Abstract
Cannabinoids are a group of compounds present in Cannabis plant (Cannabis sativa L.). They mediate their physiological and behavioral effects by activating specific cannabinoid receptors. With the recent discovery of the cannabinoid receptors (CB1 and CB2) and the endocannabinoid system, research in this field has expanded exponentially. Cannabinoids have been shown to act as potent immunosuppressive and anti-inflammatory agents and have been shown to mediate beneficial effects in a wide range of immune-mediated diseases such as multiple sclerosis, diabetes, septic shock, rheumatoid arthritis, and allergic asthma. Cannabinoid receptor 1 (CB1) is mainly expressed on the cells of the central nervous system as well as in the periphery. In contrast, cannabinoid receptor 2 (CB2) is predominantly expressed on immune cells. The precise mechanisms through which cannabinoids mediate immunosuppression is only now beginning to be understood and can be broadly categorized into four pathways: apoptosis, inhibition of proliferation, suppression of cytokine and chemokine production and induction of T regulatory cells (T regs). Studies from our laboratory have focused on mechanisms of apoptosis induction by natural and synthetic cannabinoids through activation of CB2 receptors. In this review, we will focus on apoptotic mechanisms of immunosuppression mediated by cannabinoids on different immune cell populations and discuss how activation of CB2 provides a novel therapeutic modality against inflammatory and autoimmune diseases as well as malignancies of the immune system, without exerting the untoward psychotropic effects.
Keywords: Cannabinoids, Apoptosis, Immune suppression
Introduction
Cannabinoids are a group of compounds found in the marijuana plant (Cannabis sativa L.). Marijuana has been used both for recreational and medicinal purposes for several centuries. Cannabinoids have been shown to be effective in the treatment of nausea and vomiting associated with cancer chemotherapy, anorexia and cachexia seen in HIV/AIDS patients, as well as neuropathic pain, and spasticity in multiple sclerosis (Guzman 2003; Tramer et al. 2001; Inui 2002; Pollmann and Fenenberg 2008). More recently, the anti-inflammatory properties of cannabinoids are drawing significant attention. In the last 15 years, studies with marijuana cannabinoids led to the discovery of cannabinoid receptors (CB1 and CB2) and their endogenous ligands, which make up what is known as the endocannabinoid system. Both CB1 and CB2 are heterotrimeric Gi/o-protein coupled receptors. CB1 is expressed in the tissues of both the central nervous system (CNS) and the periphery, with a predominant expression on presynaptic nerves. CB2, sometimes referred to as the “peripheral cannabinoid receptor”, is mainly expressed on immune cells but can also be found on other peripheral tissues such as the retina and in the CNS (Croxford and Yamamura 2005; Mackie 2006). The endocannabinoid system modulates many functions such as movement, memory, appetite, regulation of body temperature, pain and immune functions. The natural and synthetic cannabinoids are derived from marijuana plants, whereas endocannabinoids are metabolites of arachidonic acid (Klein and Cabral 2006). Table 1 shows a summary of some well-known natural and synthetic cannabinoids, as well as their source and receptors.
Table 1.
Some well-known natural and synthetic cannabinoids and their receptors.
| Cannabinoids | Source | Receptor/role |
|---|---|---|
| Anandamide (AEA) | Expressed in CNS (hippocampus, striatum, cerebellum) and periphery (spleen, kidney, skin, uterus) | CB1 > CB2 |
| 2-Arachidonylglycerol (2-AG) | CNS > immune cells | CB1 ≈ CB2 |
| Δ9-Tetrahydrocannabinol (THC) | Cannabis derived | CB1 ≈ CB2 |
| Δ8-Tetrahydrocannabinol (THC) | Cannabis derived | CB1 ≈ CB2 |
| Cannabinol | Cannabis derived | CB1 ≈ CB2 |
| Cannabidiol | Cannabis derived | CB2a |
| HU-210 | Synthetic | CB1 ≈ CB2 |
| HU-308 | Synthetic | CB2 select agonist |
| JWH-133 | Synthetic | CB2 select agonist |
| HU-211 | Synthetic | N/A |
| CP-55,940 | Synthetic | Analog of THC |
| WIN-55,212-2 | Synthetic | CB1 select agonist |
| SR141716 | Synthetic | CB1 antagonist |
| SR144528 | Synthetic | CB2 antagonist |
≈ Has similar affinity.
Several reports have shown the receptor-independent action of cannabidiol; however, in some cases CBD has been shown to act via CB2.
There is a ‘hierarchy’ of cannabinoid receptor expression within the immune system (B cells > Natural Killer cells > Monocytes > Neutrophils > CD8 leukocytes > CD4 leukocytes), and the level of expression is dependent on the stimulus and the activation state of the cell (Lee et al. 2001). For instance, stimulation of splenocytes with LPS led to CB2 mRNA downregulation, whereas CD40 co-stimulation resulted in CB2 mRNA upregulation (Lee et al. 2001). In vitro studies on human and mouse T cells demonstrated that low doses of THC stimulated T cell activation, while high doses of THC inhibited responses to LPS, T cell mitogens and anti-CD3 antibody (Klein et al. 1995). It is not clear if cannabinoids have a direct or indirect effect on B cells; however, the biphasic role of cannabinoids has also been shown in B cell studies. Derocq et al. (1995) demonstrated increased B cell proliferation as a response to THC and CP55,940; whereas Klein et al. (1995) showed decreased response to LPS by B cells upon cannabinoid treatment. Anandamide (AEA), an endogenous cannabinoid, inhibited proliferation of T and B lymphocytes after mitogen stimulation and induced apoptosis in low doses (Schwarz et al. 1994). Several studies have shown that cannabinoids negatively regulate phagocytosis, cell-spreading and antigen presentation by macrophages (Cabral and Mishkin 1989; Lopez-Cepero et al. 1986; McCoy et al. 1995).
The mechanism of immunosuppression by cannabinoids has been investigated both in vitro and in vivo studies; however, there still remain many questions. In general, it is known that since cannabinoids bind to CB1 and CB2, they exert their effects through inhibition of adenylate cyclase activity; thereby blocking forskolin-stimulated cAMP activation. This process leads to decreased activity of protein kinase A, and subsequently to lesser binding of transcription factors to CRE consensus sequences, and a dysfunction in IL-2 production (Condie et al. 1996). In NK cell studies, Zhu and colleagues (1995) demonstrated that when pretreated with THC, NK cells had a defect in IL-2 receptor with decreased IL-2 binding sites; therefore, IL-2 stimulated cells were unable to kill EL-4 tumor cells. Yebra et al. (1992) showed that decreased proliferation of thymocytes upon THC treatment was due to inhibition of Ca2+ stabilization within the cell. There are few reports also showing receptor-independent actions of cannabinoids, such as in B cells and rat cortical neurons (Hampson et al. 1998; Chen and Buck 2000).
Cannabinoids clearly modulate immune responses during inflammatory processes and their immunosuppressive effects have been studied in many disease models such as multiple sclerosis, diabetes, septic shock, rheumatoid arthritis, and allergic asthma (Croxford and Yamamura 2005; Klein and Cabral 2006). Studies in these disease models along with many in vitro experiments show that cannabinoids exert their immunosupressive properties in four main ways: (1) induction of apoptosis, (2) inhibition of cell proliferation, (3) inhibition of cytokine and chemokine production, and (4) induction of regulatory T cells (T regs). There have been many published reviews on the effect of cannabinoids on the immune system especially discussing second and third pathways mentioned above (Klein et al. 1998, 2000; Klein and Cabral 2006). In this review, we focus on cannabinoids and immune cell apoptosis, and we review the effects of natural and synthetic cannabinoids on different immune cell populations.
Apoptosis is the process of programmed cell death that can be induced by intrinsic factors, extrinsic factors or both (Hengartner 2000; Igney and Krammer 2002). Because apoptosis eliminates damaged, harmful and unwanted cells, it is significant in biological processes including development, morphogenesis, and homeostasis. During apoptosis, many morphological changes occur such as membrane blebbing, cell shrinkage, mitochondria leakage, and nuclear fragmentation. Molecular changes underlie these morphological changes, and they make up the two different pathways of apoptosis: (1) intrinsic pathway – via mitochondria, and (2) extrinsic pathway – through death receptors (Hengartner 2000; Igney and Krammer 2002). Briefly, the intrinsic pathway is initiated by an imbalance in anti-apoptotic and pro-apoptotic members of Bcl-2 family of proteins that regulate the permeability of the mitochondrial membrane. The imbalance towards the latter leads to cytochrome c leakage into the cytosol. Cytochrome c then combines with pro-caspase 9, ATP and APAF-1 to form the apoptosome. The apoptosome results in the formation of active caspase 9, cleaves pro-caspase 3 into active caspase 3, and leads to apoptosis (Kroemer and Reed 2000). The extrinsic pathway is triggered with the ligation of death receptors such as tumor necrosis factor receptor family (i.e. CD95), and results in the formation of Death Inducing Signaling Complex (DISC). DISC contains caspase 8 and caspase 10 as the initiator caspases, and these caspases activate caspase 3, resulting in apoptosis (Hengartner 2000).
Cannabinoid-induced apoptosis in T cells
While marijuana smoking or exposure to marijuana cannabinoid may cause lung injury, Delta9-etrahydrocannabinol (THC) in experimental models has been shown to suppress immune functions and increase susceptibility to infections (Tashkin et al. 2002). In vitro studies revealed that THC may exert a direct effect on immune cells by inhibiting the proliferation of lymphocytes in culture, although the precise mechanisms remained unclear. In 1994, anandamide, an endogenous cannabinoid receptor ligand was shown to induce apoptosis in human lymphocyte cultures (Schwarz et al. 1994). This study was also extended to murine macrophages and T cells by showing that THC treatment of cultured immune cells triggered apoptosis through the regulation of Bcl-2 and caspase activity (Zhu et al. 1998). While these studies opened new avenues to further investigate apoptosis as a mechanism of in vivo immunotoxicity induced by THC, there were no additional studies to demonstrate these effects in vivo. One of the reasons could have been that apoptosis detection in vivo is difficult because the apoptotic cells are rapidly and efficiently cleared through phagocytosis. Studies from our laboratory established this fact using a number of drugs and chemicals including 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD) and dexamethasone (Kamath et al. 1997; Camacho et al. 2001; Singh et al. 2008). In these studies, we noted that if the immune cells exposed to an apoptotic chemical in vivo, are cultured in vitro for an additional 12–24 h, they exhibit marked increase in apoptosis because of their inability to be cleared in vitro by phagocytes.
We therefore conducted a systematic investigation on whether cannabinoids induce apoptosis in vivo, using normal mice and demonstrated conclusively that THC and other cannabinoids do so, which may account for the immunotoxicity in vivo. In these studies, we demonstrated that THC administration in vivo (10 mg/kg body weight) decreased the cellularity in the spleen and thymus, affecting several cell populations such as T cells, B cells and macrophages. The conclusive evidence for apoptosis not only came from the observation that the in vivo exposed cells underwent enhanced spontaneous apoptosis in vitro but also that exposure of mice to a pan caspase inhibitor prior to THC administration blocked apoptotic effects of THC in vivo (McKallip et al. 2002b).
In addition, extensive in vitro studies demonstrated that naïve or mitogen-stimulated splenocytes, underwent apoptosis when cultured with THC as measured by TUNEL assay and AnnexinV/PI staining. At a lower concentration (10 μM) THC only induced AnnexinV + cells, which is indicative of early apoptotic cells. At higher concentrations of THC (i.e. 20 μM), the splenocytes were both AnnexinV and PI positive, which represent late apoptotic as well as necrotic cells. One interesting finding was that in cultures that had been treated with THC alone, and in the absence of mitogen, the levels of apoptosis were greater when compared to those seen in cultures that contained both THC and mitogen (McKallip et al. 2002b). These results suggested that THC may affect naïve lymphocytes to a greater degree than activated lymphocytes. Furthermore, it was noted that activated lymphocytes downregulated expression of CB2, thereby explaining decreased sensitivity of activated lymphocytes to THC. Use of CB2 antagonists blocked THC-induced apoptosis in these cells, while CB1 antagonists failed to show a significant effect. These data indicated that THC-induced apoptosis in splenocytes in a CB2 receptor-dependent way. Similar results were observed when the experiments were repeated with thymocytes (McKallip et al. 2002b).
The fact that activation of CB2 triggers apoptosis in immune cells suggested that targeting CB2 may constitute a novel approach to treat inflammatory and autoimmune diseases. Such a treatment would not exert psychotropic effects and may selectively target immune cells. To this end, Lombard et al. (2007) from our laboratory tested the effects of a CB2 synthetic agonist, JWH-015, on T and B cells. It was noted that JWH-015 in a dose-dependent fashion not only inhibited proliferation but also induced apoptosis in naïve- and activated-splenocytes and -thymocytes. In this study, the results confirmed that both the death receptor pathway and the intrinsic pathway were involved, because caspase 8 and caspase 9 inhibitor treatments were able to block the apoptosis induced by JWH-015. In vivo administration of JWH-015 (150 mg/kg body weight) inhibited the proliferation of splenocytes ex vivo, when stimulated with ConA. In addition, JWH-015 decreased the antigen-specific response in mice that were exposed to Staphylococcal Enterotoxin A, determined by decreased proliferation of SEA-responsive Vβ3+ and Vβ11+ T cells (Lombard et al. 2007).
Recently, a group of investigators studied the effects of a non-psychoactive cannabinoid, cannabidiol (CBD), on immune cell apoptosis (Lee et al. 2008). They demonstrated that CBD induced apoptosis in CD4+ and CD8+ T cell populations in a time- and dose-dependent manner. Treatment of splenocytes with 4–8 μM CBD increased apoptosis significantly, and this was determined by the percentage of hypodiploid cells and TUNEL positive cells. Furthermore, the investigators showed that apoptosis was due to the formation of reactive oxygen species (ROS), activation of caspase 8 and caspase 3. The same group also reported that CBD had similar effects on murine thymocytes and EL-4 cells.
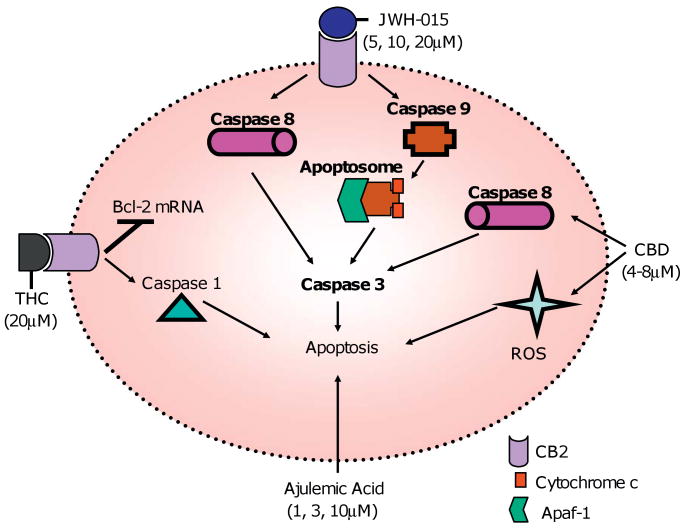
Ajulemic acid (Aja) is a metabolite of THC, and it is a potent analgesic and anti-inflammatory agent (George et al. 2008). Aja belongs to a class of cannabinoids called carboxy tetrahydrocannabinols. In controlled trials of patients with neuropathic pain, Aja proved as effective as morphine and in rats, it significantly reduced severity of adjuvant-induced arthritis (Karst et al. 2003; Zurier et al. 1998). One study investigated the effects of Aja on human peripheral blood T lymphocytes. This study showed that Aja reduced T cell proliferation by ∼75%, and induced apoptosis in a dose-dependent manner (at 1, 3 and 10 μM). Incubation of the cells with Aja (10 μM) for 6 h increased caspase 3 activity 9-fold, and DNA fragmentation 2.471.3-fold. There was a wide range in the observed effects of Aja on T cells from donors, and this suggested that each donor's cells had different levels of sensitivity to Aja induced apoptosis (Fig. 1).
Fig. 1.
Natural and synthetic cannabinoids induce apoptosis in T cells. THC (20 μM) and JWH-015 (5, 10, 20 μM) induce apoptosis in T cells through ligation of CB2 receptor. JWH-015 activates both the intrinsic pathway and the extrinsic pathway, and THC downregulates Bcl-2 mRNA and induces caspase 1 activity. It is not known if CBD and Aja exert their effects through receptor-dependent pathways; however, one report has shown that CBD may mediate its effect through CB2 receptor (McKallip et al. 2006).
The ability of cannabinoids to trigger apoptosis immune cells in vivo suggested that THC can be very effective against inflammatory diseases. In a ConA-induced hepatitis model, we illustrated that THC was able to alleviate liver damage in ConA-treated mice through induction of increased levels of apoptosis in activated T cells (Hegde et al. 2008). Interestingly, however, we also noted that THC treatment increased the number of Foxp3 + T reg cells. Such T regs may have inhibited the induction of cytokines induced in vivo by ConA. Together, these studies demonstrated that T regs, unlike other T cells, may be resistant to apoptosis induced by THC and may suppress the activation of T cells that escape apoptosis. Further studies are necessary to address this possibility.
The endocannabinoid system also plays a role during disease processes. In the blood of patients with septic shock, 4-fold increase in AEA and 2-AG was detected (Wang et al. 2001), and this was also seen in LPS-induced hypotension (Varga et al. 1998). Use of CB1 antagonists in the hypotension model, alleviated the effects of cannabonids, showing that these compounds contributed to the degree of inflammation. On the other hand, WIN compounds had protective roles in septic shock, since they decreased infiltration of lymphocytes and inhibited cytokine production (Di Fillippo et al. 2004). Once again, this example demonstrates the biphasic role of cannabinoids in general.
Sensitivity of B cells to cannabinoid-induced apoptosis
B cells are the antibody producing cells of the immune system, and they express the highest amount of CB2 on their cell surface. Several reports have shown that cannabinoids have different effects on B cells such as decreased cell proliferation and reduced antibody production (reviewed by Croxford and Yamamura 2005). There have also been studies on marijuana smoking populations, and the results from these studies vary significantly, because many factors such as the age and the genetic background of the users, the amount of marijuana consumed, and the duration of use were all different. Some reports from human studies showed reduced B cell numbers along with decreased IgG and IgM (El-Gohary and Eid 2004), while others showed no change in B cell numbers and increase in IgE levels (Rachelefsky et al. 1976).
Derocq et al. (1995) demonstrated that the activity of cannabinoids is not restricted to immunosuppression, and nanomolar concentrations of synthetic (CP55,940 and WIN55212-2) and natural (THC) cannabinoids increase proliferation in human tonsillar B cells co-stimulated with either anti-Igs or anti CD40-antibodies. They argued that this effect of cannabinoids was mediated through CB2 receptor since blocking of CB1 receptor with SR141716A did not inhibit the proliferation. The investigators mentioned that they also tested the cannabinoids at 1–100μM range, and these indicated high concentrations inhibited proliferation.
A receptor-independent mechanism of cannabinoid-induced cell death has been shown in cultured human B lymphoblastoid cells (Chen and Buck 2000). The investigators showed that THC, cannabinol, and cannabidiol protected human B lymphoblastoid cells (5/2) from serum-deprived cell death at submicromolar concentrations. In addition, they illustrated that the action of cannabinoids did not correlate with their binding affinity to the CB1 and CB2 receptors and there was no stereoselectivity, which suggested a receptor-independent action of cannabinoids. They also performed experiments with α-tocopherol, the most potent isomer of the antioxidant vitamin E and with retinod anhydroretinol, which induces oxidative cell death, either in the presence or absence of THC. Their results demonstrated that similar to the effect of α-tocopherol, and unlike retinoid anhydroretinol, THC was able to protect 5/2 cells from oxidative stress and cell death.
Cannabinoid-induced apoptosis in antigen-presenting cells
Dendritic cells (DCs) are the most potent and specialized antigen-presenting cells of the immune system, and cannabinoids have been shown to induce apoptosis in these cells via CB1 and CB2 receptors. Do et al. (2004) demonstrated that THC (5 μM or greater) and AEA (20 μM) induced apoptosis in bone marrow-derived dendritic cells from C57BL/6 mice that were cultured in serum-free medium, through the engagement of both receptors. It was shown that THC treatment led to many molecular changes such as activation of caspases 2, 8, and 9, cleavage of Bid (a Bcl2-family protein), cytochrome c release, a decrease in mitochondrial membrane potential, and an increase in phosphorylation of Iκβ-α. This study also demonstrated the in vivo effects of THC administration, and showed that there was a decrease in the number of splenic DCs, and in the expression of MHCII on DCs.
Lu et al. (2006) studied the immunosuppressive effects of THC in Legionella pneumophila (Lp) infected dendritic cells, and focused on Th1 immune suppression. Their results showed that Lp loading in vitro into the dendritic cells, and subsequent administration into mice led to immunization. However, when the dendritic cells were pretreated with THC, the immunization potential of Lp-loaded cells was suppressed. THC treatment suppressed IL-12p40 production by the dendritic cells as well as inhibited expression of maturation markers such as MHCII, CD86, and CD40. The investigators questioned whether this decrease in cytokines was due to increase in apoptosis of the dendritic cells upon THC treatment, and they demonstrated that 10 μM THC did not induce cell death in DCs after 24 h. Their results contradict Do et al.'s (2004) results; however, this may be attributed to differences in experimental design. For example, Do et al. used serum-free conditions in order to minimize the inhibitory effects of serum on cannabinoid action, while Lu et al. used serum in their culture conditions. In addition, Do et al. used DCs from the bone marrow of C57BL/6 mice, while Lu et al. used DCs from the bone marrow of Balb/c mice.
THC also induced apoptosis in naïve macrophages as well as in LPS-activated macrophages isolated from the peritoneal cavity of Balb/c mice (Zhu et al. 1998). The investigators tested the effects of THC on macrophages with agarose gel electrophoresis and TUNEL methods, and showed that at 5 μg/ml concentration, the drug caused DNA fragmentation but no membrane damage. The higher drug concentration (10 μg/ml) caused significant DNA fragmentation and membrane damage after 24 h of treatment. In terms of the mechanism of action, the researchers focused on two genes: Bcl-2 and caspase-1. Caspase-1 processes premature IL-1β into mature IL-1β, and this aids in apoptosis (Zhu et al. 1994), and when they used caspase inhibitor Ac-Tyr-Val-Ala-L- aspartic acid aldehyde, DNA fragmentation induced by THC was suppressed. Upon activation, macrophages upregulated Bcl-2 expression, but THC treatment decreased the level of Bcl-2 at the mRNA level. Similar results were also observed in splenocytes.
George et al. (2008) studied the effect of Aja in the precursor cells of osteoclastogenesis, which are monocytes and macrophages, and in osteoclasts themselves. They used bone marrow cells incubated with macrophage stimulating factor (M-CSF), and a mouse macrophage cell line as precursor cells, and they added RANKL to the precursor cells in order to obtain pure osteoclast cells in vitro. In this study, they demonstrate that Aja induced apoptosis both in precursor monocytes and macrophages as well as in osteoclasts at concentrations between 15 and 30 μM.
Effect of cannabinoids on malignant immune cells
In 2002, studies from our laboratory suggested for the first time that targeting CB2 receptors on cancers of immune origin may constitute a novel approach to treat such malignancies (McKallip et al. 2002a). We first investigated the ability of cannabinoids to induce apoptosis in malignant cells of immune origin including lymphomas and leukemias (Lombard et al. 2005; McKallip et al. 2006, 2002a). It was demonstrated that human leukemia and lymphoma cell lines such as Jurkat cells and Molt-4 expressed CB2 receptors but express little or no significant levels of CB1. These cells were found to be susceptible to apoptosis induced by THC, HU-210, anandamide, and JWH-015 (McKallip et al. 2002a). In the same study, it was noted that apoptosis was also induced by THC and anandamide in murine tumor cells such as EL-4 and LSA. In vivo administration of 3 and 5 mg/kg bodyweight THC was also effective in killing EL-4 tumor cells that were injected into the peritoneal cavity, and upon THC treatment, 25% of the mice survived the tumor challenge. Primary lymphoblastic human leukemia cells were also tested for their susceptibility to THC-induced apoptosis, and the cells taken from two different donors showed increased apoptosis upon THC treatments at 5 μM or greater concentrations (McKallip et al. 2002a). Another observation by this study was that serum interfered with the effects of THC; therefore in serum-containing medium 10 μM or greater concentrations were needed to induce apoptosis; however, in serum-free conditions 3 μM or higher concentrations were sufficient to induce a significant increase in apoptosis.
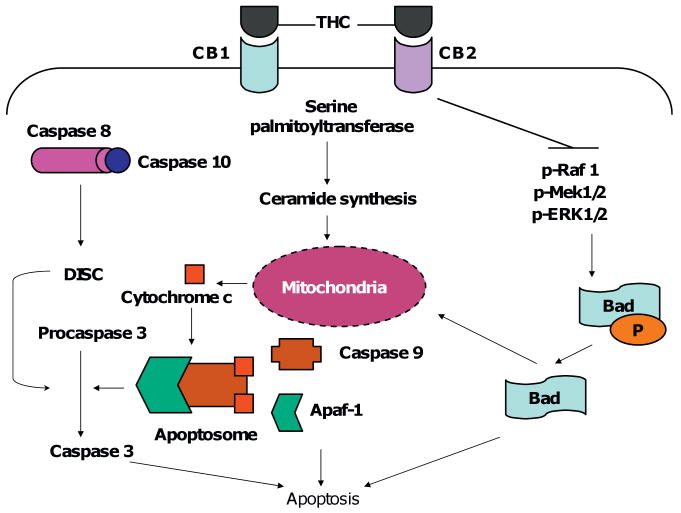
It is well established that THC induces apoptosis in Jurkat leukemia cell lines, and this occurs through three different mechanisms (Herrera et al. 2006; Jia et al. 2006; Lombard et al. 2005). The first mechanism was shown by Lombard et al. (2005), where the investigators examined the effect of THC on wild type as well as on FADD deficient, caspase 8 deficient, and caspase 9 deficient Jurkat cells. Their study demonstrated that THC-induced apoptosis in Jurkat cells occurred primarily through the intrinsic pathway including caspase 9 activation, and the release of cytochrome c into the cytosol. However, the degree of apoptosis in FADD deficient and caspase 8 deficient cells showed a partial decrease, when measured by TUNEL. THC treatment of the cells led to activation of caspases 2, 8, 9, and 10 in that order, and cleavage of Bid occurred 2 h after treatment. Overall, these studies demonstrated that THC-induced apoptosis occurred through cross-talk between the extrinsic and intrinsic pathways, with the intrinsic pathway playing the primary role (Lombard et al. 2005).
The second proposed mechanism came from a research group in Spain, and in this particular study, the researchers investigated if ceramide accumulation is involved in THC-induced apoptosis in Jurkat cells (Herrera et al. 2006). They demonstrated that upon 2 μM THC treatment, ceramide levels were increased within the cell. They inhibited ceramide synthesis with a pharmacological inhibitor or transfected the cells with a dominant negative form of the enzyme that catalyzes ceramide production, and demonstrated that this led to decreased levels of apoptosis in the presence of THC. Apoptosis was mediated through loss of mitochondrial membrane potential, cytochrome c release, and caspase activation. When a pan caspase inhibitor was used, apoptosis was prevented but there still was loss of the membrane potential of the mitochondria. Therefore, this study showed that the first step in the apoptotic pathway was ceramide production, and that this led to loss of membrane potential, and caspase activation, respectively (Herrera et al. 2006).
Our studies have further demonstrated that THC interferes with MAP kinase signaling pathway, which plays a critical role in apoptosis of Jurkat cells (Jia et al. 2006). Specifically, THC inhibited the MAPK/MEK/ERK signaling pathway, which resulted in translocation of Bad into mitochondria, and eventually apoptosis. It was also demonstrated that THC-induced apoptosis in Jurkat T cells in a dose-dependent manner, and the use of CB1 and CB2 antagonists (SR141716 and SR144528, respectively) significantly decreased the amount of apoptosis induced by THC. It was shown that THC treatment led to reduced phosphorylation of Raf-1, MEK1/2, and ERK1/2, with no change in total proteins, and that this process was caspase-independent. Furthermore, interruption of Raf-1/MEK/ERK/RSK signaling pathway, and not the PI3K/Akt pathway, played a role in dephosphorylation and translocation of a Bcl-2 family protein, Bad, into the mitochondria. This paper shed more light on the mechanism of THC induced apoptosis in leukemic T cell lines, and demonstrated that ligation of CB1 and CB2 receptors by THC led to: (1) the disruption of a main cell survival pathway, (2) translocation of Bad into the mitochondria, and (3) apoptosis (Fig. 2).
Fig. 2.
THC induces apoptosis in Jurkat cells via three different mechanisms. (1) THC binds to CB1 and CB2, and leads to ceramide synthesis by serine palmitoyltransferase. (2) Ceramide enters the mitochondria and results in cytochrome c leakage into the cytosol. (3) Cytochrome c combines with Apaf-1, caspase 9, and forms the apoptosome. (4) The apoptosome converts procaspase 3 to active caspase 3, resulting in apoptosis. THC also activates the extrinsic pathway (caspases 8 and 10), and inhibits Raf/Mek/Erk pathway. The inhibition of the cell survival pathway leads to dephosphorylation of Bad, and translocation of this Bcl-2 protein into the mitochondria, and subsequently to apoptosis.
In another study from our laboratory, McKallip et al. (2006) investigated cannabidiol-induced apoptosis in mouse (EL-4) and human (Jurkat) leukemia cells, and demonstrated that CBD-induced apoptosis occurred through (1) ligation of the CB2 receptor, (2) activation of caspases 8, 9 and 3, (3) cleavage of Bid, (4) increased cytochrome c in the cytosol, (5) increased ROS production, and (6) increased NADPH oxidases Nox4 and p22phox. In addition, the investigators showed that CBD treatment led to reduced p-p38 levels but p-ERK and p-JNK levels were unaffected. In vivo, this study demonstrated that CBD treatment decreased tumor burden and increased apoptotic tumors in C57BL/6 mice.
Maccarrone et al. (2000) studied the effect of endocannabinoids on human lymphoma U937 cells and showed that anandamide but not other endocannabinoids such as 2-AG and LEA (linoleoylethanolamide) caused apoptosis in these cells in a dose- and time-dependent manner. AEA was effective in 0.25–1 μM range, and there was a 6-fold increase in apoptosis after 48 h compared to the controls. Treatment of cells with CB1 and CB2 antagonists did not affect the degree of apoptosis, while the treatment of cells with a selective vanilloid receptor antagonist, capsazepine, led to a 30% decrease in apoptosis induced by AEA. When the investigators treated the cells with a vanilloid receptor agonist, capsaicin, there was a 5-fold increase in the amount of apoptotic bodies. Overall, this study illustrated that cannabinoids may not always exert their effects through CB1 or CB2 alone.
Effect of cannabinoids on non-immune cells
Cannabinoids affect cell fate in many other cell types such as transformed neural cells, breast cancer cells, hepatocytes, and prostate cancer cells (Guzman et al. 2002; Hegde et al. 2008). As discussed previously, the presence of the cannabinoid receptors on the cell surface is a key determinant on how the cannabinoids will affect a certain cell population. One study explored the effects of THC on murine and human breast cancer cell lines as well as in an in vivo model of breast cancer in mice (McKallip et al. 2005). The investigators demonstrated that mouse mammary carcinoma 4T1, and human breast cancer cell lines MCF-7 and MDA-MB-231 expressed low to undetectable levels of CB1 and CB2; therefore were resistant to THC-induced cell death. In addition, this study demonstrated that THC created an immune-compromised host, and helped in 4T1 tumor growth and metastasis in C57BL/6 mice. Immunosuppression was mediated through CB2 receptor, and a switch from Th1 cytokines to Th2 cytokines such as IL-4 and IL-10 occurred upon THC treatment.
Hegde et al. (2008) examined the apoptotic effects of THC on normal mouse hepatocyte cells, BNL.CL.2, and demonstrated that these cells are resistant to THC-induced apoptosis as well. In this study, the splenocytes were used as positive control, and the investigators showed that the doses that induced cell death in splenocytes were unable to cause any changes in cell viability in hepatocytes. Furthermore, the investigators collected supernatants from ConA-activated splenocyte cultures (CS) and tested the ability of THC to protect hepatocytes from CS-induced cell death. They showed that THC decreased the percentage of apoptotic cells in a dose-dependent manner. These results are in agreement with a study that investigated the effect of a select CB2 agonist, HU-308, in ischemia/reperfusion injury model in C57BL/6 mice, where they established that HU-308 suppressed apoptosis in hepatocytes measured by caspase 3 activity and DNA fragmentation assay (Rajesh et al. 2007).
One study targeted the cannabinoid receptors in order to treat melanoma in a mouse model (Blazquez et al. 2006). They showed that melanoma cells express CB1 and CB2 receptors, and that receptor activation by THC and WIN-55,212-2 led to melanoma cell-growth inhibition both in vitro and in vivo. Furthermore, they demonstrated that THC and WIN-55,212-2 caused cell cycle arrest in melanoma cells via inhibition of Akt and hypophosphorylation of the tumor suppressor protein, retinoblastoma (Rb).
Conclusion
Cannabinoids have been approved in some countries for the treatment of chemotherapy-induced nausea and vomiting, and clinical trials show cannabinoids' effectiveness in pain inhibition and appetite stimulation (Guzman 2003; Tramer et al. 2001). Anti-inflammatory therapeutic potential of cannabinoids is also evident from the studies discussed in this review paper. At the optimal concentrations, cannabinoids do induce apoptosis in immune cells, alleviating inflammatory responses and protecting the host from acute and chronic inflammation. The cumulative effect of cannabinoids on all cell populations of the immune system can be beneficial, when there is a need for immune suppression. For example, in patients with autoimmune diseases such as multiple sclerosis, arthritis and lupus, or in those with septic shock, where the disease is caused by activated immune cells, targeting the immune cells via CB2 agonists may trigger apoptosis and act as anti-inflammatory therapy. CB2 select agonists are not psychoactive and because CB2 is expressed primarily in immune cells, use of CB2 agonists could provide a novel therapeutic modality against autoimmune and inflammatory diseases. Clearly, additional research is necessary to validate these studies in humans through clinical trials. However, in other instances, such as in patients with breast cancer in which cannabinoid receptors may not be expressed by the cancer cells, cannabinoids may worsen the disease, because the immune system is weakened, and the breast cancer cells are resistant to cannabinoid-induced apoptosis. Thus, it is critical to balance the immunsuppressive effects with the anticancer properties, which require careful dose–response studies on these clinical outcomes. Overall, there exists a biphasic role for cannabinoids, and it depends on three key factors: (1) the type of cannabinoid, (2) the dose of cannabinoid, and (3) the type of cell that the cannabinoid is acting on. The degree of cannabinoid receptor expression may play role in some cases; however, the receptor-independent mechanism of action is evident for cannabinoids in some cell populations. In addition to the use of exogenous cannabinoids, in vivo manipulation of endocannabinoids may also offer novel treatment opportunities against cancer and autoimmune diseases.
Acknowledgments
Supported in part by NIH Grants R01AI053703, R01ES09098, R01 AI058300, R01DA016545, R01HL058641 and P01AT003961.
References
- Blazquez C, Carracedo A, Barrado L, Real PJ, Fernandez-Luna JL, Velasco G, et al. Cannabinoid receptors as novel targets for the treatment of melanoma. Faseb J. 2006;20(14):2633–2635. doi: 10.1096/fj.06-6638fje. [DOI] [PubMed] [Google Scholar]
- Di Filippo C, Rossi F, Rossi S, D'Amico M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN. J Leukoc Biol. 2004;75(3):453–459. doi: 10.1189/jlb.0703303. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Mishkin EM. Delta-9-tetrahydrocannabinol inhibits macrophage protein expression in response to bacterial immunomodulators. J Toxicol Environ Health. 1989;26(2):175–182. doi: 10.1080/15287398909531243. [DOI] [PubMed] [Google Scholar]
- Camacho IA, Hassuneh MR, Nagarkatti M, Nagarkatti PS. Enhanced activation-induced cell death as a mechanism of 2,3,7,8-tetrachlorodibenzo-p-dioxin(TCDD)-induced immunotoxicity in peripheral T cells. Toxicology. 2001;165(1):51–63. doi: 10.1016/s0300-483x(01)00391-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Buck J. Cannabinoids protect cells from oxidative cell death: a receptor-independent mechanism. J Pharmacol Exp Ther. 2000;293(3):807–812. [PubMed] [Google Scholar]
- Condie R, Herring A, Koh WS, Lee M, Kaminski NE. Cannabinoid inhibition of adenylate cyclase-mediated signal transduction and interleukin 2 (il-2) expression in the murine t-cell line, el4.Il-2. J Biol Chem. 1996;271(22):13175–13183. doi: 10.1074/jbc.271.22.13175. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166(1–2):3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Derocq JM, Segui M, Marchand J, Le Fur G, Casellas P. Cannabinoids enhance human b-cell growth at low nanomolar concentrations. FEBS Lett. 1995;369(2–3):177–182. doi: 10.1016/0014-5793(95)00746-v. [DOI] [PubMed] [Google Scholar]
- Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers nf-kappab-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol. 2004;173(4):2373–2382. doi: 10.4049/jimmunol.173.4.2373. [DOI] [PubMed] [Google Scholar]
- El-Gohary M, Eid MA. Effect of cannabinoid ingestion (in the form of bhang) on the immune system of high school and university students. Hum Exp Toxicol. 2004;23(3):149–156. doi: 10.1191/0960327104ht426oa. [DOI] [PubMed] [Google Scholar]
- George KL, Saltman LH, Stein GS, Lian JB, Zurier RB. Ajulemic acid, a nonpsychoactive cannabinoid acid, suppresses osteoclastogenesis in mononuclear precursor cells and induces apoptosis in mature osteoclast-like cells. J Cell Physiol. 2008;214(3):714–720. doi: 10.1002/jcp.21263. [DOI] [PubMed] [Google Scholar]
- Guzman M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3(10):745–755. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- Guzman M, Sanchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol Ther. 2002;95(2):175–184. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci USA. 1998;95(14):8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde VL, Hegde S, Cravatt BF, Hofseth LJ, Nagarkatti M, Nagarkatti PS. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: Involvement of regulatory t cells. Mol Pharmacol. 2008;74(1):20–33. doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Herrera B, Carracedo A, Diez-Zaera M, Gomez del Pulgar T, Guzman M, Velasco G. The cb2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp Cell Res. 2006;312(11):2121–2131. doi: 10.1016/j.yexcr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin. 2002;52(2):72–91. doi: 10.3322/canjclin.52.2.72. [DOI] [PubMed] [Google Scholar]
- Jia W, Hegde VL, Singh NP, Sisco D, Grant S, Nagarkatti M, et al. Delta9-tetrahydrocannabinol-induced apoptosis in jurkat leukemia t cells is regulated by translocation of bad to mitochondria. Mol Cancer Res. 2006;4(8):549–562. doi: 10.1158/1541-7786.MCR-05-0193. [DOI] [PubMed] [Google Scholar]
- Kamath AB, Nagarkatti PS, Nagarkatti M. Evidence for the induction of apoptosis in thymocytes by 2,3,7,8-tetrachlorodibenzo-p-dioxin in vivo (1997) Toxicol Appl Pharmacol. 1997;142(2):367–377. doi: 10.1006/taap.1996.8049. [DOI] [PubMed] [Google Scholar]
- Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid ct-3 on chronic neuropathic pain: a randomized controlled trial. J Am Med Assoc. 2003;290(13):1757–1762. doi: 10.1001/jama.290.13.1757. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton CA, Friedman H. Cannabinoid receptors and immunity. Immunol Today. 1998;19(8):373–381. doi: 10.1016/s0167-5699(98)01300-0. [DOI] [PubMed] [Google Scholar]
- Klein TW, Lane B, Newton CA, Friedman H. The cannabinoid system and cytokine network. Proc Soc Exp Biol Med. 2000;225(1):1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]
- Klein TW, Cabral GA. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J Neuroimmune Pharmacol. 2006;1(1):50–64. doi: 10.1007/s11481-005-9007-x. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton C, Zhu W, Daaka Y, Friedman H. Delta 9-tetrahydrocannabinol, cytokines, and immunity to Legionella pneumophila. Proc Soc Exp Biol Med. 1995;209(3):205–212. doi: 10.3181/00379727-209-43897b. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6(5):513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Lee CY, Wey SP, Liao MH, Hsu WL, Wu HY, Jan TR. A comparative study on cannabidiol-induced apoptosis in murine thymocytes and el-4 thymoma cells. Int Immunopharmacol. 2008;8(5):732–740. doi: 10.1016/j.intimp.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Lee SF, Newton C, Widen R, Friedman H, Klein TW. Differential expression of cannabinoid cb(2) receptor mrna in mouse immune cell subpopulations and following b cell stimulation. Eur J Pharmacol. 2001;423(2–3):235–241. doi: 10.1016/s0014-2999(01)01122-0. [DOI] [PubMed] [Google Scholar]
- Lombard C, Nagarkatti M, Nagarkatti P. Cb2 cannabinoid receptor agonist, jwh-015, triggers apoptosis in immune cells: Potential role for cb2-selective ligands as immunosuppressive agents. Clin Immunol. 2007;122(3):259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard C, Nagarkatti M, Nagarkatti PS. Targeting cannabinoid receptors to treat leukemia: role of crosstalk between extrinsic and intrinsic pathways in delta9-tetrahydrocannabinol (thc)-induced apoptosis of jurkat cells. Leuk Res. 2005;29(8):915–922. doi: 10.1016/j.leukres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Lopez-Cepero M, Friedman M, Klein T, Friedman H. Tetrahydrocannabinol-induced suppression of macrophage spreading and phagocytic activity in vitro. J Leukoc Biol. 1986;39(6):679–686. doi: 10.1002/jlb.39.6.679. [DOI] [PubMed] [Google Scholar]
- Lu T, Newton C, Perkins I, Friedman H, Klein TW. Cannabinoid treatment suppresses the t-helper cell-polarizing function of mouse dendritic cells stimulated with Legionella pneumophila infection. J Pharmacol Exp Ther. 2006;319(1):269–276. doi: 10.1124/jpet.106.108381. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Lorenzon T, Bari M, Melino G, Finazzi-Agro A. Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J Biol Chem. 2000;275(41):31938–31945. doi: 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- McCoy KL, Gainey D, Cabral GA. Delta 9-tetrahydrocannabinol modulates antigen processing by macrophages. J Pharmacol Exp Ther. 1995;273(3):1216–1223. [PubMed] [Google Scholar]
- McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and nox4 expression. Mol Pharmacol. 2006;70(3):897–908. doi: 10.1124/mol.106.023937. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, Grant S, et al. Targeting cb2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood. 2002a;100(2):627–634. doi: 10.1182/blood-2002-01-0098. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Lombard C, Martin BR, Nagarkatti M, Nagarkatti PS. Delta(9)-tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J Pharmacol Exp Ther. 2002b;302(2):451–465. doi: 10.1124/jpet.102.033506. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Nagarkatti M, Nagarkatti PS. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol. 2005;174(6):3281–3289. doi: 10.4049/jimmunol.174.6.3281. [DOI] [PubMed] [Google Scholar]
- Pollmann W, Fenenberg W. Current management associated with multiple sclerosis. CNS Drugs. 2008;22(4):291–324. doi: 10.2165/00023210-200822040-00003. [DOI] [PubMed] [Google Scholar]
- Rachelefsky GS, Opelz G, Mickey MR, Lessin P, Kiuchi M, Silverstein MJ, et al. Intact humoral and cell-mediated immunity in chronic marijuana smoking. J Allergy Clin Immunol. 1976;58(4):483–490. doi: 10.1016/0091-6749(76)90192-5. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Pan H, Mukhopadhyay P, Bátkai S, Osei-Hyiaman D, Haskó G, Liaudet L, Gao B, Pacher P. J Leukoc Biol. 2007;82(6):1382–1389. doi: 10.1189/jlb.0307180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz H, Blanco FJ, Lotz M. Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J Neuroimmunol. 1994;55(1):107–115. doi: 10.1016/0165-5728(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Singh NP, Nagarkatti M, Nagarkatti P. Primary peripheral T cells become susceptible to 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated apoptosis in vitro upon activation and in the presence of dendritic cells. Mol Pharmacol. 2008;73(6):1722–1735. doi: 10.1124/mol.107.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin DP, Baldwin GC, Sarafian T, Dubinett S, Roth MD. Respiratory and immunologic consequences of marijuana smoking. J Clin Pharmacol. 2002;42(11):71S–81S. doi: 10.1002/j.1552-4604.2002.tb06006.x. [DOI] [PubMed] [Google Scholar]
- Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. Br Med J. 2001;323(7303):16–21. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. Faseb J. 1998;12(11):1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Ito Y, Hashiguchi T, Kitajima I, Yamakuchi M, et al. Simultaneous measurement of anandamide and 2-arachidonoylglycerol by polymyxin b-selective adsorption and subsequent high-performance liquid chromatography analysis: Increase in endogenous cannabinoids in the sera of patients with endotoxic shock. Anal Biochem. 2001;294(1):73–82. doi: 10.1006/abio.2001.5015. [DOI] [PubMed] [Google Scholar]
- Yebra M, Klein TW, Friedman H. Delta 9-tetrahydrocannabinol suppresses concanavalin A induced increase in cytoplasmic free calcium in mouse thymocytes. Life Sci. 1992;51(2):151–160. doi: 10.1016/0024-3205(92)90009-e. [DOI] [PubMed] [Google Scholar]
- Zhu W, Friedman H, Klein TW. Delta9-tetrahydrocannabinol induces apoptosis in macrophages and lymphocytes: involvement of bcl-2 and caspase-1. J Pharmacol Exp Ther. 1998;286(2):1103–1109. [PubMed] [Google Scholar]
- Zhu W, Igarashi T, Friedman H, Klein TW. Delta 9-tetrahydrocannabinol (THC) causes the variable expression of IL2 receptor subunits. J Pharmacol Exp Ther. 1995;274(2):1001–1007. [PubMed] [Google Scholar]
- Zhu W, Newton C, Daaka Y, Friedman H, Klein TW. Delta 9-tetrahydrocannabinol enhances the secretion of interleukin 1 from endotoxin-stimulated macrophages. J Pharmacol Exp Ther. 1994;270(3):1334–1339. [PubMed] [Google Scholar]
- Zurier RB, Rossetti RG, Lane JH, Goldberg JM, Hunter SA, Burstein SH. Dimethylheptyl-thc-11 oic acid: a nonpsychoactive antiinflammatory agent with a cannabinoid template structure. Arthritis Rheum. 1998;41(1):163–170. doi: 10.1002/1529-0131(199801)41:1<163::AID-ART20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]