Abstract
Fibrin glue has been used surgically for decades for hemostasis as well as a sealant. It has also been researched as both a gel for cell delivery and a vehicle for drug delivery. The drug delivery applications for fibrin glue span tissue engineering to chemotherapy and involve several mechanisms for drug matrix interactions and control of release kinetics. Additionally, drugs or factors can be loaded in the gel via impregnation and tethering to the gel through covalent linkages or affinity based systems. This review highlights recent research of fibrin glue as a drug delivery vehicle.
Keywords: Fibrin glue, drug delivery, growth factor delivery, gene delivery, tissue engineering
1. Introduction
Fibrin glue has been studied for decades, originally for its use surgically as a hemostatic agent and as a sealant.[1–6] More recently it has been investigated for cell delivery in tissue engineering applications where it is used to encapsulate cells.[7–12] Due to fibrin glue’s current clinical use and acceptance, the material has also gained attention as a possible means to deliver drug therapies. Here many of those applications are discussed in the realm of analgesia, chemotherapy, infection, gene delivery and regenerative medicine. All of these disciplines benefit from the use of local delivery of factors or drugs, and due to ease of use and biomimetic capabilities of fibrin glue, it has been viewed as a possible candidate for such systems.
Fibrin glue consists of two components, a fibrinogen solution and a thrombin solution rich in calcium. The fibrinogen component is obtained primarily through one of two methods, cryoprecipitation or ethanol fractionation, from pooled human plasma.[13] Cryoprecipitation uses a citrate buffer in a cold environment to precipitate the relevant proteins, which are then lyophilized and sterilized before reconstitution. Additionally, cryoprecipitation is used in combined blood bank and surgery settings to create autologous fibrinogen solution. The ethanol fractionation method precipitates the fibrinogen with 10% ethanol, which is then washed with a solvent and detergent mixture to remove viral components, which could be present and is similarly lyophilized before reconstitution. The thrombin component initially used commercial bovine thrombin, but has more recently become human thrombin in commercial products. When the two components are mixed, the thrombin enzymatically cleaves fibrinogen to form fibrin and factor XIII to factor XIIIa which then crosslinks fibrin to form a gel.[14] The physiologic nature of this process allows for inclusion of other endogenous factors, which can act, to aid in cell binding, proliferation, differentiation or migration. Not only does it serve as an attractive surgical material to bind tissues, which also contain these same factors, but also due to the inherent interactions between cells or factors and the extracellular matrix like composition of the gel, fibrin glue can act biomimetically as a wound bed.
Fibrin glue has several attractive features, which lend itself to the application of controlled release. Firstly, several of the parameters of the components can be modified to change the gel’s structure, mechanical properties, and degradation. Sierra[13], in a review of fibrin sealant chemistry and material properties, describes the various changes, which can be made to fibrin gel through varying the concentration of solutes in the two components. For instance, fiber diameter and pore size are dependent on crosslinking time, which can be varied by thrombin concentration or ionic strength of the solution. Additionally, shear modulus and tensile strength can be modified by varying levels of calcium and fibrinogen, respectively. Most formulations of fibrin glue include antifibrinolytic agents to delay or slow fibrinolysis, which leads to destruction of the gel. These include, epsilon-aminocaproic acid C1-esterase inhibitor and aprotinin, of which aprotinin is most common.[14–16]
In addition to varied release kinetics involving the gel, fibrin glue is an attractive drug delivery system due to the interactions it can have with endogenous factors. Fibrin serves as a binding reservoir for several growth factors such as vascular endothelial growth factor (VEGF)[17], transforming growth factor-B1 (TGF-β1)[18], basic fibroblastic growth factor (bFGF)[19], and growth fractor binding proteins such as insulin-like growth factor-binding protein-3 for insulin-like growth factor (IGF)[20]. Due to these interactions several studies focus on the ability of fibrin glue to modulate release kinetics of these factors based upon relative concentrations of components of the fibrin glue.
2. Loaded Factor, Drug and Vector Systems
In the simplest formulation of fibrin glue as a drug delivery gel, exogenous drugs or factors are added to one of the components before addition of the other allowing the drug or factor to be distributed throughout the solution before crosslinking. This model of drug delivery has been studied extensively due to the potential for rapid translation to clinical use and simplicity of implementation. For example, Huang et al. describe the preparation necessary for a loaded gel to be made.[21] The simplicity in design of these drug delivery systems allows easy translation and application of many permutations of the system in many settings. Many drugs or factors can have an effect on the release from the gel under these circumstances, the greatest of which is affinity of the drug or factor for components of fibrin glue. This difference is primarily decided by the natural occurrence of the factor and its role in potential wound healing, where physiologic interaction between the factor and fibrin clots can occur.
2.1 Growth Factors
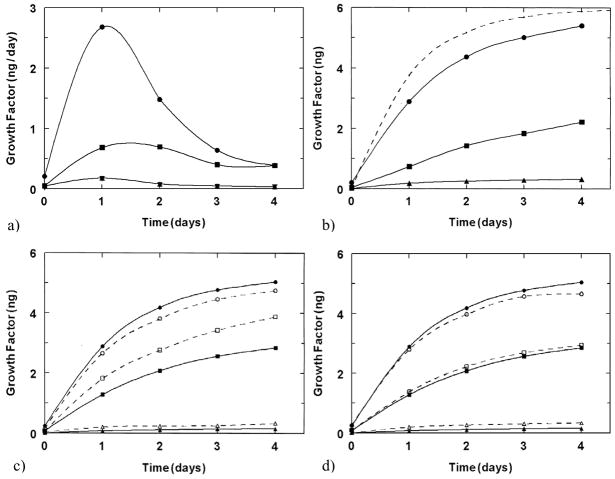
The inclusion of bioactive factors into fibrin glue has been extensively studied in tissue engineering, where fibrin serves as the scaffold, which can interact with exogenous growth or regulatory factors. In a study by Wong et al.[22], this method was utilized comparing the release of bFGF with two isoforms of VEGF with different amino acid lengths. This study illustrates the relative level of interaction between the fibrin gel and these three different factors, with bFGF maintaining the highest retention in the gel for fibrin, suggesting the most affinity and VEGF121 with the least retention as shown in figures 1a and 1b. In addition this study, compares the release kinetics of these growth factors from fibrin gels, which include other extracellular matrix components, fibronectin and heparin, in figures 1c and 1d, respectively. While no difference in release was observed for heparin, the inclusion of fibronectin into the gel resulted in greater release of VEGF165 from the gel.[22] This study illustrates the biomimetic capabilities of this material, impinged upon the natural interactions between fibrin glue components and growth factors relevant to tissue engineering and other therapies. Similarly, in a study by Becker et al.[23], the binding affinity of VEGF to fibrin glue was quantified at 152.6 pg/ml. While this study did not analyze the possibility of varying compositions of the gel, the binding affinity determined does correspond to physiologic levels of the factor.
Figure 1.
Growth factor release from fibrin glue. a) Release per day of growth factors: VEGF165 (■) and bFGF (▼) or VEGF121 (●) and bFGF (▲) from fibrin gels of 10 mg and 10 U of fibrinogen and thrombin, respectively, and 6 ng of each growth factor. b) Cumulative release of growth factors shown in a). Dashed line represents the release kinetics if no interaction between factor and components of the gel is present. c) Release of growth factors: VEGF121 (●, ○), VEGF165 (■, □) and bFGF (▲, △) with low (dashed lines) and high (solid lines) levels of fibrinogen. d) Release of growth factors: VEGF121 (●, ○), VEGF165 (■, □) and bFGF (▲, △) with (dashed) and without (solid) heparin. (Reproduced with permission from [22]).
In a similar study, Catelas et al.[18] observed the interaction of TGF-β1 with components of fibrin and the effect on release kinetics. This study showed a decrease in release from the gel with increased concentration of fibrinogen in the initial component solutions, indicating an interaction between TGF-β1 and fibrin in the gel. The authors hypothesized that this binding affinity is physiologic, because TGF-β1 is released from platelets into a clot to promote cell proliferation and differentiation. When released from platelets the factor undoubtedly encounters fibrinogen or fibrin in the clot, which may serve as a temporary storage depot increasing the duration of factor activity in the area. [18] While these hypotheses have yet to be proven, the activity between TGF-β1 and fibrin glue allow varied release kinetics tailored to desired profiles through the optimization of factor: fibrinogen: thrombin ratios.
Composite materials with fibrin glue have also been studied. In a study by Drinnan et al.[24], a poly(ethylene glycol) (PEG) and fibrin glue gel composite was studied with varied formulations of the PEG chain. Additionally, in this study dual factor release was studied with TGF-β1 and the BB isoform of platelet derived growth factor (PDGF). This study showed that through altering the PEG of the composite gel, varied release of both factors could be achieved. The individual release of each factor was controlled by different mechanisms, diffusion and degradation. The TGF-β1 was covalently linked to the gel while the PDGF was entrapped. The covelent link of the TGF-β1 degraded and released the TGF-β1 at the same rate as the gel degraded, while the PDGF diffused out of the gel, resulting in varying release kinetics for each factor. In another study using composite materials, scaffolds of polycaprolactone and fibrin glue with TGF-β1 were placed subcutaneously, intramuscularly and subperiostally and they were examined histologically. Scaffolds loaded with TGF-β1 resulted in more mesenchymal stem cell recruitment and in some cases differentiation than those without any TGF-β1.[25] This study did not analyze the release kinetics of their scaffolds but did show positive outcomes for TGF-β1 activity through greater recruitment of mesenchymal stem cells.
There are multitudes of studies looking at various biological factors and signals loaded into fibrin glue. The majority of these studies load the drug into the fibrinogen component before crosslinking[22, 26–29], but the thrombin component can be loaded as well, as in the study by Woodruff et al.[30] In this study heparan sulfate was loaded into the thrombin component before mixing to increase bone regeneration in a rat calvarial defect. Initial in vitro results indicated similar level and profile of the release kinetics with low and high levels of thrombin.[30] One could hypothesize through this study that the level of interaction between the proteins of fibrin glue and heparan sulfate are low due to the lack of change with altered composition of the gel as is seen above.
In two studies by Arkudas et al.[26, 27], fibrin glue served as a suitable delivery vehicle resulting in increased vascularization of subcutaneous and arteriovenous loop implants in rats when loaded with VEGF and bFGF, but failed to show a difference in vascularization with differing levels of fibrinogen in the composition. These results from an in vivo study seem to draw different conclusions than those for a similar system described by Wong et al.[22] above in vitro. This discrepancy could be due to several factors including the presence of many other factors in vivo, which alter the release kinetics, or simply the lot of growth factors and fibrin glue, which as indicated by Catelas et al.[18] can have significant effects on release kinetics.
Utilizing other growth factors, Gao et al.[29] created a fibrin gel with nerve growth factor (NGF), which performed better than fibrin glue or NGF alone in a sciatic nerve defect in rats in regeneration of a nerve defect. The performance of these implants were measured histomorphometrically, electrophysiologically and functionally of which fibrin glue with NGF performed better compared to each component alone.[29] In a similar study, nerve autograft with fibrin glue impregnated with acidic fibroblastic growth factor was used to regenerate a defect in the hemispinal cord of monkeys.[31] Another study of neural regeneration utilized glial cell derived neurotrophic factor (GDNF). This study put GDNF loaded fibrin glue spheres along with pieces of fetal brain tissue into the anterior chambers of rats and documented increased staining of neurons in the group with GDNF versus fibrin glue alone.[32] The breadth of tissue types in these studies indicates the possible realms for fibrin glue beyond clotting and coagulation disciplines.
Furthermore, factors, whose affinity for components of fibrin glue is low but high retention within the gel is desired, can be altered to increase retention in the gel. Schmoekel et al.[33] chemically modified bone morphogenetic protein-2 (BMP-2) to allow greater retention in the gel. In this study, recombinant human BMP-2, which is normally glycosylated, was not glycosylated altering the solubility of the drug in aqueous solution. Release kinetics showed much greater retention in the fibrin gel after subjection to washes with non-glycosylated BMP-2 versus glycosylated BMP-2. Additionally, when implanted into a rat cranial defect, results indicated a possible increase in bone formation with non-glycosylated BMP-2 versus glycosylated BMP-2, but there was no statistical significance.[33] In another study by the same group, the dose effect of the non-glycosylated BMP-2 was found with significant differences between 2 and 5 μg of non-glycosylated BMP-2 but no such difference between 5 and 10 μg.[34] Modification of factors can extend beyond affecting solubility, as shown in two studies by Erzurum et al.[35] and Shireman et al.[36], where two different mutations of FGF-1 reduce the susceptibility of the growth factor to cleavage by thrombin and thus extend its duration of bioactivity. This is especially important in drug delivery utilizing fibrin glue due to the high levels of thrombin used to form the gel. Results indicate higher levels of bioactivity, as shown by increased proliferation of smooth muscle cells and endothelial cells at the same concentration as unmodified FGF-1, as well as a lower maximal effect level than unmodified FGF-1.
In addition to these therapeutic delivery capacities of the material, fibrin glue has also been investigated as barrier material against the widespread diffusion of exogenous factors. In two studies performed by Patel et al.[37, 38], fibrin glue is used to seal collagen sponges soaked in a BMP-2 solution for spinal fusion. A complication of using BMP-2 in spinal fusion is nerve root compression due to undesirable bone growth thought to occur by the diffusion of BMP-2. First, release kinetics of collagen sponges loaded with BMP-2 were measured as the sponge alone, placed on a layer of fibrin glue, or encapsulated in fibrin glue. After 8 days, the sponge alone had released approximately 100% of the BMP-2, while both the sponge on the layer of fibrin glue and the encapsulated sponge had released approximately 50% and 20%, respectively, suggesting that BMP-2 diffused into and remained in the fibrin gel due to the retention in the layer with the sponge on top. In vivo, fibrin glue, placed around the nerve roots before the BMP-2 soaked collagen sponge, maintained greater nerve activity measured by electrophysiology.
2.2 Synthetic Drugs
In addition to natural factors, synthetic drugs have been investigated for use in fibrin glue as a drug delivery system. Because of fibrin glue’s frequency of use in surgical repairs as an adhesive or sealant, surgically relevant drugs have been investigated for release from areas where the material may already be utilized. For example, in a study by Zhibo and Miaobo[39], release of lidocaine from fibrin glue for pain reduction was tested in humans after breast augmentation. Patients who received fibrin glue with lidocaine in the subpectoral pocket experienced less pain than those who received the same amount of lidocaine or fibrin glue alone. While release kinetics where not performed in this study, functionally the fibrin glue improved the gross effect of the drug. Fibrin glue can also be used as a sealant in vascular repairs, such as the placement of a synthetic graft. Infection of synthetic vascular grafts is a common complication, so in a study by Fujimoto et al.[40], vancomycin was added to the fibrinogen component of fibrin glue and applied to Dacron grafts in rats. The level of local drug was measured in these groups and compared to those with a Dacron graft where vancomycin was administered intravenously. The targeting index, the ratio of local drug in the targeted therapy versus intravenous administration, for fibrin glue was 12.11, indicating much more drug in the area of interest with fibrin glue used as a carrier. Osada et al.[41] performed a similar study where sisomicin was incorporated into fibrin glue around Dacron grafts in rats. Blood and local concentrations of sisomicin were compared to that of intravenous administration of the drug, and while intravenous administration of sisomicin resulted in higher concentrations in the blood at all time points, inclusion of sisomicin loaded fibrin glue resulted in higher local concentrations of the drug initially after material placement. These studies are clinically relevant due to the toxic effects of high concentrations of antibiotics, which are sometimes necessary when intravenous administration is used versus targeted therapy.
Another avenue of drug delivery is chemotherapeutic agents used in cancer. Yoshida et al.[42] conducted a study comparing the release of fluorouracil, mitomycin C and enocitabine from fibrin glue with and without aprotinin. In this study, fluorouracil and mitomycin C were released rapidly independent of aprotinin, while enocitabine was released slowly in the presence of aprotinin. The results were analyzed with respect to the drug’s hydrophobicity, protein binding and molecular weight where only the hydrophobicity showed a correlation of increasing retention with increasing hydrophobicity. Another study observing the release kinetics of chemotherapeutic agents from fibrin glue, looked at doxorubicin from a composite gel of fibrin with sodium alginate. This study showed the burst release of doxorubicin solution as well was that of doxorubicin in fibrin glue, but a steady duration of release from a fibrin gel in the presence of sodium alginate as shown in figure 2.[43] While in this study fibrin glue does not directly control the release, the study illustrates the possibility of fibrin glue as a carrier for other drug delivery systems such as microparticles and composite gels.
Figure 2.
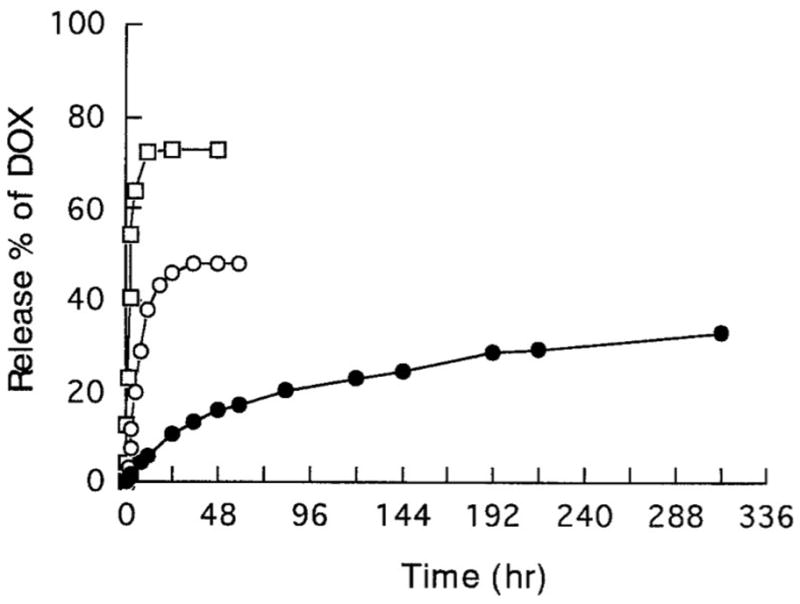
Profile of release of doxorubicin from phosphate buffered saline (□), fibrin glue (○), fibrin glue with sodium alginate (●) through dialysis membrane. (Reproduced with permission from [43]).
While fibrin glue has shown promise in several of the above areas in delivering a depot of drugs, the release kinetics attainable for some drugs using fibrin is not sufficient. In a study by Yau et al.[44] meta-iodobenzylguanidine (MIBG) was loaded into a fibrin gel for long-term release. MIBG inhibits arginine dependent mono(ADP-ribosyl)ation and might prevent neointimal hyperplasia after angioplasty. Inclusion of MIBG into a fibrin gel showed release of the drug within the first few days of implantation. This duration of release is not suitable for the purposes of this drug and therefore does not support the use of fibrin glue as a mode of delivery.
2.3 Gene Delivery Vectors
Fibrin glue has also been used to deliver genes by incorporating gene delivery vectors. Extended release duration is especially important for gene delivery vectors, which can transpose a short acting function such as adenoviral vectors whose transcriptional activity declines rapidly necessitating continued release of the vector to achieve long term effect. This was the goal of a study by Breen et al.[45], which sought to establish the effect of fibrin glue on the transfection efficiency of adenoviral vectors. In a rabbit ear model, exposed cartilage was covered with nothing, fibrin glue, an adenoviral vector encoding β-galactosidase or fibrin glue with the adenoviral vector. Shortly after placement the number of cells expressing β-galactosidase was shown to be increased in those cells, which received the gene delivery vector. Additionally, the group with the adenoviral vector in fibrin glue showed more transfected cells than those with the vector alone. The difference between the adenoviral vector group and the adenoviral vector in fibrin glue group, declined over time.
Research to reduce closure of venous grafts used in coronary artery bypass surgery has led to both pharmacologic and mechanical means to maintain patency of grafts. Gene delivery has been one means to establish lengthened pharmacologic therapy to the graft. In a study by Wan et al.[46], fibrin glue with adenoviral vectors were tested in a rabbit graft model using the jugular vein and common carotid. Grafts were left untreated, given the adenoviral vector encoding for β-galactosidase, supported mechanically with fibrin glue, or supported with fibrin glue containing the adenoviral vector. Grafts with fibrin glue and the vector showed greater transfection for longer durations than those with the vector alone. Additionally, groups with fibrin glue showed decreased intimal area compared to unsupported groups.[46] This study showed efficacy of the fibrin glue not only as a delivery vehicle for adenoviral vectors but the advantage of the structure and support the gel can provide. Teraishi et al.[47] performed a study, which also utilizes the unique structural properties of fibrin glue. Fibrin glue was administered endoscopically through a multiluminal tube to the esophagus of pigs along with adenoviral vectors. Through the use of nitrogen gas pumped through the tube, the fibrin glue was sprayed onto the esophageal epithelium, utilizing the adhesive properties of the gel to target the vectors to the surface of the tissue. Figure 3 qualitatively illustrates the effectiveness of the spray at transfecting the epithelium of the esophagus with β-galactosidase.[47]
Figure 3.
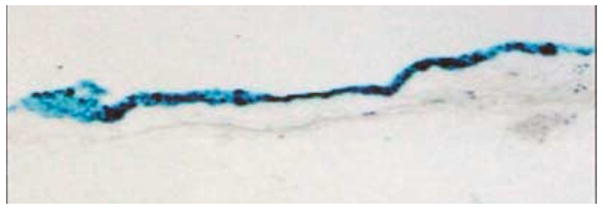
Staining of pig esophageal epithelium after spraying with adenovirus encoding β-galactosidase in fibrin glue through endoscope. (Reproduced with permission from [47]).
3. Tethered Factor and Drug Systems
In addition to using fibrin glue as a carrier of drugs, drugs or bioactive factors can be anchored to the gel through covalent linkages and affinity binding. Figure 4 illustrates the structural differences between the two types of tethering. Many of these linkages, whether affinity or covalently based, use transglutaminase or other sequences which crosslink with fibrin naturally during clot formation.[48–54] Through this linkage the release of the drug or factor can be based upon several parameters, including the degradation of the fibrin glue or of the linkage, the affinity between the drug and the tether or the relative amount of sites for interaction between the gel and the drug.
Figure 4.
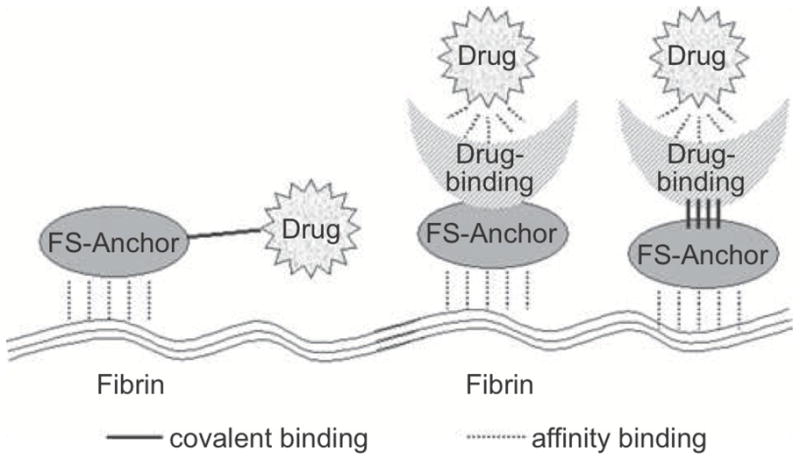
Illustration showing the structural differences between covalent linkage and affinity based drug delivery systems. (Reproduced with permission from [49]).
3.1 Affinity Based Systems
Many endogenous factors have natural binding sites on other molecules, which serve as reservoirs. When present inside a material, these molecules can bind factors and release them over time based on the factor and molecule affinity.
Wood et al.[52] performed a study in which GDNF was linked to fibrin glue via affinity-based mechanisms. In the study a peptide linker with transglutaminase and a heparin-binding domain was crosslinked into the gel. This affinity based system works by the heparin binding to the linker and the GDNF associating with the heparin. In this technique the kinetics of release can be altered through various methods but in this study the heparin was varied. Comparing retention of GDNF in the gel, the gels with heparin, the peptide linker, and GDNF were significantly greater in retention compared to GDNF in fibrin alone, fibrin with heparin, and fibrin with the peptide linker.[52] Using the same linker, Willerth et al.[51] linked a heparin-binding domain to a fibrin gel through factor XIIIa substrate from a2-plasmin inhibitor. Heparin acted as a reservoir of neurotrophin-3 (NT-3), Sonic hedgehog (Shh), and platelet derived growth factor (PDGF). Again, by altering the amount of heparin included in the gel and thus changing the ratio of heparin to peptide, the release kinetics were altered.[51]
3.2 Covalent Linkage Systems
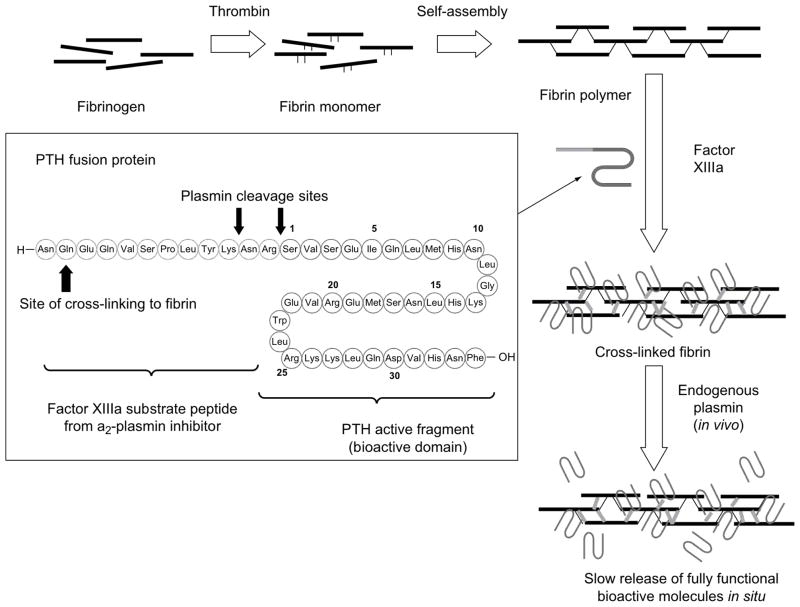
Covalent linkages have also been shown to bind drugs or factors to fibrin glue. The use of covalent linkages between factors and gels has been employed extensively to control drug release by gel degradation or through the use of enzyme sensitive linkages. In a study by Arrighi et al.[48], parathyroid hormone was tethered to fibrin by transglutaminase with a plasmin sensitive link as shown in figure 5. The study showed decreased activity of the hormone when linked to fibrin glue with the link, which increased upon release of the drug from the gel by enzymatic degradation of the link.[48] In a similar study performed by Schmoekel et al.[50] BMP-2 was bound to fibrin glue via a transglutaminase domain again with a plasmin sensitive link. In this study the bound BMP-2 showed greater retention in the gel compared to the unbound BMP-2. Furthermore, these tethered factor gels resulted in greater bone growth in a rat cranial model and faster and more extensive bone bridging in a canine pancarpal fusion model again compared to unbound BMP-2.[50]
Figure 5.
Crosslinking process of fibrinogen to fibrin and finally crosslinked fibrin. Inset shows amino acid sequence of tethered parathyroid hormone with fibrin crosslinking, plasmin cleavage and PTH domains. (Reproduced with permission from [48]).
In two studies by Zhang et al.[55, 56], growth factors are covalently attached to fibrin gels via incubation with fibrinogen, which has been modified with poly(ethylene glycol) (PEG). This conjugation utilized benzotriazole carbonate derivative on the ends of PEG, which allow the molecule to bind to both fibrinogen and growth factors. These studies attached hepatocyte growth factor and stromal cell-derived factor-1alpha and showed greater cell viability and migration, respectively, in a murine acute myocardial infarction model. Unlike the studies utilizing plasmin sensitive linkages, the PEG linkage is non-degradable and thus release kinetics depend on gel degradation.
4. Conclusions
Fibrin glue has many options for the delivery of drugs or factors, whose release kinetics can be tailored through composition, affinity and covalent linkage. While endogenous factors present in normal wound healing physiology have preexisting affinities for the proteins of the gel, the number of available proteins can be varied to alter release kinetics through concentration and competition. However, there are drawbacks to this system, in that not all drugs have interactions with the material or its components to control their release and the length of delivery does not make it applicable to long term drug depot use. Taking these drawbacks into consideration, the variability of fibrin glue and its ease of implementation lend the material to development of drug delivery systems, which utilize fibrin glue’s crosslinking and biomimetic structure and composition.
Acknowledgments
We acknowledge support from the National Institutes of Health (R21 AR056076) and the Armed Forces Institute for Regenerative Medicine (W81XWH-08-2-0032) for work involving the use of drug delivery systems for tissue engineering. PPS acknowledges support from the Robert and Janice McNair Foundation.
Abbreviations
- aFGF
acidic fibroblastic growth factor
- bFGF
basic fibroblastic growth factor
- BMP-2
bone morphogenetic protein-2
- FGF-1
fibroblastic growth factor-1
- GDNF
glial cell derived neurotrophic factor
- NGF
nerve growth factor
- NT-3
neurotrophin-3
- PEG
poly(ethylene glycol)
- PDGF
platelet derived growth factor
- Shh
sonic hedgehog
- TGF-β1
transforming growth factor beta-1
- VEGF
vascular endothelial growth factor
- VEGF121
121 amino acid length vascular endothelial growth factor
- VEGF165
165 amino acid length vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antonelli M, Cicconetti F, Vivino G, Gasparetto A. Closure of a Tracheoesophageal Fistula by Bronchoscopic Application of Fibrin Glue and Decontamination of the Oral Cavity. Chest. 1991;100:578–579. doi: 10.1378/chest.100.2.578. [DOI] [PubMed] [Google Scholar]
- 2.Radosevich M, Goubran HA. Burnouf, Fibrin sealant: Scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997;72:133–143. doi: 10.1046/j.1423-0410.1997.7230133.x. [DOI] [PubMed] [Google Scholar]
- 3.Flahiff C, Feldman D, Saltz R, Huang S. Mechanical-Properties of Fibrin Adhesives for Blood-Vessel Anastomosis. J Biomed Mater Res. 1992;26:481–491. doi: 10.1002/jbm.820260406. [DOI] [PubMed] [Google Scholar]
- 4.Jackson MR, Friedman SA, Carter AJ, Bayer V, Burge JR, MacPhee MJ, Drohan WN, Alving BM. Hemostatic efficacy of a fibrin sealant-based topical agent in a femoral artery injury model: A randomized, blinded, placebo-controlled study. J Vasc Surg. 1997;26:274–280. doi: 10.1016/s0741-5214(97)70189-7. [DOI] [PubMed] [Google Scholar]
- 5.Marx G, Mou X. Characterizing fibrin glue performance as modulated by heparin, aprotinin, and factor XIII. J Lab Clin Med. 2002;140:152–160. doi: 10.1067/mlc.2002.126413. [DOI] [PubMed] [Google Scholar]
- 6.Mouritzen C, Dromer M, Keinecke HO. The Effect of Fibrin Gluing to Seal Bronchial and Alveolar Leakages After Pulmonary Resections and Decortications. Eur J Cardiothorac Surg. 1993;7:75–80. doi: 10.1016/1010-7940(93)90184-d. [DOI] [PubMed] [Google Scholar]
- 7.Cox S, Cole M, Tawil B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: Dependence on fibrinogen and thrombin concentration. Tissue Eng. 2004;10:942–954. doi: 10.1089/1076327041348392. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Collins SF, Suggs LJ. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6004–6014. doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Zhang G, Drinnan CT, Geuss LR, Suggs LJ. Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta Biomater. 2010 doi: 10.1016/j.actbio.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Mogford JE, Tawil B, Jia S, Mustoe TA. Fibrin sealant combined with fibroblasts and platelet-derived growth factor enhance wound healing in excisional wounds. Wound Repair Regen. 2009;17:405–410. doi: 10.1111/j.1524-475X.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 11.Ho W, Tawil B, Dunn JCY, Wu BM. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12:1587–1595. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 12.Mooney R, Tawil B, Mahoney M. Specific Fibrinogen and Thrombin Concentrations Promote Neuronal Rather Than Glial Growth When Primary Neural Cells Are Seeded Within Plasma-Derived Fibrin Gels. Tissue Eng Part A. doi: 10.1089/ten.TEA.2009.0372. [DOI] [PubMed] [Google Scholar]
- 13.Sierra DH. Fibrin Sealant Adhesive Systems: A Review of Their Chemistry, Material Properties and Clinical Applications. J Biomater App. 1993;7:309. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 14.Matras H. Fibrin seal: the state of the art. J Oral Maxillofac Surg. 1985;43:605–611. doi: 10.1016/0278-2391(85)90129-6. [DOI] [PubMed] [Google Scholar]
- 15.Beduschi R, Beduschi MC, Wojno KJ, Jhung M, Williams AL, Wolf JS. Antifibrinolytic additives to fibrin glue for laparoscopic wound closure in urinary tract. J Endourol. 1999;13:283–287. doi: 10.1089/end.1999.13.283. [DOI] [PubMed] [Google Scholar]
- 16.Pipan CM, Glasheen WP, Matthew TL, Gonias SL, Hwang LJ, Jane JA, Spotnitz WD. Effects of antifibrinolytic agents on the life span of fibrin sealant. J Surg Res. 1992;53:402–407. doi: 10.1016/0022-4804(92)90068-b. [DOI] [PubMed] [Google Scholar]
- 17.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–3778. [PubMed] [Google Scholar]
- 18.Catelas I, Dwyer JF, Helgerson S. Controlled release of bioactive transforming growth factor beta-1 from fibrin gels in vitro. Tissue Eng Part C Methods. 2008;14:119–128. doi: 10.1089/ten.tec.2007.0262. [DOI] [PubMed] [Google Scholar]
- 19.Sahni A, Odrljin T, Francis CW. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J Biol Chem. 1998;273:7554–7559. doi: 10.1074/jbc.273.13.7554. [DOI] [PubMed] [Google Scholar]
- 20.Campbell PG, Durham SK, Hayes JD, Suwanichkul A, Powell DR. Insulin-like growth factor-binding protein-3 binds fibrinogen and fibrin. J Biol Chem. 1999;274:30215–30221. doi: 10.1074/jbc.274.42.30215. [DOI] [PubMed] [Google Scholar]
- 21.Huang MC, Lo MJ, Lin YL, Chang SE, Huang WC, Kuo WC, Tsai MJ, Kuo HS, Shih YH, Cheng H. Functional recovery after the repair of transected cervical roots in the chronic stage of injury. J Neurotrauma. 2009;26:1795–1804. doi: 10.1089/neu.2008.0529. [DOI] [PubMed] [Google Scholar]
- 22.Wong C, Inman E, Spaethe R, Helgerson Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003;89:573–582. [PubMed] [Google Scholar]
- 23.Becker JC, Domschke W, Pohle T. Biological in vitro effects of fibrin glue: fibroblast proliferation, expression and binding of growth factors. Scand J Gastroenterol. 2004;39:927–932. doi: 10.1080/00365520410003371. [DOI] [PubMed] [Google Scholar]
- 24.Drinnan CT, Zhang G, Alexander MA, Pulido AS, Suggs LJ. Multimodal release of transforming growth factor-beta1 and the BB isoform of platelet derived growth factor from PEGylated fibrin gels. J Controlled Release. 2010 doi: 10.1016/j.jconrel.2010.03.026. in press. [DOI] [PubMed] [Google Scholar]
- 25.Huang Q, Goh JC, Hutmacher DW, Lee EH. In vivo mesenchymal cell recruitment by a scaffold loaded with transforming growth factor beta1 and the potential for in situ chondrogenesis. Tissue Eng. 2002;8:469–482. doi: 10.1089/107632702760184727. [DOI] [PubMed] [Google Scholar]
- 26.Arkudas A, Tjiawi J, Saumweber A, Beier JP, Polykandriotis E, Bleiziffer O, Horch RE, Kneser U. Evaluation of blood vessel ingrowth in fibrin gel subject to type and concentration of growth factors. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arkudas A, Pryymachuk G, Hoereth T, Beier JP, Polykandriotis E, Bleiziffer O, Horch RE, Kneser U. Dose-finding study of fibrin gel-immobilized vascular endothelial growth factor 165 and basic fibroblast growth factor in the arteriovenous loop rat model. Tissue Eng Part A. 2009;15:2501–2511. doi: 10.1089/ten.tea.2008.0477. [DOI] [PubMed] [Google Scholar]
- 28.Catelas I, Sese N, Wu BM, Dunn JCY, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12:2385–2396. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 29.Gao C, Ma S, Ji Y, Wang J, Li J. Siatic nerve regeneration in rats stimulated by fibrin glue containing nerve growth factor: an experimental study. Injury. 2008;39:1414–1420. doi: 10.1016/j.injury.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Woodruff MA, Rath SN, Susanto E, Haupt LM, Hutmacher DW, Nurcombe V, Cool SM. Sustained release and osteogenic potential of heparan sulfate-doped fibrin glue scaffolds within a rat cranial model. J Mol Histol. 2007;38:425–433. doi: 10.1007/s10735-007-9137-y. [DOI] [PubMed] [Google Scholar]
- 31.Levi AD, Dancausse H, Li X, Duncan S, Horkey L, Oliviera M. Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J Neurosurg. 2002;96:197–205. doi: 10.3171/spi.2002.96.2.0197. [DOI] [PubMed] [Google Scholar]
- 32.Cheng H, Hoffer B, Strömberg I, Russell D, Olson L. The effect of glial cell line-derived neurotrophic factor in fibrin glue on developing dopamine neurons. Exp Brain Res. 1995;104:199–206. doi: 10.1007/BF00242006. [DOI] [PubMed] [Google Scholar]
- 33.Schmökel HG, Schense JC, Weber FE, Grätz KW, Gnägi D, Müller R, Hubbell JA. Bone healing in the rat and dog with nonglycosylated BMP-2 demonstrating low solubility in fibrin matrices. J Orthop Res. 2004;22:376–381. doi: 10.1016/S0736-0266(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 34.Schmökel HG, Weber FE, Seiler G, von Rechenberg B, Schense JC, Schawalder P, Hubbell J. Treatment of nonunions with nonglycosylated recombinant human bone morphogenetic protein-2 delivered from a fibrin matrix. Vet Surg. 2004;33:112–118. doi: 10.1111/j.1532-950x.2004.04018.x. [DOI] [PubMed] [Google Scholar]
- 35.Erzurum VZ, Bian JF, Husak VA, Ellinger J, Xue L, Burgess WH, Greisler HP. R136K fibroblast growth factor-1 mutant induces heparin-independent migration of endothelial cells through fibrin glue. J Vasc Surg. 2003;37:1075–1081. doi: 10.1067/mva.2003.177. [DOI] [PubMed] [Google Scholar]
- 36.Shireman PK, Xue L, Maddox E, Burgess WH, Greisler HP. The S130K fibroblast growth factor-1 mutant induces heparin-independent proliferation and is resistant to thrombin degradation in fibrin glue. J Vasc Surg. 2000;31:382–390. doi: 10.1016/s0741-5214(00)90168-x. [DOI] [PubMed] [Google Scholar]
- 37.Patel VV, Zhao L, Wong P, Pradhan BB, Bae HW, Kanim L, Delamarter RB. An in vitro and in vivo analysis of fibrin glue use to control bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth. Spine J. 2006;6:397–403. doi: 10.1016/j.spinee.2005.11.006. discussion 404. [DOI] [PubMed] [Google Scholar]
- 38.Patel VV, Zhao L, Wong P, Kanim L, Bae HW, Pradhan BB, Delamarter RB. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31:1201–1206. doi: 10.1097/01.brs.0000217650.90861.99. [DOI] [PubMed] [Google Scholar]
- 39.Zhibo X, Miaobo Z. Effect of sustained-release lidocaine on reduction of pain after subpectoral breast augmentation. Aesthet Surg J. 2009;29:32–34. doi: 10.1016/j.asj.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Fujimoto K, Yamamura K, Osada T, Hayashi T, Nabeshima T, Matsushita M, Nishikimi N, Sakurai T, Nimura Y. Subcutaneous tissue distribution of vancomycin from a fibrin glue/Dacron graft carrier. J Biomed Mater Res. 1997;36:564–567. doi: 10.1002/(sici)1097-4636(19970915)36:4<564::aid-jbm16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 41.Osada T, Yamamura K, Yano K, Fujimoto K, Mizuno K, Sakurai T, Nabeshima T. Distribution and serum concentration of sisomicin released from fibrin glue-sealed dacron graft in the rat and human. J Biomed Mater Res. 2000;52:53–57. doi: 10.1002/1097-4636(200010)52:1<53::aid-jbm7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida H, Yamaoka Y, Shinoyama M, Kamiya A. Novel drug delivery system using autologous fibrin glue--release properties of anti-cancer drugs. Biol Pharm Bull. 2000;23:371–374. doi: 10.1248/bpb.23.371. [DOI] [PubMed] [Google Scholar]
- 43.Kitazawa H, Sato, Adachi I, Masuko Y, Horikoshi I. Microdialysis assessment of fibrin glue containing sodium alginate for local delivery of doxorubicin in tumor-bearing rats. Biol Pharm Bull. 1997;20:278–281. doi: 10.1248/bpb.20.278. [DOI] [PubMed] [Google Scholar]
- 44.Yau L, Molnar P, Moon MC, Buhay S, Werner JP, Molnar K, Saward L, Del Rizzo D, Zahradka P. Meta-iodobenzylguanidine, an inhibitor of arginine-dependent mono(ADP-ribosyl)ation, prevents neointimal hyperplasia. J Pharmacol Exp Ther. 2008;326:717–724. doi: 10.1124/jpet.108.137513. [DOI] [PubMed] [Google Scholar]
- 45.Breen A, Dockery P, O’Brien T, Pandit A. Fibrin scaffold promotes adenoviral gene transfer and controlled vector delivery. J Biomed Mater Res A. 2009;89:876–884. doi: 10.1002/jbm.a.32039. [DOI] [PubMed] [Google Scholar]
- 46.Wan L, Li D, Wu Q. Perivenous application of fibrin glue as external support enhanced adventitial adenovirus transfection in rabbit model. J Surg Res. 2006;135:312–316. doi: 10.1016/j.jss.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 47.Teraishi F, Umeoka T, Saito T, Tsukagoshi T, Tanaka N, Fujiwara T. A novel method for gene delivery and expression in esophageal epithelium with fibrin glues containing replication-deficient adenovirus vector. Surg Endosc. 2003;17:1845–1848. doi: 10.1007/s00464-003-8146-5. [DOI] [PubMed] [Google Scholar]
- 48.Arrighi I, Mark S, Alvisi M, von Rechenberg B, Hubbell JA, Schense JC. Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials. 2009;30:1763–1771. doi: 10.1016/j.biomaterials.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 49.Morton TJ, Fürst W, van Griensven M, Redl H. Controlled release of substances bound to fibrin-anchors or of DNA. Drug Deliv. 2009;16:102–107. doi: 10.1080/10717540802605608. [DOI] [PubMed] [Google Scholar]
- 50.Schmökel HG, Weber FE, Schense JC, Grätz KW, Schawalder P, Hubbell JA. Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices. Biotechnol Bioeng. 2005;89:253–262. doi: 10.1002/bit.20168. [DOI] [PubMed] [Google Scholar]
- 51.Willerth SM, Rader A, Sakiyama-Elbert SE. The effect of controlled growth factor delivery on embryonic stem cell differentiation inside fibrin scaffolds. Stem Cell Res. 2008;1:205–218. doi: 10.1016/j.scr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood MD, Borschel GH, Sakiyama-Elbert SE. Controlled release of glial-derived neurotrophic factor from fibrin matrices containing an affinity-based delivery system. J Biomed Mater Res A. 2009;89:909–918. doi: 10.1002/jbm.a.32043. [DOI] [PubMed] [Google Scholar]
- 53.Zhao W, Han Q, Lin H, Gao Y, Sun W, Zhao Y, Wang B, Chen B, Xiao Z, Dai J. Improved neovascularization and wound repair by targeting human basic fibroblast growth factor (bFGF) to fibrin. J Mol Med. 2008;86:1127–1138. doi: 10.1007/s00109-008-0372-9. [DOI] [PubMed] [Google Scholar]
- 54.Zhao W, Han Q, Lin H, Sun W, Gao Y, Zhao Y, Wang B, Wang X, Chen B, Xiao Z, Dai J. Human basic fibroblast growth factor fused with Kringle4 peptide binds to a fibrin scaffold and enhances angiogenesis. Tissue Eng Part A. 2009;15:991–998. doi: 10.1089/ten.tea.2008.0240. [DOI] [PubMed] [Google Scholar]
- 55.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 56.Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A. 2008;14:1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]