates nitric oxide (NO) by generation of superoxide anions (Wolin et al., 1990; Marczin et al., 1992). MB is likely to be a more specific and potent inhibitor of NOS than of sGC, since direct NO-donating compounds in the presence of MB can still partially activate cGMP signaling pathways (Mayer et al., 1993).
However, in recent years, it has become clear that several pharmacological actions of MB are mediated by mechanisms other than inhibition of the cGMP pathway. Regardless of its mode of action, MB proved invaluable in various branches of medicine and biology. In the following chapters, special emphasis will be given to the cellular and molecular basis of MB actions in the nervous system.
3. Adverse Effects
In adults, large doses of MB (>7.0 mg/kg) administered intravenously have been reported to cause nausea, abdominal and chest pain, cyanosis, methemoglobinemia, sweating, dizziness, headache, and confusion (Harvey, 1980). In neonates, MB toxicities include hemolytic anemia, respiratory distress, and phototoxicity (Cragan, 1999). MB administration during breast feeding is also discouraged since MB easily crosses the blood-milk barrier (Ziv and Heavner, 1984). However, a review of the literature indicates that, especially in neonates, the most likely cause for MB toxicity is excessive dose administration (Albert et al., 2003). From the toxicological point of view, MB has an enviable safety record. In one notable pediatric case report (Blass and Fung, 1976), a child was given a dose that was 16 times the recommended maximum. The child's skin was intensely blue but there were no other documented ill effects.
Intra-amniotic MB instillation has also been reported to cause Heinz body hemolytic anemia (Sills and, Zinkham, 1994) and liver damage (McFadyen, 1992). Importantly, MB has been associated with a high percentage of fetal intestinal atresia when used in the second trimester of pregnancy (Dolk, 1991; van der Pol et al., 1992; Gluer; 1995). In preclinical studies the intra-amniotic instillation of MB produced teratogenic effects including cleft palate, digit malformations, increased incidence of fetal mortality, and neuronal tube malformations (Iyengar and Lal, 1985; Tiboni et al., 2001). Intra-amniotic MB injection is currently not recommended in obstetrics due to its association with above mentioned teratogenic effects (for a review Cragan, 1999).
In preclinical in vitro studies, MB is employed at concentrations ranging from 0.1 nM to 10 μM and toxic effects are reported at levels higher than 100 μM. The oral LD50 of MB has been estimated as 1,180 mg/kg in rats and 3,500 mg/kg in mice (Lewis, 1992); the intraperitoneal LD50 as 150 mg/kg in mice and 180 mg/kg in rats; and the intravenous LD50 as 77 mg/kg in mice, 1,250 mg/kg in rats, and 42.3 mg/kg in sheep (Burrows, 1984; Lewis, 1992; Cragan, 1999). The results of preclinical toxicity studies by the National Toxicology Program (2008) indicated that long-term MB administration (1 to 24 months) in rodents is associated with lymphomas as well as various intestinal malignancies.
5. Methylene Blue and Neurohistology
In 1886, Ehrlich carried out the first MB staining of nervous tissue by injecting it intravenously into a living animal (Ehrlich, 1886). In the following years, various methods for both vital and supra-vital MB staining of neuronal structures were developed and employed in numerous studies (for a review Barbosa and Peters, 1971). Importantly, camera lucida drawings used for Cajal's neuron theory are also based on his early MB-studies (for a review Garcia-Lopez et al., 2007). MB has been used to locate muscle spindles (Hines and Tower, 1928), cutaneous nerve endings (Weddell, 1941), motor end plates (Coers, 1952; Cheng, 1954) and nodes of Ranvier (Müller, 1992). MB has a high affinity towards nervous tissue and binds indiscriminately to motor, sensory and autonomous nerve fibers (Kristiansen, 1989). However, it selectively stains certain subpopulations of neurons in the brain, e.g. cerebellar Purkinje cells, where it binds to their dendritic spines (Müller, 1992) or polymorphic nerve cells in the hippocampal cornu ammonis, after supra-vital (intracardiac injection in deeply anesthetized animals) administration (Müller, 1998) and has been employed routinely for the staining of neuronal structures in clinical and histochemical studies (Frimer et al., 1970; Kristiansen, 1989; Müller, 1990; 1998; Seif et al., 2004). At the ultrastructural level, it was shown that MB appears as electron-dense precipitates staining the cytoplasm and chromatin homogeneously. Moreover, MB accumulates in the shape of large, drop-like structures in the cytoplasm and binds to material at the plasma membrane, possibly belonging to perineuronal nets (Müller, 1998).
Pharmacokinetic studies of its organ distribution in rodents have shown that the concentration of MB is 10–20 times higher in the brain than in the circulation one hour following systemic administration, indicating a rapid and extensive accumulation of MB in the nervous system (Kristiansen, 1989; Peter et al., 2000). The cellular mechanism(s) underlying the high affinity uptake of MB into nervous tissue is not completely elucidated. It is likely that multiple factors play a role in this process. The presence of a sulfur atom in the MB molecule has been suggested as the reason for its high affinity to neural structures (Müller, 1998). MB is a cation whereas the reduced (leuco) form has a pKa of 5.8, resulting in very low ionization (3%) at physiological pH. It has been suggested that MB is reduced to its uncharged leuco-form by a reductase associated with the plasma membrane, immediately before entering nerve cells, followed by intracellular reoxidation to the oxidized (blue) form (Müller, 1998). Ehrlich suggested that neuronal structures showed a high affinity for the reduced (leuko) form of MB and demonstrated this phenomenon by exposing tissue sections to air or by treating them with iron chloride. This resulted in the return of the blue stain (Ehrlich, 1886). Color alterations between oxidized and reduced forms of MB have also been utilized successfully in monitoring neuronal activity levels (Fischer and Zeman, 1959).
6. Methylene Blue in Anesthesiology
Local applications of MB at high concentrations not only stain nerve endings and fibers but also disrupt their function. This property of MB has been utilized for the treatment of sensory pathologies such as pain and itching (Kristiansen, 1989). In fact, MB was first employed as an analgesic in 1890 (Ehrlich and Leppmann, 1890). Ehrlich, together with the German psychiatrist Arthur Leppmann, used this dye successfully in the treatment of neuritic and rheumatic diseases. Local MB applications have been used for the functional blockade of sensory nerve endings in the treatment of pain (Zakaria et al., 2005; Tan and Seow-Choen, 2007; Peng et al., 2007) and intractable itching (Wolloch and Dintsman, 1979; Eusebio et al., 1990; Mentes et al., 2004; Sutherland et al., 2009). Furthermore, the hyperalgesia induced by intrathecal injections of prostaglandin E2 and prostaglandin F2 was inhibited by low μM concentrations of MB (Minami et al., 1995; Ferreira and Lorenzetti, 1996). However, intrathecal application of MB is absolutely contraindicated; in cats, epidural injections of MB induced paraplegia, axonal swelling, and inflammation of the leptomeninges (Poppers et al., 1970). Several earlier clinical studies have described neurotoxic adverse effects of intrathecal MB; neurological sequelae included quadriplegia, paraplegia, radiculopathy, cauda equina syndrome, encephalopathy, optic neuritis, and meningeal irritation (Arieff and Pyzik, 1960; Evans and Keegan, 1960; Schultz and Schwartz, 1970; Steiner and Steiner-Milz, 1986). Although the mechanism for these detrimental effects of MB is not clear, it is likely that anatomical regions with limited blood perfusion, such as the spinal cord, are highly sensitive to the well-known vasoconstrictive effects of MB (Sharr et al., 1978). Furthermore, in vitro exposure of organotypic slice cultures of the immature rat cerebellum to high MB concentrations resulted in a progressive destruction of differentiating cells (Garthwaite and Garthwaite, 1988), suggesting that MB may have additional neurotoxic effects at concentrations above 100 μM.
In addition to its local analgesic actions, MB also decreases minimum alveolar concentration of general anesthetics such as sevoflurane (Masaki and Kondo, 1999), isoflurane (Vutskits et al., 2008), and the propofol (Licker et al., 2008). In fact, phenothiazine-class compounds were employed initially to potentiate the effects of anesthetics and sedatives (for reviews Kristiansen, 1989; Frankenburg and Baldessarini, 2008).
7. Methylene Blue and Ifosfamide Encephalopathy
In neuro-oncology, MB has recently been introduced into the treatment of ifosfamide-induced encephalopathy (Küpfer et al., 1994; Zulian et al., 1995; Aeschlimann et al., 1996; Pelgrims et al., 2000). Ifosfamide is an alkylating agent used on a wide range of solid tumors (sarcomas and germ cell tumors) and hematologic malignancies. However, neuropsychiatric toxicity, commonly known as ifosfamide encephalopathy, is one of the most serious adverse effects of ifosfamide, occurring in 5-30 % of patients treated with this agent (Alici-Evcimen and Breitbart, 2007). Clinical symptoms may range from mild confusion to stupor, coma, hallucinations, seizures, and death. Both oral and intravenous administrations of MB have been shown to prevent and reverse ifosfamide-induced encephalopathy (Küpfer et al., 1994; Ferrero et al., 1995). For this purpose, prophylactic use of MB has also been suggested for preventing the recurrence of encephalopathy by subsequent administrations of ifosfamide (Küpfer et al., 1994; Alici-Evcimen and Breitbart, 2007; Di Cataldo et al., 2009).
The pathophysiology of ifosfamide encephalopathy is still poorly understood. However, chloroacetaldehyde (CAA) accumulation in the nervous system and defects in mitochondrial fatty acid oxidation due to defective electron transfer to flavoproteins appear to be involved (Ajithkumar et al., 2007; Alici-Evcimen and Breitbart, 2007). Since MB has a redox potential close to that of oxygen and can be reduced by components of the electron transport chain, it is very efficient in cycling between oxidized and reduced forms of redox factors in the mitochondria (Clark et al., 1925; Ajithkumar et al., 2007). MB restores mitochondrial respiratory chain function by serving as an alternative electron acceptor, restoring hepatic gluconeogenesis by reversing NADH inhibition, preventing the transformation of chloroethylamine into CAA, and inhibiting multiple amine oxidase activities, thereby preventing the formation of CAA (Ajithkumar et al., 2007). In order to compensate for the inhibition of gluconeogenesis caused by ifosfamide, MB is usually administered in combination with a glucose infusion (Alici-Evcimen and Breitbart, 2007). In the 1930s it was suggested that defective electron transfer in cyanide poisoning can be corrected by MB (Brooks, 1936; Wendel, 1935). These findings have led to the development of the current antidotal treatment of cyanide poisoning (Gracia and Shepherd, 2004).
8. Methylene Blue and the Neuroendocrine System
MB has been shown to modulate the physiological actions of hormones involved in the hypothalamo-pituitary-peripheral axis (Nedvídkova et al., 1995; 2000; 2001). For example, MB raises blood thyroxine and causes a feedback decrease in the serum thyroid stimulating hormone level (Nedvídkova et al., 1995). It appears that an MB-induced increase in thyroid peroxidase activity enhances the iodination of thyronines, with subsequent increases in the synthesis of thyroxine (Nedvídkova et al., 2000). As a redox modulating agent, MB acts as an alternative electron acceptor in the mitochondria1 oxidation chain and in tissue oxidases, particularly xanthine oxidase. MB also inhibited the activity of aldehyde dehydrogenases in human erythrocyte and leukocytes, and the rat liver mitochondria (Helander et al., 1993). Although this effect was suggested to protect against the metabolic redox effects of ethanol (Ryle et al., 1985), it has been shown in cultured rat astrocytes that MB-induced acetaldehyde accumulation during chronic ethanol exposure potentiates the toxic effects of ethanol in vitro (Signorini-Allibe et. al., 2005). In vivo studies in rats indicate that MB significantly increases the metabolism of ethanol to CO2, indicating that it can speed up ethanol elimination (Vonlanthen et al., 2000). However this effect was not observed in humans, when MB was given in a dose (50 mg orally) that can be safely administered (Vonlanthen et al., 2000)
Similar to thyroid hormone, the actions of estrogens are also modulated by MB (Hirsch et al., 1989). Estradiol-induced adenohypophyseal enlargement and the enhancement of prolactin secretion are antagonized by MB (Nedvídkova et al., 2000; 2001). Treatment with MB also prevents estradiol-induced decreases in anterior pituitary dopamine levels and increases in D2 receptor number. MB alone reduces anterior pituitary weight, increases anterior pituitary dopamine concentrations and decreases anterior pituitary D2 receptor number with a corresponding reduction in its affinity (Nedvídkova et al., 2000; 2001). It is likely that inhibition of estradiol actions in the anterior pituitary by MB is mediated by elevations in dopamine levels induced by the actions of MB on receptor binding and monoamine oxidase activity (see below).
9. Molecular Targets of Methylene Blue
At the cellular level, MB modulates the functions of various integral membrane proteins involved in transports of solutes such as glucose (Louters et al., 2006; Scott et al., 2009) and ions such as Na+, K+, and H+ (Puppi et al., 1986; Sanders et al., 1989; Visarius et al., 1997; Shah et al., 2006; Furian et al., 2007). In addition, the functions of voltage-sensitive Na+ (Oxford, 1977; Starkus et al., 1984; 1993; Kress et al., 1997), Ca2+ (Thuneberg, 1990) and Ca2+-activated K+ channels (Nemeth et al., 1985; Stockand and Samson, 1996; Saitow and Nakaoka, 1997) are also modulated by MB. As a result of MB actions on ion channels, excitability of neurons is altered significantly. Depolarizing effects of MB on neuronal structures have been demonstrated in several earlier studies (Burmistrov et al., 1967; Nozdrachev et al., 1984; Nemeth et al., 1985; Kress et al., 1997). Inhibition of Ca2+-activated K+ channels (Nemeth et al., 1985), activation of Ca2+ channels (Saitow and Nakaoka, 1997), and facilitation of Na+ channel inactivation (Oxford, 1977; Kress et al., 1997) are suggested to be possible mechanisms for MB-induced depolarizations. It appears that the actions of MB, reported in the majority of these studies, are not mediated by the cGMP pathway. Molecular mechanisms mediating the effects of MB on these ion channels are complex and depend on multiple factors including the route of MB application (Oxford, 1977; Armstrong and Croop, 1982; Starkus et al., 1984; 1993), light exposure (Pooler, 1968; Starkus et al., 1993; Kress et al., 1997), membrane potential (Valenzeno et al., 1993) and the redox state of cells (Nanasi and Dely, 1995). Among these factors, light exposure deserves mention since it is particularly important during in vitro experiments in which various light sources are used routinely. Some of the depolarizing actions of MB can be induced by visible light illumination. When exposed to light, MB becomes photosensitized, leading to the release of cytotoxic, highly active, and short-lived oxygen-derived species such as singlet oxygen (see section 3). These photooxidation products are known to alter the functional properties of several enzymes, proteins, and ion channels by oxidation of amino acids such as histidine, methionine, tryptophan, and tyrosine (Weil et al., 1951; Chatterjee and Noltmann, 1967). Furthermore, these cytotoxic species induce direct oxidative damage to cellular organelles and promote apoptosis (for a review, Triesscheijn et al., 2006).
10. Methylene Blue and Neurotransmitter Systems
MB influences neuronal communication by altering cholinergic (Cook, 1926; Pfaffendorf et al., 1997), monoaminergic (Ramsay et al., 2007), and glutamatergic (Vutskits et al., 2008) synaptic neurotransmission both in the central and the peripheral nervous systems. MB modulates the cholinergic system at multiple levels. At the receptor level, MB competitively displaces the binding of muscarinic acetylcholine receptor antagonists such as [3H]quinuclidinyl benzylate in cardiac myocytes (KD= 187 nM; Abi-Gerges et al., 1997) and [3H]-N-Methylscopolamine (KD= 550 nM; Pfaffendorf et al., 1997) in cardiac membrane homogenates. However, cognitive deficits induced by scopolamine (a muscarinic antagonist) are reversed by MB (Deiana et al., 2009), suggesting that MB can still exert beneficial effects when muscarinic receptors are blocked.
At the synaptic level, MB increases acetylcholine concentrations by inhibiting the function of acetylcholinesterase (AChE), an enzyme that hydrolyzes acetylcholine. In fact, MB shares this action mechanism with other AChE inhibitors presently used as long-term symptomatic treatment in patients with Alzheimer's Disease (see below). Earlier studies indicated that AChE activity in cow erythrocytes was competitively inhibited by MB with an IC50 of 0.57 μM (Augustinsson, 1950). Interestingly, the extent of inhibition by MB was decreased significantly when the incubation time of MB was increased. Since the reduced form of MB was ineffective on AChE activity, it was concluded that the formation of leucoMB during prolonged incubation periods causes decreased efficacy of MB in in vitro assay systems. In a more recent study, MB inhibited the esterase activity of human plasma and bovine AChE concentration-dependently with IC50 values of 1.1 μM, and 0.42 μM, respectively (Pfaffendorf et. al., 1997).
The second major form of cholinesterase is butyrylcholinesterase (BuChE, also known as pseudocholinesterase; plasma cholinesterase, or formally, acylcholine acylhydrolase), found primarily in the liver and to a lesser extent in the brain. The two forms, AChE and BuChE, differ genetically, structurally, and in their kinetics. Butyrylcholine is not a physiological substrate in the mammalian brain, which makes the function of BuChE difficult to interpret. Nevertheless, MB inhibits the activity of BuChE in a concentration-dependent manner with IC50 values ranging between 0.4 μM and 5.3 μM (Pfaffendorf et. al., 1997; Kucukkilinc and Ozer, 2007, Yucel et al., 2008). Finally, in addition to MB, several other phenothiazine derivatives also block the activity of BuChE with Kd values ranging from 4 nM to 10 μM (Radic et al., 1993; Debord et al., 2002; Darvesh et al., 2005; 2007). It appears that the extent of MB inhibition of cholinesterase activity differs significantly in an isoenyme- (AChE versus BuChE) and species-dependent manner (Augustinsson, 1950; Pfaffendorf et. al., 1997).
MB can also affect the metabolism of choline, a precursor for acetylcholine synthesis, by inhibiting the activity of choline oxidase (COx), an enzyme that oxidizes choline to betaine aldehyde (Tacal and Ozer, 2006). COx activity has been detected in the brain (Li et al., 2007) and intraperitoneal injection of this enzyme impairs learning and memory in mice (Ikarashi et al., 2000). However, the physiological importance of MB inhibition of COx is not clear since choline deficiencies are completely compensated in a mouse knock-out model (Li et al., 2007).
MB also modulates the glutamatergic system. In rat hippocampal slices, glutamatemediated synaptic transmission is abolished by relatively high concentrations (5-50 μM) of MB (Vutskits et al., 2008). Although glutamatergic transmission plays important roles in learning and memory and MB is known to improve cognitive functions in animal models (Martinez et al., 1978; Callaway et al., 2002, 2004; Gonzalez-Lima and Bruchey, 2004; Wrubel et al., 2007), currently there are no studies on the actions of MB on glutamate receptors, neither ionotropic nor metabotropic.
Both glutamate and dopamine have been implicated in the pathogenesis of psychoses and MB has been the lead compound for the development of classical antipsychotics such as chlorpromazine (for reviews; Kristiansen, 1989; Wainwright, 2003). Considering the similarities in the chemical structures of MB and antipsychotics of the phenothiazine class, it is likely that MB modulates the activity of dopamine receptors. Although there are no current studies on the actions of MB on dopamine receptors, the antipsychotic effects of MB have been known for more than a century (Bodoni, 1899). Knowing that MB acts on nerve cells, Bodoni used MB to effectively calm psychotic agitation, also reporting that many other physicians in the Genova region were administering it for the same indication (Healey, 2002). More recent preclinical (Klamer et al., 2004) and clinical studies have confirmed the beneficial effects of MB in the treatment of psychotic disorders (Narsapur and Naylor, 1983; Thomas and Callender, 1985; Naylor et al., 1986; 1988; Deutsch et al., 1997).
MB has also beneficial effects in brain disorders traditionally linked to disturbances in the serotonergic system. For example, MB showed antidepressant actions in clinical trials (Naylor et al., 1986; 1987). More recently, anxiolytic and antidepressant-like activities of MB were reported in various behavioral animal studies (Eroglu and Caglayan, 1997; De-Oliveira and Guimaraes, 1999; Volke et al., 2003; Patil et al., 2005). Cellular mechanisms mediating antidepressant actions of MB have been investigated in several earlier studies. Wegener et al. (2000) demonstrated that NOS inhibitors including MB increased extracellular levels of serotonin (5-HT) and dopamine in the rat ventral hippocampus after local or systemic administration, whereas the NO precursor L-arginine had the opposite effect. In another study, 7-nitroindazole, a neuronal NOS inhibitor, potentiated the effect of bupropion, a dopamine reuptake inhibitor, and this effect was antagonized by L-arginine (Dhir and Kulkarni, 2007). Thus, these studies suggest that endogenous NO exerts a negative control over monoamine levels and that inhibition of NO synthesis may underlie the antidepressant action of MB.
However, contradictory evidence also exists concerning the influence of NO on monoamine levels. For example, L-arginine has been shown to induce dopamine release from the striatum and to increase the extracellular levels of 5-HT and dopamine in the medial preoptic area in vivo (Strasser et al., 1994; Lorrain and Hull, 1993). Therefore, in addition to the cGMP pathway, additional molecular targets are likely to play a role in the antidepressant actions of MB.
The inhibition of monoamine oxidase (MAO) activity by MB has been known for many decades (Philpot, 1937; Philpot and Cantoni, 1941; Imaizumi et al., 1959; Ehringer et al., 1961; Cotzias et al., 1974). It was reported that MB (0.5 μM) potently inhibited the activity of MAO in guinea pig liver homogenates and potentiated the vasoconstriction induced by tyramine (Imaizumi et al., 1959). Binding of MB to MAO in mouse brain has also been shown in histochemical studies (Cotzias et al., 1974).
5-HT is considered to be a relatively specific substrate for MAO-A, but not for MAO-B and the pharmacological profiles of antidepressants are closely associated with selective inhibition of MAO-A. Recent studies indicated that MB selectively inhibits the function of MAO type A, with IC50 values ranging from to 27 to 180 nM (Oxenkrug et al., 2007; Ramsay et al., 2007). Induction of melatonin biosynthesis by MB and antidepressant effects related to MB inhibition of MAO-A have also been shown (Oxenkrug et al., 2007).
11. Methylene Blue and Serotonin Toxicity
Recently, the effects of MB on monoaminergic transmission have attracted considerable attention in unfolding the mechanisms of prolonged CNS side effects of MB observed in some patients after surgical procedures. For example, preoperative intravenous administration of MB is a widely used practice to identify neuronal structures during parathyroid surgery (Dudley et al., 1971; Gordon et al., 1975; Sherlock and Holl-Allen, 1984; Muslumanoglu et al., 1995; Kuriloff and Sanborn, 2004; Sweet and Standiford, 2007). However, during the past few years, an emerging number of clinical observations have suggested that MB infusion during parathyroid surgery can induce prolonged disorientation, dizziness, headache, tremors, seizures, and mental confusion during the postoperative period (Martindale and Stedeford, 2003; Bach et al., 2004; Kartha et al., 2006; Mathew et al., 2006; Majithia and Stearns, 2006; Khan et al., 2007; Ng et al., 2008). It was found that these symptoms usually occur in patients who are also under medication of serotonin reuptake inhibitors (SRIs). Since MB is a relatively specific inhibitor of MAO-A (Aeschlimann et al., 1996; Oxenkrug et al., 2007; Ramsay et al., 2007) in patients administered SRIs such as citalopram, clomipramine, duloxetine, imipramine, sibutramine, and venlafaxine, intravenous infusion of MB, especially at doses exceeding 5 mg/kg (Gillman, 2006; 2008; Ramsay et al., 2007; Khavandi et al., 2008), may precipitate 5-HT toxicity or 5-HT syndrome (Boyer and Shannon, 2005). For this reason, the use of MB is contraindicated in patients treated with SRIs (Parlow et al., 2008; Stanford et al., 2009).
12. Methylene Blue and Neurodegenerative Diseases
Recent clinical studies indicate that MB may also have a beneficial effect on the cognitive performance of patients with Alzheimer's disease (AD) (Wischik et al., 2008; see also Gura et al., 2008; Sullivan, 2008; Oz et al. 2009). AD, a progressive neurodegenerative disorder characterized by irreversible cognitive impairment, is the leading cause of dementia in the elderly population. Its neuropathology is characterized by senile plaques (SP), neurofibrillary tangles, and neuronal cell death (see Goedert and Spillantini, 2006; Zhu et al., 2007 for review). SP are accumulations of amyloid beta protein, which is produced by cleavage of amyloid precursor protein. Neurofibrillary tangles are intracellular cytoplasmic aggregates of hyperphosphorylated microtubule-associated tau-protein (Binder et al., 2005; Neve and McPhie, 2006). Cell death preferentially affects neurons of the basal forebrain, the main source of cholinergic innervation in the forebrain, leading to a marked reduction in cholinergic markers in the cortex. It is currently not yet clear whether improper amyloid precursor protein processing (amyloid hypothesis), abnormal tau phosphorylation (tau hypothesis) or reduced acetylcholine synthesis (cholinergic hypothesis) lies at the root of AD.
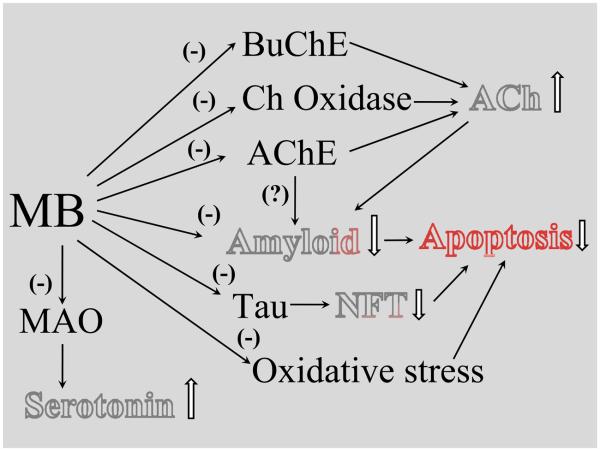
MB influences all three key neuropathological features of AD (Figure 2). Amyloid plaques are extracellular fibrillar deposits formed by aggregation of Aβ, which first forms oligomers and then fibrils. Amyloid precursor protein is an integral membrane protein, which is metabolized by several metabolic pathways. When amyloid precursor protein is cleaved extracellularly by β or γ secretase, the amyloidogenic peptide is released, whereas α secretase generates the secreted form of amyloid precursor protein α, which prevents formation of amyloidogenic fragments (Govoni et al., 2001). MB has been reported to inhibit the formation of Aβ oligomers (Necula et al., 2007a). However, data regarding fibril formation are conflicting; both inhibition (Taniguchi et al., 2005) and promotion (Necula et al., 2007a,b) of fibril formation have been described. Recently, quinacrine, a closely related derivative of MB and other thiazine-related dyes, was also reported to inhibit Aβ fibril formation (Necula et al., 2007b; Dolphin et al., 2008).
Figure 2.
A schematic view of methylene blue (MB) actions with potential beneficial effects on the progression of Alzheimer Disease. Pathological hallmarks and key neurotransmitter changes of Alzheimer Disease are highlighted. Abbreviations: AChE, acetylcholine esterase; BuChE, butyrylcholine esterase; Choline Ox, Choline oxidase; MAO, monoamine oxidase; NFT, neurofibrillary tangles.
Acetylcholine plays an important role in cognitive functions, especially memory, and depletion of cholinergic markers is an invariable feature of AD. Because AChE inhibitors increase synaptic acetylcholine levels, thereby enhancing cholinergic neurotransmission indirectly, they are currently used in the symptomatic treatment of AD (Nordberg, 2006; Holzgrabe et al., 2007). As mentioned earlier, MB increases cholinergic transmission by inhibiting both AChE (Augustinsson, 1950) and BuChE (Kucukkilinc and Ozer, 2007; Yucel et al., 2008). BuChE has been detected in neuritic plaques and tangles occurring in the brains of AD patients. Whereas brain AChE activity decreases in the course of AD, BuChE activity rises progressively (Giacobini, 2003) and BuChE possibly compensates for diminished AChE activity. When BuChE is selectively inhibited in rodents, learning performance improves and β-amyloid protein levels decrease (Greig et al., 2005). It has therefore been proposed that substances which inhibit the activities of both AChE and BuChE are most efficient in treating advanced AD (Giacobini, 2003; Geula and Darvesh, 2004).
AChE also interacts with senile plaques by forming stable and highly toxic AChE-amyloid- Aβ complexes (Nordberg, 2006). When AChE activity is inhibited, AChE-Aβ complex formation is impaired and expression of nicotinic receptors is increased, resulting in improved cognitive performance in AD patients (for reviews Nordberg, 2006, Holzgrabe et al., 2007; Inestrosa et al., 2008).
Dyes closely related to MB have been known to stain tau and beta amyloid-related cellular pathologies in AD (Mena et al., 1995) and have recently been proposed as a neuropathological tool for the rapid diagnosis of AD in tissue imprints (Luna-Munoz et al., 2008). The idea that MB may be a potential therapeutic against AD arose from the observation that tau filaments could be dissolved by a number of phenothiazines including MB and its desmethylated derivatives Azure A, Azure B, and tolonium chloride (Wischik et al., 1996). Similarly, heparin-induced assembly of tau protein into filaments is inhibited in vitro by MB and several other phenothiazines such as azure A, azure B, and quinacrine mustard (Taniguchi et al., 2005). The first and fourth repeats of the microtubule-binding domain (MBD) of the tau protein are essential for this inhibitory activity of MB upon filament formation (Hattori et al., 2008).
In addition to cholinergic disturbances, alterations in serotonergic transmission have also been reported in AD (Lorke et al., 2006). Association of AD with a loss of serotonergic neurons in the raphe nuclei, a reduction in serotonergic projection fibers in the cerebral cortex, a marked depletion in cortical 5-HT, and a significant decrease in cortical neurons expressing 5-HT receptors has been shown (Lorke et al., 2006). Elevated brain 5-HT levels as a result of MB-induced inhibition of MAO-A activity (Oxenkrug et al., 2007; Ramsay et al., 2007) may also have a beneficial effect on AD.
Other biological effects of MB that are not related to neurotransmitter systems may also influence AD-related events such as the progression of this disease (Atamna et al., 2008), memory processing, and task learning (Callaway et al., 2004; Gonzalez-Lima and Bruchey, 2004). MB has been shown to accumulate within mitochondria (Hassan and Fridovich, 1979), improve mitochondrial respiration by shuttling electrons to oxygen in the electron transport chain (Lindahl and O'berg, 1961; Visarius et al., 1997), and inhibit the production of superoxide (Kelner et al., 1988; Salaris et al. 1991). Recently, MB has been suggested to have antisenescence properties by interacting with specific mitochondrial electron carriers in its oxidized and reduced forms (Atamna et al., 2008).
Effects of MB on mitochondrial functions have also been utilized in ischemic and hypoxic conditions. MB shows significant neuroprotective effects in ischemia-reperfusion models of the brain (Wiklund et al., 2007) and the spinal cord (Bardakci et al., 2006). Similarly, retinal neurodegeneration induced by rotenone, an inhibitor of mitochondrial enzyme complex I (Zhang et al., 2006), and neurotoxic effects of methylmalonate, an inhibitor of mitochondrial enzyme complex II (Furian et al., 2007), are significantly attenuated by MB.
Transmissible spongiform encephalopathies (TSE) are fatal and incurable neurodegenerative disorders caused by prions, a class of proteins that target predominantly the CNS. Several phenothiazines closely related to MB (Amaral and Kristiansen, 2001) have been investigated for the treatment of TSEs, which include Creutzfeldt-Jakob disease in humans, bovine spongiform encephalopathy in cattle, and scrapie in sheep. Although MB was found to be ineffective in in vitro studies (Korth et al., 2001), quinacrine, a derivative of MB (Korth et al., 2001; Spilman et al., 2008), and chlorpromazine, closely related to MB (Dees et al., 1985; Korth et al., 2001; Kocisko et al., 2003), inhibited the conversion of soluble prion protein into the protease-resistant form and prion protein-related cellular toxicities. However, in-vitro effects of quinacrine vary significantly depending on the tissues, animal species, and experimental conditions (Cronier et al., 2007) and the efficacy of quinacrine has been questioned in a recent clinical study (Collinge et al., 2009).
13. Conclusions
In summary, MB has a broad range of targets encompassing multiple neurotransmitter systems, ion channels, and enzymes involved in various physiological functions of the nervous system. It appears that many of the biological effects of MB are closely associated with its unique physicochemical properties, including its redox characteristics, ionic charges, and light spectrum characteristics. MB has a high solubility in aqueous media; preclinical and clinical studies demonstrate a low toxicity profile (Küpfer et al., 1994; Riha et al., 2005). In addition, its ability to permeate cellular membranes and to cross the blood-brain barrier (Peter et al., 2000) makes MB attractive as a potential therapeutic agent. Recent clinical investigations on the biological effects of MB range from new trials on malaria treatment (Coulibaly et al., 2009) to the therapy of depression and Alzheimer's disease. However, there are still many open questions regarding the effect of MB in CNS disorders and further studies elucidating the molecular and cellular targets of MB actions are needed.
Acknowledgements
The authors cordially thank Ms. Mary Pfeiffer of NIDA/NIH for her careful editing of the manuscript and Ms. Betty Murgolo of (NIH Library) for her relentless efforts to provide most of the articles cited in this review. Currently the number of articles related to “Methylene Blue” is approaching 11,000 PubMed. Given the wide breadth of studies examining the effects of MB on biological systems, only the recent reviews and articles closely related to subjects discussed in this review have been cited in many instances and we apologize to those whose work may have been omitted due to space considerations. However, given the importance of the studies during the 19th century, we have also tried to cover the seminal studies of this era not covered by PubMed.
REFERENCES
- Abi-Gerges N, Eschenhagen T, Hove-Madsen L, Fischmeister R, Mery PF. Methylene blue is a muscarinic antagonist in cardiac myocytes. Mol Pharmacol. 1997;52:482–490. doi: 10.1124/mol.52.3.482. [DOI] [PubMed] [Google Scholar]
- Aeschlimann C, Cerny T, Küpfer A. Inhibition of (mono)amine oxidase activity and prevention of ifosfamide encephalopathy by methylene blue. Drug Metab Dispos. 1996;24:1336–1339. [PubMed] [Google Scholar]
- Ajithkumar T, Parkinson C, Shamshad F, Murray P. Ifosfamide encephalopathy. Clin Oncol. 2007;19:108–114. doi: 10.1016/j.clon.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Albert M, Lessin MS, Gilchrist BF. Methylene blue: dangerous dye for neonates. J. Pediatr. Surg. 2003;38:1244–1245. doi: 10.1016/s0022-3468(03)00278-1. [DOI] [PubMed] [Google Scholar]
- Alici-Evcimen Y, Breitbart WS. Ifosfamide neuropsychiatric toxicity in patients with cancer. Psychooncology. 2007;16:956–960. doi: 10.1002/pon.1161. [DOI] [PubMed] [Google Scholar]
- Amaral L, Kristiansen JE. Phenothiazines: potential management of Creutzfeldt-Jacob disease and its variants. Int J Antimicrob Agents. 2001;18:411–417. doi: 10.1016/s0924-8579(01)00432-0. [DOI] [PubMed] [Google Scholar]
- Arieff AJ, Pyzik SW. Quadriplegia after intrathecal injection of methylene blue. JAMA. 1960;173:794–796. doi: 10.1001/jama.1960.73020250011008d. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Croop RS. Simulation of Na channel inactivation by thiazine dyes. J Gen Physiol. 1982;80:641–662. doi: 10.1085/jgp.80.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22:703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- Augustinsson K. Methylene blue as an inhibitor of acetyl-choline-esterase. Acta Chem Scand. 1950;4:536–542. [Google Scholar]
- Bach KK, Lindsay FW, Berg LS, Howard RS. Prolonged postoperative disorientation after methylene blue infusion during parathyroidectomy. Anesth Analg. 2004;99:1573–1574. doi: 10.1213/01.ANE.0000134860.73875.CF. [DOI] [PubMed] [Google Scholar]
- Barbosa P, Peters TM. The effects of vital dyes on living organisms with special reference to methylene blue and neutral red. Histochem J. 1971;3:71–93. doi: 10.1007/BF01686508. [DOI] [PubMed] [Google Scholar]
- Bardakci H, Kaplan S, Karadeniz U, Ozer C, Bardakci Y, Ozogul C, Birincioglu CL, Cobanoglu A. Methylene blue decreases ischemia-reperfusion (I/R)-induced spinal cord injury: an in vivo study in an I/R rabbit model. Eur Surg Res. 2006;38:482–488. doi: 10.1159/000096007. [DOI] [PubMed] [Google Scholar]
- Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer's disease. Biochimica et Biophysica Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Blass N, Fung D. Dyed but not dead-methylene blue overdose. Anesthesiology. 1976;45:458–459. doi: 10.1097/00000542-197610000-00020. [DOI] [PubMed] [Google Scholar]
- Bodoni P. Dell'azione sedativa del bleu di metilene in varie forme di psicosi. Clin Med Ital. 1899;24:217–222. [Google Scholar]
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- Brooks MM. Methylene Blue as an Antidote for Cyanide and Carbon Monoxide Poisoning. The Scientific Monthly. 1936;43:585–586. [Google Scholar]
- Buchholz K, Schirmer RH, Eubel JK, Akoachere MB, Dandekar T, Becker K, Gromer S. Interactions of methylene blue with human disulfide reductases and their orthologues from Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52:183–191. doi: 10.1128/AAC.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budavari S, O'Neil MJ, Smith A, Heckelman PE, Kinneary JF, editors. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Vol. 12. Merck; New Jersey: 1996. p. 1035. [Google Scholar]
- Burmistrov IuM, Liudkovskaia RG, Shuranova ZhP. Electric activity of crayfish neurons during vital staining with methylene blue. Biofizika. 1969;14:495–501. [PubMed] [Google Scholar]
- Burrows GE. Methylene blue: effects and disposition in sheep. J Vet Pharmacol Ther. 1984;7:225–231. doi: 10.1111/j.1365-2885.1984.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Callaway NL, Riha PD, Wrubel KM, McCollum D, Gonzalez-Lima F. Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats. Neurosci Lett. 2002;332:83–86. doi: 10.1016/s0304-3940(02)00827-3. [DOI] [PubMed] [Google Scholar]
- Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav. 2004;77:175–181. doi: 10.1016/j.pbb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Caro H. 1877 Engl. Pat. 3751. 9/10.
- Caetano W, Haddad PS, Itri R, Severino D, Vieira VC, Baptista MS, Schröder AP, Marques CM. Photo-induced destruction of giant vesicles in methylene blue solutions. Langmuir. 2007;23:1307–1314. doi: 10.1021/la061510v. [DOI] [PubMed] [Google Scholar]
- Cawein M, Behlen CH, 2nd, Lappat EJ, Cohn JE. Hereditary diaphorase deficiency and methemoglobinemia. Arch Intern Med. 1964;113:578–85. doi: 10.1001/archinte.1964.00280100086014. [DOI] [PubMed] [Google Scholar]
- Chatterjee GC, Noltmann EA. Dye-sensitized photooxidation as a tool for the elucidation of critical amino acid residues in phosphoglucose isomerase. Eur J Biochem. 1967;2:9–18. doi: 10.1111/j.1432-1033.1967.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Conn HJ. Biological Stains. 7th Ed. Williams & Wilkins; Baltimore: 1961. [Google Scholar]
- Cheng KK. Intra-arterial injection of methylene blue for staining nerve endings in striated muscles. Nature. 1954;173:492–493. doi: 10.1038/173492b0. [DOI] [PubMed] [Google Scholar]
- Clark WM, Cohen B, Gibbs HD. Studies on Oxidation-Reduction: VIII. Methylene Blue. U.S. Public Health Reports. 1925;40:1131–1201. [Google Scholar]
- Clifton J, II, Leikin JB. Methylene blue. Am J Ther. 2003;10:289–291. doi: 10.1097/00045391-200307000-00009. [DOI] [PubMed] [Google Scholar]
- Coers C. The vital staining of muscle biopsies with methylene blue. J. Neurol. Neurosurg. Psychiat. 1952;15:211–215. doi: 10.1136/jnnp.15.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Gorham M, Hudson F, Kennedy A, Keogh G, Pal S, Rossor M, Rudge P, Siddique D, Spyer M, Thomas D, Walker S, Webb T, Wroe S, Darbyshire J. Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial. Lancet Neurol. 2009;8:334–344. doi: 10.1016/S1474-4422(09)70049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RP. The antagonism of acetylcholine by methylene blue. J Physiol. 1926;62:160–165. doi: 10.1113/jphysiol.1926.sp002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias GC, Tang LC, Ginos JZ. Monoamine oxidase and cerebral uptake of dopaminergic drugs. Proc Natl Acad Sci U S A. 1974;71:2715–2719. doi: 10.1073/pnas.71.7.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly B, Zoungrana A, Mockenhaupt FP, Schirmer RH, Klose C, Mansmann U, Meissner PE, Müller O. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomised controlled trial. PLoS One. 2009;4:e5318. doi: 10.1371/journal.pone.0005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragan JD. Teratogen update: methylene blue. Teratology. 1999;60:42–48. doi: 10.1002/(SICI)1096-9926(199907)60:1<42::AID-TERA12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Cronier S, Beringue V, Bellon A, Peyrin JM, Laude H. Prion strain- and species-dependent effects of antiprion molecules in primary neuronal cultures. J Virol. 2007;81:13794–13800. doi: 10.1128/JVI.01502-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvesh S, McDonald RS, Penwell A, Conrad S, Darvesh KV, Mataija D, Gomez G, Caines A, Walsh R, Martin E. Structure-activity relationships for inhibition of human cholinesterases by alkyl amide phenothiazine derivatives. Bioorg Med Chem. 2005;13:211–222. doi: 10.1016/j.bmc.2004.09.059. [DOI] [PubMed] [Google Scholar]
- Darvesh S, McDonald RS, Darvesh KV, Mataija D, Conrad S, Gomez G, Walsh R, Martin E. Selective reversible inhibition of human butyrylcholinesterase by aryl amide derivatives of phenothiazine. Bioorg Med Chem. 2007;15:6367–6378. doi: 10.1016/j.bmc.2007.06.060. [DOI] [PubMed] [Google Scholar]
- Debord J, Merle L, Bollinger JC, Dantoine T. Inhibition of butyrylcholinesterase by phenothiazine derivatives. J Enzyme Inhib Med Chem. 2002;17:197–202. doi: 10.1080/1475636021000003165. [DOI] [PubMed] [Google Scholar]
- Dees C, Wade WF, German TL, Marsh RF. Inactivation of the scrapie agent by ultraviolet irradiation in the presence of chlorpromazine. J Gen Virol. 1985;66:845–849. doi: 10.1099/0022-1317-66-4-845. [DOI] [PubMed] [Google Scholar]
- Deiana S, Harrington CR, Wischik CM, Riedel G. Methylthioninium chloride reverses cognitive deficits induced by scopolamine: comparison with rivastigmine. Psychopharmacology (Berl) 2009;202:53–65. doi: 10.1007/s00213-008-1394-2. [DOI] [PubMed] [Google Scholar]
- De-Oliveira RW, Guimarães FS. Anxiolytic effect of methylene blue microinjected into the dorsal periaqueductal gray matter. Braz J Med Biol Res. 1999;32:1529–1532. doi: 10.1590/s0100-879x1999001200012. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Paul SM, Tomasino V, Koetzner L, Morn CB, Mastropaolo J. 7-Nitroindazole and methylene blue, inhibitors of neuronal nitric oxide synthase and NO-stimulated guanylate cyclase, block MK-801-elicited behaviors in mice. Neuropsychopharmacology. 1996;15:37–43. doi: 10.1016/0893-133X(95)00153-5. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Schwartz BL, Fay-McCarthy M, Rosenberg PB, Fearing K. Methylene blue adjuvant therapy of schizophrenia. Clin Neuropharmacol. 1997;20:357–363. doi: 10.1097/00002826-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of bupropion, a dopamine reuptake inhibitor. Eur J Pharmacol. 2007;568:177–185. doi: 10.1016/j.ejphar.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Di Cataldo A, Astuto M, Rizzo G, Bertuna G, Russo G, Incorpora G. Neurotoxicity during ifosfamide treatment in children. Med Sci Monit. 2009;15:CS22–25. [PubMed] [Google Scholar]
- DiSanto AR, Wagner JG. Pharmacokinetics of highly ionized drugs. II. Methylene blue--absorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci. 1972;61:1086–90. doi: 10.1002/jps.2600610710. [DOI] [PubMed] [Google Scholar]
- Dolk H. Methylene blue and atresia or stenosis of ileum and jejunum. EUROCAT Working Group. Lancet. 1991;338:1021–1022. doi: 10.1016/0140-6736(91)91885-x. [DOI] [PubMed] [Google Scholar]
- Dolphin GT, Chierici S, Ouberai M, Dumy P, Garcia J. A multimeric quinacrine conjugate as a potential inhibitor of Alzheimer's beta-amyloid fibril formation. Chembiochem. 2008;14:952–963. doi: 10.1002/cbic.200700602. [DOI] [PubMed] [Google Scholar]
- Dudley NE. Methylene blue for rapid identification of the parathyroids. Br Med J. 1971;3:680–681. doi: 10.1136/bmj.3.5776.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O, Lechner K. The effect of methylene blue on monoamine oxidase and the catechol amine and 5-hydroxytryptamine metabolism of the brain. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1961;241:568–582. [PubMed] [Google Scholar]
- Ehrlich P. Über die Methylenblaureaction der lebenden Nervensubstanz. Dtsch Med Wochenschr. 1886;12:49–52. [Google Scholar]
- Ehrlich P, Leppmann A. Über schmerzstillende Wirkung des Methylenblau. In: Himmelweil F, Marquardt M, Dale H, editors. The collected papers of Paul Ehrlich. I. Pergamon Press; London: 1890. pp. 555–8. 1956. reprinted from Dtsch Med Wochenschr 1890. [Google Scholar]
- Eroglu L, Caglayan B. Anxiolytic and antidepressant properties of methylene blue in animal models. Pharmacol Res. 1997;36:381–385. doi: 10.1006/phrs.1997.0245. [DOI] [PubMed] [Google Scholar]
- Eusebio EB, Graham J, Mody N. Treatment of intractable pruritus ani. Dis Colon Rectum. 1990;33:770–772. doi: 10.1007/BF02052324. [DOI] [PubMed] [Google Scholar]
- Evans JP, Keegan HR. Danger in the use of intrathecal methylene blue. JAMA. 1960;174:856–859. doi: 10.1001/jama.1960.03030070034007. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB. Intrathecal administration of prostaglandin E2 causes sensitization of the primary afferent neuron via the spinal release of glutamate. Inflamm Res. 1996;45:499–502. doi: 10.1007/BF02311085. [DOI] [PubMed] [Google Scholar]
- Fischer R, Zeman W. Neuronal dye-sorption as a histochemical indicator of nervous activity. Nature. 1959;183:1337–1338. doi: 10.1038/1831337a0. [DOI] [PubMed] [Google Scholar]
- Frankenburg FR, Baldessarini RJ. Neurosyphilis, malaria, and the discovery of antipsychotic agents. Harv Rev Psychiatry. 2008;16:299–307. doi: 10.1080/10673220802432350. [DOI] [PubMed] [Google Scholar]
- Frimer ML, Cohen MM, Harrison RC, Holubitsky IB. The selective nerve stain leucomethylene blue as an intraoperative aid to achieving complete vagotomy. Gut. 1970;11:881–882. doi: 10.1136/gut.11.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furian AF, Fighera MR, Oliveira MS, Ferreira AP, Fiorenza NG, de Carvalho Myskiw J, Petry JC, Coelho RC, Mello CF, Royes LF. Methylene blue prevents methylmalonate-induced seizures and oxidative damage in rat striatum. Neurochem Int. 2007;50:164–171. doi: 10.1016/j.neuint.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Garcia-Lopez P, Garcia-Marin V, Freire M. The discovery of dendritic spines by Cajal in 1888 and its relevance in the present neuroscience. Prog Neurobiol. 2007;83:110–130. doi: 10.1016/j.pneurobio.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Garthwaite G, Garthwaite J. Cyclic GMP and cell death in rat cerebellar slices. Neuroscience. 1988;26:321–326. doi: 10.1016/0306-4522(88)90148-0. [DOI] [PubMed] [Google Scholar]
- Geula C, Darvesh S. Butyrylcholinesterase, cholinergic neurotransmission and the pathology of Alzheimer's disease. Drugs Today (Barc) 2004;40:711–721. doi: 10.1358/dot.2004.40.8.850473. [DOI] [PubMed] [Google Scholar]
- Giacobini E. Cholinesterases: new roles in brain function and in Alzheimer's disease. Neurochem Res. 2003;28:515–522. doi: 10.1023/a:1022869222652. [DOI] [PubMed] [Google Scholar]
- Gillman PK. Methylene blue implicated in potentially fatal serotonin toxicity. Anaesthesia. 2006;61:1013–1014. doi: 10.1111/j.1365-2044.2006.04808.x. [DOI] [PubMed] [Google Scholar]
- Gillman PK. Methylene blue is a potent monoamine oxidase inhibitor. Can J Anaesth. 2008;55:311–312. doi: 10.1007/BF03017212. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Gluer S. Intestinal atresia following intraamniotic use of dyes. Eur J Pediatr Surg. 1995;5:240–242. doi: 10.1055/s-2008-1066215. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Bruchey AK. Extinction memory improvement by the metabolic enhancer methylene blue. Learn Mem. 2004;11:633–640. doi: 10.1101/lm.82404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DL, Airan MC, Thomas W, Seidman LH. Parathyroid identification by methylene blue infusion. Br J Surg. 1975;62:747–749. doi: 10.1002/bjs.1800620919. [DOI] [PubMed] [Google Scholar]
- Govoni S, Lanni C, Racchi M. Advances in understanding the pathogenetic mechanisms of Alzheimer's disease. Functional Neurology. 2001;16:17–30. [PubMed] [Google Scholar]
- Gracia R, Shepherd G. Cyanide Poisoning and Its Treatment. Pharmacotherapy. 2004;24:1358–1365. doi: 10.1592/phco.24.14.1358.43149. [DOI] [PubMed] [Google Scholar]
- Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, Yu QS, Mamczarz J, Holloway HW, Giordano T, Chen D, Furukawa K, Sambamurti K, Brossi A, Lahiri DK. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc Natl Acad Sci U S A. 2005;102:17213–17218. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gura T. Hope in Alzheimer's fight emerges from unexpected places. Nat Med. 2008;14:894. doi: 10.1038/nm0908-894. [DOI] [PubMed] [Google Scholar]
- Guttmann P, Ehrlich P. Über die Wirkung des Methylenblau bei Malaria. Berlin Klin Woch. 1891;28:953–956. [Google Scholar]
- Harvey SC. Antiseptics and Disinfectants; Fungicides, Ectoparasiticides. In: Goodman AG, Goodman LS, Gilman A, Mayer SE, Melmon KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. MacMillan Publishing Co., Inc.; New York: 1980. p. 980. [Google Scholar]
- Hassan HM, Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979;196:385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hattori M, Sugino E, Minoura K, In Y, Sumida M, Taniguchi T, Tomoo K, Ishida T. Different inhibitory response of cyanidin and methylene blue for filament formation of tau microtubule-binding domain. Biochem Biophys Res Commun. 2008;374:158–163. doi: 10.1016/j.bbrc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Healy D. The Creation of Psychopharmacology. Harvard University Press; Cambridge, MA: 2002. [Google Scholar]
- Helander A, Cronholm T, Tottmar O. Inhibition of aldehyde dehydrogenases by methylene blue. Biochem Pharmacol. 1993;46:2135–2158. doi: 10.1016/0006-2952(93)90601-r. [DOI] [PubMed] [Google Scholar]
- Herman MI, Chyka PA, Butler AY, Rieger SE. Methylene blue by intraosseous infusion for methemoglobinemia. Ann Emerg Med. 1999;33:111–113. doi: 10.1016/s0196-0644(99)70427-0. [DOI] [PubMed] [Google Scholar]
- Hines M, Tower SS. Studies on the innervation of skeletal muscles. II. Of muscle spindles in certain muscles of the kitten. Johns Hopkins Hospital Bulletin. 1928;42:264–295. [Google Scholar]
- Hirsch JI, Banks WL, Jr, Sullivan JS, Horsley JS. Effect of methylene blue on estrogen-receptor activity. Radiology. 1989;171:105–107. doi: 10.1148/radiology.171.1.2467322. [DOI] [PubMed] [Google Scholar]
- Holzgrabe U, Kapkova P, Alptuzun V, Scheiber J, Kugelmann E. Targeting acetylcholinesterase to treat neurodegeneration. Expert Opin Ther Targets. 2007;11:161–179. doi: 10.1517/14728222.11.2.161. [DOI] [PubMed] [Google Scholar]
- Ikarashi Y, Kuribara H, Shiobara T, Takahashi A, Ishimaru H, Maruyama Y. Learning and memory in mice treated with choline oxidase, a hydrolytic enzyme for choline. Pharmacol Biochem Behav. 2000;65:519–522. doi: 10.1016/s0091-3057(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Imaizumi R, Omori K, Unoki A, Sano K, Watari Y, Namba J, Inui K. Physiological significance of monoamine oxidase. Jpn. J. Pharmacol. 1959;8:87–95. doi: 10.1254/jjp.8.87. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Dinamarca MC, Alvarez A. Amyloid-cholinesterase interactions. Implications for Alzheimer's disease. FEBS J. 2008;275:625–632. doi: 10.1111/j.1742-4658.2007.06238.x. [DOI] [PubMed] [Google Scholar]
- Iyengar B, Lal SK. Methylene blue and organised differentiation in the chick embryo. Acta Anat. 1985;123:220–223. doi: 10.1159/000146005. [DOI] [PubMed] [Google Scholar]
- Kartha SS, Chacko CE, Bumpous JM, Fleming M, Lentsch EJ, Flynn MB. Toxic metabolic encephalopathy after parathyroidectomy with methylene blue localization. Otolaryngol Head Neck Surg. 2006;135:765–768. doi: 10.1016/j.otohns.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Khan MA, North AP, Chadwick DR. Prolonged postoperative altered mental status after methylene blue infusion during parathyroidectomy: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W9–W11. doi: 10.1308/147870807X160434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khavandi A, Whitaker J, Gonna H. Serotonin toxicity precipitated by concomitant use of citalopram and methylene blue. Med J Aust. 2008;189:534–535. doi: 10.5694/j.1326-5377.2008.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Klamer D, Engel JA, Svensson L. Phencyclidine-induced behaviour in mice prevented by methylene blue. Basic Clin. Pharmacol. Toxicol. 2004;94:65–72. doi: 10.1111/j.1742-7843.2004.pto940203.x. [DOI] [PubMed] [Google Scholar]
- Kristiansen JE. Dyes, antipsychotic drugs, and antimicrobial activity. Fragments of a development, with special reference to the influence of Paul Ehrlich. Dan. Med. Bull. 1989;36:178–185. [PubMed] [Google Scholar]
- Kelner MJ, Bagnell R, Hale B, Alexander NM. Potential of methylene blue to block oxygen radical generation in reperfusion injury. Basic Life Sci. 1988;49:895–898. doi: 10.1007/978-1-4684-5568-7_146. [DOI] [PubMed] [Google Scholar]
- Kocisko DA, Baron GS, Rubenstein R, Chen J, Kuizon S, Caughey B. New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products. J Virol. 2003;77:10288–10294. doi: 10.1128/JVI.77.19.10288-10294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth C, May BC, Cohen FE, Prusiner SB. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc Natl Acad Sci U S A. 2001;98:9836–9841. doi: 10.1073/pnas.161274798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki A, Watanabe J. Dose dependency of apparent volumes of distribution for methylene blue in rabbits. J Pharmacobiodyn. 1981;4:49–57. doi: 10.1248/bpb1978.4.49. [DOI] [PubMed] [Google Scholar]
- Kress M, Petersen M, Reeh PW. Methylene blue induces ongoing activity in rat cutaneous primary afferents and depolarization of DRG neurons via a photosensitive mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:619–625. doi: 10.1007/pl00005098. [DOI] [PubMed] [Google Scholar]
- Kucukkilinc T, Ozer I. Multi-site inhibition of human plasma cholinesterase by cationic phenoxazine and phenothiazine dyes. Arch Biochem Biophys. 2007;461:294–298. doi: 10.1016/j.abb.2007.02.029. [DOI] [PubMed] [Google Scholar]
- Küpfer A, Aeschlimann C, Wermuth B, Cerny T. Prophylaxis and reversal of ifosfamide encephalopathy with methylene-blue. Lancet. 1994;343:763–764. doi: 10.1016/s0140-6736(94)91839-2. [DOI] [PubMed] [Google Scholar]
- Kuriloff DB, Sanborn KV. Rapid intraoperative localization of parathyroid glands utilizing methylene blue infusion. Otolaryngol. Head Neck Surg. 2004;131:616–622. doi: 10.1016/j.otohns.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Lee SK, Mills A. Novel photochemistry of leuco-Methylene Blue. Chem. Commun. (Camb) 2003;18:2366–2347. doi: 10.1039/b307228b. [DOI] [PubMed] [Google Scholar]
- Lewis RJ. Sax's Dangerous Properties of Industrial Chemicals. 8th ed. Vol. 2. Van Nostrand Reinhold; New York: 1992. p. 481. [Google Scholar]
- Li Z, Agellon LB, Vance DE. Choline redistribution during adaptation to choline deprivation. J Biol Chem. 2007;282:10283–10289. doi: 10.1074/jbc.M611726200. [DOI] [PubMed] [Google Scholar]
- Licker M, Diaper J, Robert J, Ellenberger C. Effects of methylene blue on propofol requirement during anaesthesia induction and surgery. Anaesthesia. 2008;63:352–357. doi: 10.1111/j.1365-2044.2007.05354.x. [DOI] [PubMed] [Google Scholar]
- Lorke DE, Lu G, Cho E, Yew DT. Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of Alzheimer and normal aging patients. BMC Neurosci. 2006;7:36. doi: 10.1186/1471-2202-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Hull EM. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport. 1993;5:87–89. doi: 10.1097/00001756-199310000-00024. [DOI] [PubMed] [Google Scholar]
- Louters LL, Dyste SG, Frieswyk D, Tenharmsel A, Vander Kooy TO, Walters L, Whalen T. Methylene blue stimulates 2-deoxyglucose uptake in L929 fibroblast cells. Life Sci. 2006;78:586–591. doi: 10.1016/j.lfs.2005.05.082. [DOI] [PubMed] [Google Scholar]
- Luna-Munoz J, Peralta-Ramirez J, Chávez-Macías L, Harrington CR, Wischik CM, Mena R. Thiazin red as a neuropathological tool for the rapid diagnosis of Alzheimer's disease in tissue imprints. Acta Neuropathol. 2008;116:507–515. doi: 10.1007/s00401-008-0431-x. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Luo D, Das S, Vincent SR. Effects of methylene blue and LY83583 on neuronal nitric oxide synthase and NADPH-diaphorase. Eur J Pharmacol. 1995;290:247–251. doi: 10.1016/0922-4106(95)00084-4. 1995. [DOI] [PubMed] [Google Scholar]
- Majithia A, Stearns MP. Methylene blue toxicity following infusion to localize parathyroid adenoma. J Laryngol Otol. 2006;120:138–140. doi: 10.1017/S0022215105005098. [DOI] [PubMed] [Google Scholar]
- Marczin N, Ryan US, Catravas JD. Methylene blue inhibits nitrovasodilator- and endothelium-derived relaxing factor-induced cyclic GMP accumulation in cultured pulmonary arterial smooth muscle cells via generation of superoxide anion. J Pharmacol Exp Ther. 1992;263:170–179. [PubMed] [Google Scholar]
- Martindale SJ, Stedeford JC. Neurological sequelae following methylene blue injection for parathyroidectomy. Anaesthesia. 2003;58:1041–1042. doi: 10.1046/j.1365-2044.2003.03415_23.x. [DOI] [PubMed] [Google Scholar]
- Martinez JL, Jr., Jensen RA, Vasquez BJ, McGuinness T, McGaugh JL. Methylene blue alters retention of inhibitory avoidance responses. Physiol Psychol. 1978;6:387–390. [Google Scholar]
- Masaki E, Kondo I. Methylene blue, a soluble guanylyl cyclase inhibitor, reduces the sevoflurane minimum alveolar anesthetic concentration and decreases the brain cyclic guanosine monophosphate content in rats. Anesth Analg. 1999;89:484–489. doi: 10.1097/00000539-199908000-00045. [DOI] [PubMed] [Google Scholar]
- Mathew S, Linhartova L, Raghuraman G. Hyperpyrexia and prolonged postoperative disorientation following methylene blue infusion during parathyroidectomy. Anaesthesia. 2006;61:580–583. doi: 10.1111/j.1365-2044.2006.04619.x. [DOI] [PubMed] [Google Scholar]
- May JM, Qu Z, Whitesell RR. Generation of oxidant stress in cultured endothelial cells by methylene blue: protective effects of glucose and ascorbic acid. Biochem Pharmacol. 2003;66:777–784. doi: 10.1016/s0006-2952(03)00408-8. [DOI] [PubMed] [Google Scholar]
- Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol. 1993;45:367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J Biol Chem. 1970;245:1374–1377. [PubMed] [Google Scholar]
- McFadyen I. The dangers of intra-amniotic methylene blue. Br J Obstet Gynaecol. 1992;99:89–90. doi: 10.1111/j.1471-0528.1992.tb14458.x. [DOI] [PubMed] [Google Scholar]
- Mellish KJ, Cox RD, Vernon DI, Griffiths J, Brown SB. In vitro photodynamic activity of a series of methylene blue analogues. Photochem Photobiol. 2002;75:392–397. doi: 10.1562/0031-8655(2002)075<0392:ivpaoa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mena R, Edwards P, Pérez-Olvera O, Wischik CM. Monitoring pathological assembly of tau and beta-amyloid proteins in Alzheimer's disease. Acta Neuropathol. 1995;89:50–56. doi: 10.1007/BF00294259. [DOI] [PubMed] [Google Scholar]
- Mentes BB, Akin M, Leventoglu S, Gultekin FA, Oguz M. Intradermal methylene blue injection for the treatment of intractable idiopathic pruritus ani: results of 30 cases. Tech Coloproctol. 2004;8:11–14. doi: 10.1007/s10151-004-0043-y. [DOI] [PubMed] [Google Scholar]
- Mihai R, Mitchell EW, Warwick J. Dose-response and postoperative confusion following methylene blue infusion during parathyroidectomy. Can J Anaesth. 2007;54:79–81. doi: 10.1007/BF03021907. [DOI] [PubMed] [Google Scholar]
- Minami T, Nishihara I, Ito S, Sakamoto K, Hyodo M, Hayaishi O. Nitric oxide mediates allodynia induced by intrathecal administration of prostaglandin E2 or prostaglandin F2 alpha in conscious mice. Pain. 1995;61:285–290. doi: 10.1016/0304-3959(94)00183-F. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006;176:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- Müller T. Supravital staining of murine brain with methylene blue according to the Cajal method: a simple and reliable preparation technique for embedding in paraffin. J Neurosci Methods. 1990;32:223–226. doi: 10.1016/0165-0270(90)90144-5. [DOI] [PubMed] [Google Scholar]
- Müller T. Methylene blue supravital staining: an evaluation of its applicability to the mammalian brain and pineal gland. Histol Histopathol. 1998;13:1019–1026. doi: 10.14670/HH-13.1019. [DOI] [PubMed] [Google Scholar]
- Muslumanoglu M, Terzioglu T, Ozarmagan S, Tezelman S, Guloglu R. Comparison of preoperative imaging techniques (thallium technetium scan and ultrasonography) and intraoperative staining (with methylene blue) in localizing the parathyroid glands. Radiol Med (Torino) 1995;90:444–447. [PubMed] [Google Scholar]
- Nahlieli O, Levy Y. Intravital staining with methylene blue as an aid to facial nerve identification in parotid gland surgery. J Oral Maxillofac Surg. 2001;59:355–356. doi: 10.1053/joms.2001.21014. [DOI] [PubMed] [Google Scholar]
- Nanasi PP, Dely M. Effects of methylene blue and ascorbate on transmembrane potential in frog skeletal muscle. Gen Pharmacol. 1995;26:1307–1311. doi: 10.1016/0306-3623(95)00007-n. [DOI] [PubMed] [Google Scholar]
- Narsapur SL, Naylor GJ. Methylene blue. A possible treatment for manic depressive psychosis. J Affect Disord. 1983;5:155–1561. doi: 10.1016/0165-0327(83)90008-3. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program NTP Toxicology and Carcinogenesis Studies of Methylene Blue Trihydrate (CAS No. 7220-79-3) in F344/N Rats and B6C3F1 Mice (Gavage Studies) Natl. Toxicol. Program Tech. Rep. Ser. 2008;540:1–224. [PubMed] [Google Scholar]
- Naylor GJ, Martin B, Hopwood SE, Watson Y. A two-year double-blind crossover trial of the prophylactic effect of methylene blue in manic-depressive psychosis. Biol Psychiatry. 1986;21:915–920. doi: 10.1016/0006-3223(86)90265-9. [DOI] [PubMed] [Google Scholar]
- Naylor GJ, Smith AH, Connelly P. A controlled trial of methylene blue in severe depressive illness. Biol Psychiatry. 1987;22:657–659. doi: 10.1016/0006-3223(87)90194-6. [DOI] [PubMed] [Google Scholar]
- Naylor GJ, Smith AH, Connelly P. Methylene blue in mania. Biol Psychiatry. 1988;24:941–942. doi: 10.1016/0006-3223(88)90229-6. [DOI] [PubMed] [Google Scholar]
- Necula M, Breydo L, Milton S, Kayed R, van der Veer WE, Tone P, Glabe CG. Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization. Biochemistry. 2007a;46:8850–8860. doi: 10.1021/bi700411k. [DOI] [PubMed] [Google Scholar]
- Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007b;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- Nedvidkova J, Sterzl I, Haluzík M, Schreiber V. An increase in the blood thyroxine level after methylene blue in rats: the interaction with carbimazole. Endocr Res. 1995;21:709–717. doi: 10.3109/07435809509030485. [DOI] [PubMed] [Google Scholar]
- Nedvidkova J, Pacak K, Haluzík M, Nedvídek J. The regulation of adenohypophyseal prolactin secretion: effect of triiodothyronine and methylene blue on estrogenized rat adenohypophysis. Physiol Res. 2000;49:S27–S35. [PubMed] [Google Scholar]
- Nedvidkova J, Pacak K, Haluzík M, Nedvídek J, Schreiber V. The role of dopamine in methylene blue-mediated inhibition of estradiol benzoate-induced anterior pituitary hyperplasia in rats. Neurosci Lett. 2001;304:194–198. doi: 10.1016/s0304-3940(01)01752-9. [DOI] [PubMed] [Google Scholar]
- Neve RL, McPhie DL. The cell cycle as a therapeutic target for Alzheimer's disease. Pharmacology and Therapeutics. 2006;111:99–113. doi: 10.1016/j.pharmthera.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Ng BK, Cameron AJ, Liang R, Rahman H. Serotonin syndrome following methylene blue infusion during parathyroidectomy: a case report and literature review. Can J Anaesth. 2008;55:36–41. doi: 10.1007/BF03017595. [DOI] [PubMed] [Google Scholar]
- Nemeth PR, Daly K, Erde S, Wood JD. Effects of methylene blue on electrical behavior of myenteric neurons. Experientia. 1985;41:259–261. doi: 10.1007/BF02002626. [DOI] [PubMed] [Google Scholar]
- Nordberg A. Mechanisms behind the neuroprotective actions of cholinesterase inhibitors in Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S12–18. doi: 10.1097/01.wad.0000213804.59187.2d. [DOI] [PubMed] [Google Scholar]
- Nozdrachev AD, Kachalov IuP, Chernysheva ON, Murav'ev VI. Effect of methylene blue on spontaneous and evoked reactions in the autonomic neuromuscular junction. Fiziol Zh SSSR Im I M Sechenova. 1984;70:449–455. [PubMed] [Google Scholar]
- O'Leary JL, Petty J, Harris AB, Inukai J. Supravital staining of mammalian brain with intra-arterial methylene blue followed by pressurized oxygen. Stain Technol. 1968;43:197–201. doi: 10.3109/10520296809115068. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF, Sablin SO, Requintina PJ. Effect of methylene blue and related redox dyes on monoamine oxidase activity; rat pineal content of N-acetylserotonin, melatonin, and related indoles; and righting reflex in melatonin-primed frogs. Ann N Y Acad Sci. 2007;1122:245–252. doi: 10.1196/annals.1403.017. [DOI] [PubMed] [Google Scholar]
- Oxford GS, Pooler JP, Narahashi T. Internal and external application of photodynamic sensitizers on squid giant axons. J Membr Biol. 1977;36:159–173. doi: 10.1007/BF01868149. [DOI] [PubMed] [Google Scholar]
- Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer's disease. Biochem Pharmacol. 2009 doi: 10.1016/j.bcp.2009.04.034. in press. [DOI] [PubMed] [Google Scholar]
- Parlow JL, van Vlymen JM. The methylene blues: anticipating adverse reactions to non-anesthetic drugs. Can J Anaesth. 2008;55:1–5. doi: 10.1007/BF03017590. [DOI] [PubMed] [Google Scholar]
- Patil CS, Singh VP, Kulkarni SK. Peripheral and central activation of nitric oxide-cyclic GMP pathway by sildenafil. Inflammopharmacology. 2005;13:467–478. doi: 10.1163/156856005774649359. [DOI] [PubMed] [Google Scholar]
- Pelgrims J, De Vos F, Van den Brande J, Schrijvers D, Prové A, Vermorken JB. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: report of 12 cases and a review of the literature. Br J Cancer. 2000;82:291–294. doi: 10.1054/bjoc.1999.0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Zhang Y, Hou S, Wu W, Fu X. Intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Eur Spine J. 2007;16:33–38. doi: 10.1007/s00586-006-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter C, Hongwan D, Kupfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000;56:247–250. doi: 10.1007/s002280000124. [DOI] [PubMed] [Google Scholar]
- Pfaffendorf M, Bruning TA, Batnik HD, van Zwieten PA. The interaction between methylene blue and the cholinergic system. Br J Pharmacol. 1997;122:95–98. doi: 10.1038/sj.bjp.0701355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot FJ. Some observations on the oxidation of tyramine in the liver. Biochem J. 1937;31:856–861. doi: 10.1042/bj0310856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot FJ, Cantoni G. Adrenaline destruction and methylene blue. J Pharmacol Exp Ther. 1941;71:95–103. [Google Scholar]
- Poppers PJ, Mastri AR, Lebeaux M, Covino BG. The effect of Methylene blue on neural tissue. Anethesiology. 1970;33:335–340. doi: 10.1097/00000542-197009000-00014. [DOI] [PubMed] [Google Scholar]
- Pooler J. Light-induced changes in dye-treated lobster giant axons. Biophys J. 1968;8:1009–1026. doi: 10.1016/S0006-3495(68)86535-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppi A, Wittmann I, Dely M. Inverse modulation of extracellular Na+- and K+-activities by ascorbate or methylene blue. Gen Physiol Biophys. 1986;5:187–191. [PubMed] [Google Scholar]
- Radic Z, Pickering NA, Vellom DC, Camp S, Taylor P. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry. 1993;32:12074–12084. doi: 10.1021/bi00096a018. [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Dunford C, Gillman PK. Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction. Br J Pharmacol. 2007;152:946–951. doi: 10.1038/sj.bjp.0707430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengelshausen J, Burhenne J, Fröhlich M, Tayrouz Y, Singh SK, Riedel KD, Müller O, Hoppe-Tichy T, Haefeli WE, Mikus G, Walter-Sack I. Pharmacokinetic interaction of chloroquine and methylene blue combination against malaria. Eur. J. Clin Pharmacol. 2004;60:709–715. doi: 10.1007/s00228-004-0818-0. [DOI] [PubMed] [Google Scholar]
- Riedel W, Lang U, Oetjen U, Schlapp U, Shibata M. Inhibition of oxygen radical formation by methylene blue, aspirin, or alpha-lipoic acid, prevents bacterial-lipopolysaccharide-induced fever. Mol Cell Biochem. 2003;247:83–94. doi: 10.1023/a:1024142400835. [DOI] [PubMed] [Google Scholar]
- Riha PD, Bruchey AK, Echevarria DJ, Gonzalez-Lima F. Memory facilitation by methylene blue: Dosedependent effect on behavior and brain oxygen consumption. Eur J Pharmacol. 2005;511:151–158. doi: 10.1016/j.ejphar.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Rowley M, Riutort K, Shapiro D, Casler J, Festic E, Freeman WD. Methylene Blue-Associated Serotonin Syndrome: A ‘Green’ Encephalopathy After Parathyroidectomy. Neurocrit Care. 2009 doi: 10.1007/s12028-009-9206-z. in press. [DOI] [PubMed] [Google Scholar]
- Ryle PR, Chakraborty J, Thomson AD. The effect of methylene blue on the hepatocellular redox state and liver lipid content during chronic ethanol feeding in the rat. Biochem J. 1985;232:877–882. doi: 10.1042/bj2320877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitow F, Nakaoka Y. The photodynamic action of methylene blue on the ion channels of Paramecium causes cell damage. Photochem Photobiol. 1997;65:902–907. doi: 10.1111/j.1751-1097.1997.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Salaris SC, Babbs CF, Voorhees WD. Methylene blue as an inhibitor of superoxide generation by xanthine oxidase. A potential new drug for the attenuation of ischemia/reperfusion injury. Biochem Pharmacol. 1991;42:499–506. doi: 10.1016/0006-2952(91)90311-r. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Burke EP, Stevens RJ. Effects of methylene blue on rhythmic activity and membrane potential in the canine proximal colon. Am J Physiol. 1989;256:779–784. doi: 10.1152/ajpgi.1989.256.4.G779. [DOI] [PubMed] [Google Scholar]
- Schultz P, Schwarz GA. Radiculomyelopathy following intrathecal instillation of methylene blue. A hazard reaffirmed. Arch Neurol. 1970;22:240–244. doi: 10.1001/archneur.1970.00480210050006. [DOI] [PubMed] [Google Scholar]
- Scott J, Tidball A, Uitvlugt JM, Lucia M, Vander Griend DA, Louters LL. Methyl-beta-cyclodextrin directly binds methylene blue and blocks both its cell staining and glucose uptake stimulatory effects. Biochimie. 2009;91:271–276. doi: 10.1016/j.biochi.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Seif C, Martínez Portillo FJ, Osmonov DK, Böhler G, van der Horst C, Leissner J, Hohenfellner R, Juenemann KP, Braun PM. Methylene blue staining for nerve-sparing operative procedures: an animal model. Urology. 2004;63:1205–1208. doi: 10.1016/j.urology.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Shah DI, Santani DD, Goswami SS. A novel use of methylene blue as a pharmacological tool. J Pharmacol Toxicol Methods. 2006;54:273–277. doi: 10.1016/j.vascn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Shanmugam G. Vasoplegic syndrome-the role of methylene blue, Eur. J. Cardiothorac. Surg. 2005;28:705–710. doi: 10.1016/j.ejcts.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Sharr MM, Weller RO, Brice JG. Spinal cord necrosis after intrathecal injection of methylene blue. J Neurol Neurosurg Psychiatry. 1978;41:384–386. doi: 10.1136/jnnp.41.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40:407–414. doi: 10.1016/s0010-440x(99)90082-2. [DOI] [PubMed] [Google Scholar]
- Sherlock DJ, Holl-Allen RT. Intravital methylene blue staining of parathyroid glands and tumours. Ann R Coll Surg Engl. 1984;66:396–398. [PMC free article] [PubMed] [Google Scholar]
- Signorini-Allibe N, Gonthier B, Lamarche F, Eysseric H, Barret L. Chronic consumption of ethanol leads to substantial cell damage in cultured rat astrocytes in conditions promoting acetaldehyde accumulation. Alcohol Alcohol. 2005;40:163–171. doi: 10.1093/alcalc/agh097. [DOI] [PubMed] [Google Scholar]
- Sills MR, Zinkham WH. Methylene blue-induced Heinz body hemolytic anemia. Arch. Pediatr. Adolesc. Med. 1994;148:306–310. doi: 10.1001/archpedi.1994.02170030076017. 1994. [DOI] [PubMed] [Google Scholar]
- Spilman P, Lessard P, Sattavat M, Bush C, Tousseyn T, Huang EJ, Giles K, Golde T, Das P, Fauq A, Prusiner SB, Dearmond SJ. A gamma-secretase inhibitor and quinacrine reduce prions and prevent dendritic degeneration in murine brains. Proc Natl Acad Sci U S A. 2008;105:10595–10600. doi: 10.1073/pnas.0803671105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus JG, Rayner MD, Fleig A, Ruben PC. Fast and slow inactivation of sodium channels: effects of photodynamic modification by methylene blue. Biophys J. 1993;65:715–726. doi: 10.1016/S0006-3495(93)81098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus JG, Heggeness ST, Rayner MD. Kinetic analysis of sodium channel block by internal methylene blue in pronased crayfish giant axons. Biophys J. 1984;46:205–218. doi: 10.1016/S0006-3495(84)84014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner HH, Steiner-Milz HG. Severe complications following the intrathecal administration of a methylene blue solution. Laryngol Rhinol Otol (Stuttg) 1986;65:699–701. [PubMed] [Google Scholar]
- Stockand JD, Sansom SC. Activation by methylene blue of large Ca(2+)-activated K+ channels. Biochim Biophys Acta. 1996;1285:123–126. doi: 10.1016/s0005-2736(96)00194-0. [DOI] [PubMed] [Google Scholar]
- Stanford SC, Stanford BJ, Gillman PK. Risk of severe serotonin toxicity following co-administration of methylene blue and serotonin reuptake inhibitors: an update on a case report of post-operative delirium. J Psychopharmacol. 2009 doi: 10.1177/0269881109105450. in press. [DOI] [PubMed] [Google Scholar]
- Strasser A, Mccarron RM, Ishii H, Stanimirovic D, Spatz M. Larginine induces dopamine release from the striatum in vivo. Neuroreport. 1994;5:2298–2300. doi: 10.1097/00001756-199411000-00023. [DOI] [PubMed] [Google Scholar]
- Streba J, Walluschb J, Yinc S. Knowledge spill-over from new to old industries: The case of German synthetic dyes and textiles (1878–1913) Explorations in Economic History. 2007;44:203–223. [Google Scholar]
- Sullivan M. Phase II Findings in AD Drug Trial ‘Not All Bad’. Clinical Psychiatry News. 2008;36:34–35. [Google Scholar]
- Sutherland AD, Faragher IG, Frizelle FA. Intradermal injection of methylene blue for the treatment of refractory pruritus ani. Colorectal Dis. 2009;11:282–287. doi: 10.1111/j.1463-1318.2008.01587.x. [DOI] [PubMed] [Google Scholar]
- Sweet G, Standiford SB. Methylene-blue-associated encephalopathy. J Am Coll Surg. 2007;204:454–458. doi: 10.1016/j.jamcollsurg.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Tacal O, Ozer I. Inhibition of choline oxidase by quinoid dyes. J Enzyme Inhib Med Chem. 2006;21:783–787. doi: 10.1080/14756360600829530. [DOI] [PubMed] [Google Scholar]
- Tan KY, Seow-Choen F. Methylene blue injection reduces pain after lateral anal sphincterotomy. Tech Coloproctol. 2007;11:68–69. [PubMed] [Google Scholar]
- Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, Hasegawa M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem. 2005;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- Tardivo J, Del Giglio A, de Oliveira C, Gabrielli D, Junqueira H, Tada D, Severino D, de Fátima Turchiello R, Baptista M. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis and Photodynamic Therapy. 2005;2:175–191. doi: 10.1016/S1572-1000(05)00097-9. [DOI] [PubMed] [Google Scholar]
- Thomas RD, Callender K. Methylene blue in treatment of bipolar illness. Biol Psychiatry. 1985;20:120–121. doi: 10.1016/0006-3223(85)90147-7. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Methylene blue as a pharmacological probe of intestinal pacemaker activity. Am J Physiol. 1990;258:992–994. doi: 10.1152/ajpgi.1990.258.6.G992. [DOI] [PubMed] [Google Scholar]
- Tiboni GM, Lamonaca D. Transplacental exposure to methylene blue initiates teratogenesis in the mouse: preliminary evidence for a mechanistic implication of cyclic GMP pathway disruption. Teratology. 2001;64:213–220. doi: 10.1002/tera.1066. [DOI] [PubMed] [Google Scholar]
- Triesscheijn M, Baas P, Schellens JH, Stewart FA. Photodynamic therapy in oncology. Oncologist. 2006;11:1034–1044. doi: 10.1634/theoncologist.11-9-1034. 2006. [DOI] [PubMed] [Google Scholar]
- Tuite EM, Kelly JM. Photochemical interactions of methylene blue and analogues with DNA and other biological substrates. J Photochem Photobiol B. 1993;21:103–124. doi: 10.1016/1011-1344(93)80173-7. [DOI] [PubMed] [Google Scholar]
- Wainwright M. The use of dyes in modern biomedicine. Biotech Histochem. 2003;78:147–155. doi: 10.1080/10520290310001602404. [DOI] [PubMed] [Google Scholar]
- Wainwright M, Crossley KB. Methylene Blue--a therapeutic dye for all seasons? J Chemother. 2002;14:431–443. doi: 10.1179/joc.2002.14.5.431. [DOI] [PubMed] [Google Scholar]
- Walter-Sack I, Rengelshausen J, Oberwittler H, Burhenne J, Mueller O, Meissner P, Mikus G. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur J Clin Pharmacol. 2009;65:179–189. doi: 10.1007/s00228-008-0563-x. 2009. [DOI] [PubMed] [Google Scholar]
- Weddel G. The pattern of cutaneous innervation in relation to cutaneous sensibility. J.f Anatomy. 1941;75:346–367. [PMC free article] [PubMed] [Google Scholar]
- Wegener G, Volke V, Rosenberg R. Endogenous nitric oxide decreases hippocampal levels of serotonin and dopamine in vivo. Br. J. Pharmacol. 2000;130:575–580. doi: 10.1038/sj.bjp.0703349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil L, Gordon WG, Buchert AR. Photooxidation of amino acids in the presence of methylene blue. Arch Biochem. 1951;33:90–109. doi: 10.1016/0003-9861(51)90084-7. [DOI] [PubMed] [Google Scholar]
- Wendel WB. Methylene blue, methemoglobin, and cyanide poisoning. J Pharmacol Exp Ther. 1935;54:283–298. [Google Scholar]
- Wiklund L, Basu S, Miclescu A, Wiklund P, Ronquist G, Sharma HS. Neuro- and cardioprotective effects of blockade of nitric oxide action by administration of methylene blue. Ann N Y Acad Sci. 2007;122:231–244. doi: 10.1196/annals.1403.016. [DOI] [PubMed] [Google Scholar]
- Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik CM, Bentham P, Wischik DJ, Seng KM. Tau aggregation inhibitor (TAI) therapy with rember™ arrests disease progression in mild and moderate Alzheimer's disease over 50 weeks. Alzheimer's and Dementia. 2008;4:T167. [Google Scholar]
- Wolin MS, Cherry PD, Rodenburg JM, Messina EJ, Kaley G. Methylene blue inhibits vasodilation of skeletal muscle arterioles to acetylcholine and nitric oxide via the extracellular generation of superoxide anion. J Pharmacol Exp Ther. 1990;254:872–876. [PubMed] [Google Scholar]
- Wolloch Y, Dintsman M. A simple and effective method of treatment for intractable pruritis ani. Am J Proctol Gastroenterol Colon Rectal Surg. 1979;30:34–36. [PubMed] [Google Scholar]
- Wrubel KM, Riha PD, Maldonado MA, McCollum D, Gonzalez-Lima F. The brain metabolic enhancer methylene blue improves discrimination learning in rats. Pharmacol Biochem Behav. 2007;86:712–717. doi: 10.1016/j.pbb.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzeno DP, Arriaga E, Trank J, Tarr M. Membrane potential can influence the rate of membrane photomodification. Photochem Photobiol. 1993;57:996–999. doi: 10.1111/j.1751-1097.1993.tb02961.x. [DOI] [PubMed] [Google Scholar]
- van der Pol JG, Wolf H, Boer K, Treffers PE, Leschot NJ, Hey HA, Vos A. Jejunal atresia related to the use of methylene blue in genetic amniocentesis in twins. Br J Obstet Gynaecol. 1992;99:141–143. doi: 10.1111/j.1471-0528.1992.tb14473.x. [DOI] [PubMed] [Google Scholar]
- Visarius TM, Stucki JW, Lauterburg BH. Stimulation of respiration by methylene blue in rat liver mitochondria. FEBS Lett. 1997;412:157–160. doi: 10.1016/s0014-5793(97)00767-9. [DOI] [PubMed] [Google Scholar]
- Volke V, Wegener G, Vasar E, Rosenberg R. Methylene blue inhibits hippocampal nitric oxide synthase activity in vivo. Brain Res. 1999;826:303–305. doi: 10.1016/s0006-8993(99)01253-6. [DOI] [PubMed] [Google Scholar]
- Volke V, Wegener G, Bourin M, Vasar E. Antidepressant- and anxiolytic-like effects of selective neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole in mice. Behav Brain Res. 2003;140:141–147. doi: 10.1016/s0166-4328(02)00312-1. [DOI] [PubMed] [Google Scholar]
- Vonlanthen R, Beer JH, Lauterburg BH. Effect of methylene blue on the disposition of ethanol. Alcohol Alcohol. 2000;35:424–426. doi: 10.1093/alcalc/35.5.424. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Briner A, Klauser P, Gascon E, Dayer AG, Kiss JZ, Müller D, Licker MJ, Morel DR. Adverse effects of methylene blue on the central nervous system. Anesthesiology. 2008;108:684–692. doi: 10.1097/ALN.0b013e3181684be4. [DOI] [PubMed] [Google Scholar]
- Yucel YY, Tacal O, Ozer I. Comparative effects of cationic triarylmethane, phenoxazine and phenothiazine dyes on horse serum butyrylcholinesterase. Arch Biochem Biophys. 2008;478:201–205. doi: 10.1016/j.abb.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Zakaria ZA, Sulaiman MR, Somchit MN, Jais AM, Ali DI. The effects of L-arginine, D-arginine, L-name and methylene blue on channa striatus-induced peripheral antinociception in mice. J Pharm Pharm Sci. 2005;8:199–206. [PubMed] [Google Scholar]
- Zhang X, Rojas JC, Gonzalez-Lima F. Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox Res. 2006;9:47–57. doi: 10.1007/BF03033307. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochimica et Biophysica Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Ziv G, Heavner JE. Permeability of the blood-milk barrier to methylene blue in cows and goats. J Vet Pharmacol Therap. 1984;7:55–59. doi: 10.1111/j.1365-2885.1984.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Zulian GB, Tullen E, Maton B. Methylene blue for ifosfamide-associated encephalopathy. N Engl J Med. 1995;332:1239–1240. doi: 10.1056/NEJM199505043321817. [DOI] [PubMed] [Google Scholar]