Abstract
Background
The National Cancer Institute (NCI) has established the Pediatric Preclinical Testing Program (PPTP) for testing drugs against in vitro and in vivo childhood cancer models to aid in the prioritization of drugs considered for early phase pediatric clinical trials.
Procedures
In vitro cytotoxicity testing employs a semi-automated fluorescence-based digital imaging cytotoxicity assay (DIMSCAN) that has a 4-log dynamic range of detection. Curve fitting of the fractional survival data of the cell lines in response to various concentrations of the agents was used to calculate relative IC50, absolute IC50, and Ymin values The panel of 23 pediatric cancer cell lines included leukemia (n=6), lymphoma (n=2), rhabdomyosarcoma (n=4), brain tumors (n=3), Ewing family of tumors (EFT, n=4), and neuroblastoma (n=4). The doubling times obtained using DIMSCAN were incorporated into data analyses to estimate the relationship between input cell numbers and final cell number.
Results
We report in vitro activity data for three drugs (vincristine, melphalan, and etoposide) that are commonly used for pediatric cancer and for the mTOR inhibitor rapamycin, an agent that is currently under preclinical investigation for cancer. To date, the PPTP has completed in vitro testing of 39 investigational and approved agents for single drug activity and two investigational agents in combination with various “standard” chemotherapy drugs.
Conclusions
This robust in vitro cytotoxicity testing system for pediatric cancers will enable comparisons to response data for novel agents obtained from xenograft studies and from clinical trials.
Keywords: Cell line models, childhood cancer, cytotoxicity, DIMSCAN, NCI PPTP
INTRODUCTION
The limited patient pool for pediatric clinical trials and economic constraints require the development of approaches to prioritizing the many new antineoplastic agents that are becoming available for clinical testing [1]. With this goal in mind, in 2003 the National Cancer Institute (NCI) established the Pediatric Preclinical Testing Program (PPTP) comprised of investigators at: St. Jude Children’s Research Hospital, Duke University, Albert Einstein College of Medicine, Children’s Cancer Institute of Australia, Children’s Hospital of Philadelphia, and Texas Tech University Health Sciences Center (TTUHSC). This program has assembled a panel of cell lines for in vitro testing and a panel of human tumor xenografts for in vivo testing that are representative of the most common childhood cancers [2]. There are 6 cell lines common to both the in vivo and in vitro panels to enable comparisons between the in vivo and in vitro results. To date, 39 agents were evaluated for their single agent in vitro activity, and two agents were tested in combination with various conventional chemotherapy drugs.
Prior extensive characterization of some of the cell line and xenograft models enriches the characterization undertaken by the consortium [3, 4]. The large number of drugs that have shown minimal or no activity in PPTP testing is similar to what has historically been observed in clinical trials. Those data, together with the molecular data in which RNA expression profiles of the models were similar to those observed in patient tumors suggest that the PPTP models and testing methods may provide data that can inform clinical trial development [5, 6]. However, future comparisons between new agent activity as defined by PPTP testing and activity observed in pediatric oncology clinical trials will be necessary to define the clinical relevance of the PPTP data.
This paper describes the establishment of the cell line panel for in vitro testing and the data analyses of the first three drugs and an investigational agent. The in vitro cytotoxicity is measured with DIMSCAN which is a semi-automated digital imaging 96 well fluorescence assay. Curve-fitting of dose response data is used to analyze the cell survival data [7]. We also incorporate doubling times of cell lines into the data analysis, which allows interpretation of in vitro results with relationship to the estimated starting number of cells. In the current manuscript, we report the in vitro cytotoxicity system that we established within PPTP, the cell lines employed, and we describe the cytotoxicity data of three clinically well-studied agents and a molecularly targeted agent under investigation in children with cancer.
METHODS
Cell Lines
The initial panel is composed of 27 cell lines; 23 used as a primary panel for cytotoxicity assays, and 4 in a secondary panel for expanded testing. Prior to beginning the in vitro assays, 50 vials of 5 million cells each were cryopreserved for the cell line panel (the Master Bank). For each cell line, cells in the Master Bank were expanded from a single original source vial to ensure that we test a consistent cell population throughout the project. There is a range of 10 passages or less between the lowest and the highest passage vials in the Master Bank (e.g., passage 24 – 34). During the drug testing phase, an additional 8 cell pellets of 3 million cells each were snap frozen for all cell lines to enable molecular characterization [8], and 10–20 frozen vials of 5 million cells each were established for the primary panel (the Working Bank). The Working Bank is our source of cells for the cytotoxicity assays; the highest passage in the Working Bank is no more than 15 passages higher than the lowest passage in the Master Bank (e.g., passage 24 – 39). The cell passage during the actual cytotoxicity assay does not exceed 20 passages from the lowest passage in the Master Bank (e.g., passage 24 – 44).
The primary panel consists of a broad range of pediatric cancers (Table I): These cell lines can be further distinguished by the clinical status of the patient at the time the original sample was obtained (Table 1), adding to the diversity of this panel as a model for pediatric cancers [3, 9–28]. The cell lines were provided by 5 different institutions: American Type Culture Collection (ATCC, Manassas, VA) (RS4;11, MOLT-4, CCRF-CEM, Kasumi-1, Ramos-RA1, RD, MV-4–11); Childrens Hospital Los Angeles, Los Angeles, CA (TC-71); The Childrens Oncology Group Cell Line repository (www.COGcell.org) (COG-LL-317, CHLA-266, CHLA-9, CHLA-10, CHLA-258, CHLA-90, CHLA-136, CHLA-119, CHLA-122); St. Jude Children’s Research Hospital, Memphis, TN (Rh41, Rh18, Rh30, BT-12, SJ-GBM2, NB-1643, NB-EBc1); and Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany) (NALM-6, Karpas-299, SUDHL-1).
Table I.
Demographics of the primary panel used for in vitro testing as part of the PPTP.
| Cell Line | Diagnosis | Treatment | Site of specimen | Age at Dx | Gender | Ref. | |
|---|---|---|---|---|---|---|---|
| Rhabdomyosarcoma | |||||||
| RD | Embryonal | Post-Tx* | muscle | 7 | F | [9] | |
| Rh41 | Alveolar | Post-Tx | lung | F | [10] | ||
| Rh18 | Embryonal | DX§ | perineum | 2 | [11] | ||
| Rh30 | Alveolar | DX | bone marrow | 16 | M | [12] | |
| Brain tumor | |||||||
| BT-12 | Atypical teratoid/rhabdoid | DX | [13] | ||||
| SJ-GBM2 | Glioblastoma multiforme | Post-Tx | brain | 5 | F | [14] | |
| CHLA-266 | Atypical teratoid/rhabdoid | DX | posterior fossa | 1.5 | F | [15] | |
| Ewings Family of Tumors (EFT) | |||||||
| CHLA-9 | PNET | DX | thorax | 14 | F | [3] | |
| CHLA-10 | PNET | Post-Tx | thorax | 14 | F | [3] | |
| CHLA-258 | PNET | Post-BMT** | lung | 12 | F | [3] | |
| TC-71 | Ewings | Post-Tx | bone marrow | 22 | M | [16] | |
| Neuroblastoma | MYCN status† | ||||||
| NB-1643 | Neuroblastoma | A | DX | adrenal mass | <3 | [17] | |
| NB-EBc1 | Neuroblastoma | NA | Post-Tx | retroperitoneal mass | <3 | [17] | |
| CHLA-90 | Neuroblastoma | NA | Post-BMT | bone marrow | 8 | M | [18] |
| CHLA-136 | Neuroblastoma | A | Post-BMT | blood | 3 | F | [19] |
| Leukemia | |||||||
| NALM-6 | Pre-B cell ALL | Post-Tx* | peripheral blood | 19 | M | [20] | |
| RS4;11 | Pre-B cell ALL | Post-Tx | bone marrow | 32 | F | [21] | |
| COG-LL-317 | T-cell ALL | Post-Tx | bone marrow | 1.7 | M | [22] | |
| MOLT-4 | T-cell ALL | Post-Tx | peripheral blood | 19 | M | [23] | |
| CCRF-CEM | T-cell ALL | Post-Tx | peripheral blood | 4 | F | [24] | |
| Kasumi-1 | AML | Post-BMT** | peripheral blood | 7 | M | [25] | |
| Lymphoma | |||||||
| Karpas-299 | Anaplastic Large Cell | Post-Tx | peripheral blood | 25 | M | [26] | |
| Ramos-RA1 | Burkitt | peripheral blood | 3 | M | [27, 28] | ||
Cell line established from patients at relapse following chemotherapy;
Cell line established from patients at relapse following myeloablative chemotherapy supported by bone marrow transplantation;
Cell line established from patient at diagnosis prior to treatment;
Genomic amplification of MYCN; A: amplified, NA: not amplified
All cell lines were grown in Iscove’s Modified Dulbecco’s Medium (IMDM, Bio Whittaker, Walkersville, MD), supplemented with 3mM L-glutamine (Gemini Bioproducts, Inc., Calabasas, CA), 5 μg/ml of insulin, 5 μg/ml of transferrin, 5 ng/ml of selenous acid (ITS Culture Supplement, Collaborative Biomedical Products, Bedford, MA), and 20% heat-inactivated fetal bovine serum (Omega Scientific, Tarzana, CA).
All cell lines were cultured at 37°C in a humidified incubator containing 95% air and 5% CO2, except for COG-LL-317, which was cultured from initial explant under bone-marrow level hypoxic conditions at 5% O2/5% CO2/90% N2 [29]. All cell lines were grown antibiotic-free to assist in the detection of mycoplasma. All cell lines except TC-71 were found to be negative for mycoplasma contamination by the University of Southern California Bioreagent Cell Culture Core Facility, using the MycoAlert assay (Cambrex, East Rutherford, NJ). Mycoplasma contamination in TC-71 was found during a routine pathogen screen prior to establishing a xenograft from a cell line, and was cleared by antibiotics prior to expansion of the master bank and working banks.
DNA genotyping
All cell lines underwent DNA genotyping using the AmpFLSTR Identifiler PCR kit, cat. # 4322288 (Applied Biosystems, Foster City, CA). A short tandem repeat (STR) profile was created for each cell line to confirm the uniqueness of each line, ruling out any possible cross-contamination and providing a basis for molecular cell line identification during testing. Tandemly repeated DNA sequences show sufficient variability among individuals in a population that they have been used in genetic mapping and human identity testing. Fifteen polymorphic STR loci (D8S1179, D21S11, D7S820, CSF1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TPOX, D18S51, D5S818, FGA) and the sex-specific locus AMEL were amplified, and the PCR products were analyzed simultaneously with size standards by using automated fluorescent detector (ABI 3130xl Genetic Analyzer, Applied Biosystems, Foster City, CA).
Drugs and Chemicals
The primary panel of 23 cell lines was tested against 4 drugs provided by the NCI: vincristine, melphalan, etoposide, and rapamycin. Drug identity was blinded until completion of the testing. Vincristine was dissolved in sterile water; DMSO (Sigma-Aldrich, St. Louis, MO) was used to dissolve etoposide, melphalan, and rapamycin. All 4 agents were prepared immediately prior to use. Fluorescein diacetate (FDA) and eosin Y were obtained from Sigma-Aldrich.
Cytotoxicity Assay
The DIMSCAN system uses digital image fluorescence microscopy to quantify live cells, which selectively accumulate FDA. It is capable of measuring cytotoxicity over a 4-log dynamic range, after digital-image thresholding and eosin-Y quenching [7, 30]. Cell lines were seeded in 150 μl of complete medium at 700 – 8,000 cells per well (dependent on cell size and doubling time) in 96-well plates. After overnight incubation, drugs were added as single agents in 100 μl of complete medium to the cells, at the following concentrations: 0.003–10 nM for vincristine, 0.01–100 μM for melphalan and etoposide, and 0.01–100 nM for rapamycin. Stock solutions of melphalan and etoposide were prepared at 10 mM in DMSO; vincristine at 1 mM in normal saline; rapamycin 1 mM in DMSO. Controls were treated with the appropriate drug vehicles (DMSO for etoposide, melphalan and rapamycin: final DMSO content of ≤0.1% at the highest concentration tested). Six replicate wells were tested per concentration as well as per control. Following 4 days (96 h) of incubation, 50 μl of 0.5% eosin-Y + 10 μg/ml of FDA were added to the wells. After 20 minutes of incubation in the dark, the fluorescence of viable cells in each well was measured using the DIMSCAN system. The results were expressed as the survival fraction of treated cells for each concentration relative to the control cells. The standard deviations (sd) for control wells were less than 15%, and for any plates in which the sd exceeded 15%, the assay was repeated.
Cytotoxicity Data Analysis
The relative IC50 (concentration that reduces cell survival by 50% of the maximum agent effect) and the absolute IC50 values (concentration that reduces cell survival to 50% of the control value) were calculated using Kaleidagraph software (Synergy Software, Reading, PA). Calculations employed non-linear regression with a sigmoidal dose-response curve: y = m1+(m2−m1)/(1+(x/m3)m4), where m1 was the lowest surviving fraction, m2 = the highest surviving fraction, m3 = an relative IC50 value provided by Kaleidagraph, and m4 = the hill slope, also provided by Kaleidagraph. We did not constrain m1 value to zero or m2 value to 100 values to accommodate the plateauing effect of some agents into data analysis.
Absolute IC50 values were calculated using the equation:
When a given IC value lay outside the concentrations tested, the calculated value was replaced with either < ”lowest concentration tested” or > ”highest concentration tested”, as appropriate. Given that relative IC50 and absolute IC50 represent different properties of the cell killing effect (Fig. 1), both relative IC50 and absolute IC50 values have been included. For example, if an agent affects less than 50% of cells compared with control, absolute IC50 cannot be determined although relative IC50 may still be generated. Absolute IC50 and relative IC50 will be the same when the maximal drug effect reaches 0% survival fraction. Relative IC50 values are not reported when Ymin values are > 75% because of the difficulty in accurately determining relative IC50 when there is little difference between the control T/C and treated T/C values at the highest concentrations of the tested agent evaluated. The Mann-Whitney test was used to test the difference of medians of relative IC50 and Ymin values between the groups of lines with similar tumor types to the remaining lines of the panel.
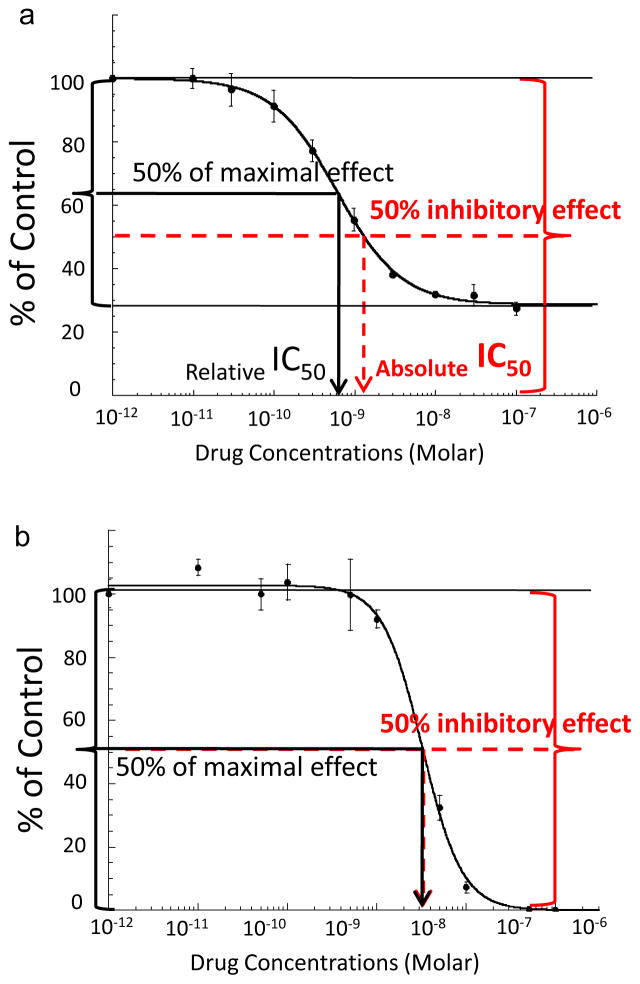
Fig. 1.
Representative graphs from Kaleidagraph software for a) rapamycin dose-response in Rh-41 showing the differences of absolute IC50 vs relative IC50, and b) melphalan dose-response in TC-71. Relative IC50 is determined at the concentration required to induce 50% effect between the baseline and maximum (black solid line and arrow). Absolute IC50 is the concentration that is inhibitory for 50% of cells (red dotted line and arrow). Dose (in a log scale) is presented on the x axis. Instead of zero for a control concentration, we used 0.001 nM to enable graphing the zero dose values on this scale. The figures illustrate the typical plateau effect of an anti-mitotic agent (a), for which the absolute IC50 and relative IC50 values are different, and a cytotoxic agent (b), for which the absolute IC50 and relative IC50 values are similar. In vitro cytotoxicity terms are in accordance with NIH Chemical Genomics Center terminology (http://www.ncgc.nih.gov/guidance/section11.html).
Assessing and Incorporating Doubling Time into the Data Analyses
Cells were seeded into 96-well plates in 150 μL of complete medium at 700 – 8,000 cells per well depending on the growth of the cell lines, and incubated for 24 hours. Then, 100 μL of complete medium was added to each well. Viable cell values were assessed at 24, 48, 72, and 96 hours of incubation using a DIMSCAN system. Doubling time was determined by fitting the fluorescence data obtained using the DIMSCAN system to the exponential curve (y=a·ebx: y= fluorescence data, x=incubation time; doubling time = ln(2)/b). The predicted fold-increase at 96 hours of cell growth was calculated using y=e(doubling time*96). Then, the predicted fold-increase was divided into 100 (the normalized value of untreated cells that all treated cells are adjusted to) to determine the fraction of viable cells (Predicted Ymin) that should be expected for a cytostatic agent. The number that compares the relative difference in final cell number compared with the starting cell number for treated cells and for control cells: (Observed Ymin-Predicted Ymin)/(100-Predicted Ymin) if Observed Ymin > Predicted Ymin; and (Predicted Ymin-Observed Ymin)/(Predicted Ymin) if Observed Ymin < Predicted Ymin) is called “Relative Input/Output (Relative I/O)” in this study. Observed Ymin is the minimum survival fraction (Treated/Control %) at the range of concentrations of the drug employed.
RESULTS
Cell Line Characteristics
The panel consists of 27 cell lines; 23 used as a primary panel for cytotoxicity assays and 4 in a secondary panel for expanded testing, representing a broad range of pediatric cancers (Table 1). The cell lines can be further distinguished by the clinical status of the patient at the time the original sample was obtained (Table I), adding to the diversity of this panel as a model for pediatric cancers [3, 9–28]. Gene expression profiling and whole-genome copy number analyses studies showed that the PPTP panel of cell lines recapitulate the molecular characteristics of their respective clinical histotypes that are common to childhood cancer [5].
Supplemental Table I shows the results of the STR profiling. The results are the number of repeats corresponding to the length of the PCR products amplified at each locus of the cell lines. RD, RS4;11, CCRF-CEM, and Kasumi-1 yielded profile matches with those listed by the repository from which we acquired them (ATCC); MOLT-4 yielded a match with another cell line, MOLT-3, derived from the same patient; the cell line pair from the same patient CHLA-9 (primary tumor before therapy) and CHLA-10 (nodal metastasis, after chemotherapy) showed the same STR profile; and COG-LL-317, CHLA-266, CHLA-258, and CHLA-136 each matched STR profiles of tissue from the corresponding patients. All other cell lines showed unique STR profiles [31, 32], in searches of the Children’s Oncology Group Cell Line and Xenograft Repository STR database (www.COGcell.org).
In vitro activity of vincristine, melphalan, etoposide, and rapamycin
There are two primary parameters that can be derived from in vitro concentration-response curves to distinguish the response of cell lines to different test agents. These are the Hill equation defined relative IC50 value, which measures the potency by which the agent exerts its effect on the cell line, and Ymin, which defines the maximum effect of the agent in decreasing cell number. Ymin values approaching 0% are indicative of a complete cytotoxic effect, whereas plateau-effect Ymin values significantly greater than 0% are consistent with a cytostatic effect or mixed cytotoxic/cytostatic effect. As described below, interpretation of the Ymin value is facilitated by its comparison to the Ymin value that would be expected for the cell line for a completely effective cytostatic agent.
Dose-response curves and relative sensitivity of the cell line panel to the four drugs are shown in Fig. 2, with relative IC50 and absolute IC50 values provided in Table II. Relative IC50 and absolute IC50 values were nearly equal to each other for vincristine, melphalan, and etoposide. This similarity is expected for cytotoxic agents that have survival fraction (Ymin) values approaching 0%. For rapamycin, differences in the median values for relative IC50 and absolute IC50 were apparent as a result of the Ymin values being much greater than 0%.
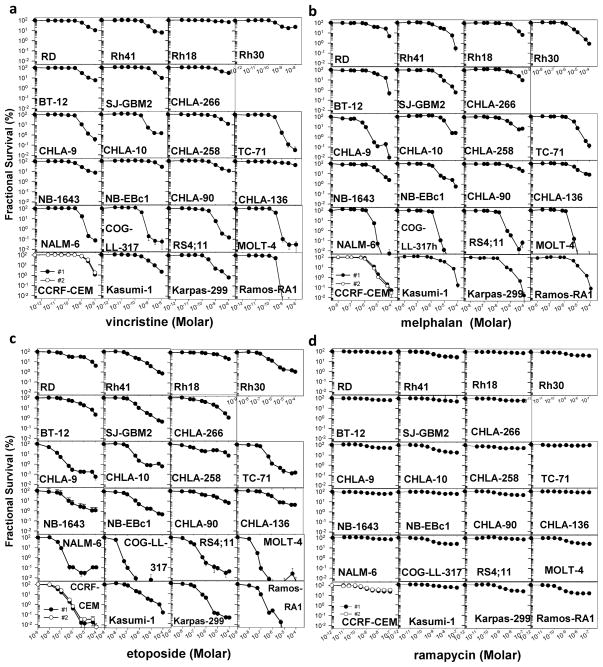
Fig. 2.
Dose-response curves to vincristine (0.003 – 10 nM, a), melphalan (0.01 – 100 μM, b), etoposide (0.01 – 100 μM, c), and rapamycin (0.01 – 100 nM, d) obtained by DIMSCAN analysis of the PPTP in vitro panel. The two plots for CCRF-CEM represent the independent testing of this cell line by two different technicians (● technician #1, ○ technician #2) that are used for quality control of the data.
Table II.
Cytotoxicity of three common chemotherapeutic agents, vincristine, melphalan, etoposide, and rapamycin against 23 pediatric cancer cell lines
| Type | Cell line | vincristine | melphalan | etoposide | rapamycin | ||||
|---|---|---|---|---|---|---|---|---|---|
| R-IC50 (nM) | A-IC50 (nM) | R-IC50 (nM) | A-IC50 (nM) | R-IC50 (nM) | A-IC50 (nM) | R-IC50 (nM) | A-IC50 (nM) | ||
| Rhabdomyo-sarcoma | RD | 1.19 ± 0.12 | 1.28 | 3.42 ± 1.09 | 4.24 | 0.19 ± 0.08 | 0.29 | 3.93 ± 0.80 | >100 |
| Rh41 | 0.54 ± 0.04 | 0.57 | 2.16 ± 0.44 | 2.53 | 0.17 ± 0.03 | 0.20 | 0.61 ± 0.04 | 1.32 | |
| Rh18 | 0.19 ± 0.28 | >10 | 17.74 ± 1.38 | 17.77 | 18.80 ± 2.27 | 18.85 | NA* | >100 | |
| Rh30 | 0.30 ± 0.07 | 0.32 | 2.05 ± 0.29 | 2.30 | 0.19 ± 0.03 | 0.21 | 0.73 ± 0.13 | 3.91 | |
| Brain tumor | BT-12 | 0.75 ± 0.05 | 0.77 | 6.32 ± 1.66 | 5.58 | 0.80 ± 0.17 | 0.97 | 1.37 ± 0.37 | >100 |
| SJ-GBM2 | 1.82 ± 0.20 | 2.03 | 2.94 ± 0.23 | 2.84 | 0.26 ± 0.01 | 0.26 | 1.41 ± 0.28 | >100 | |
| CHLA-266 | 1.38 ± 0.15 | 2.20 | 8.37 ± 2.93 | 13.14 | 0.32 ± 0.11 | 0.32 | 0.74 ± 0.09 | >100 | |
| Ewing family of tumors | CHLA-9 | 0.51 ± 0.06 | 0.50 | 0.14 ± 0.06 | 0.14 | 0.01 ± 0.00 | 0.01 | 0.85 ± 0.19 | >100 |
| CHLA-10 | 0.39 ± 0.05 | 0.40 | 7.27 ± 1.20 | 8.71 | 0.12 ± 0.01 | 0.13 | 0.62 ± 0.08 | 1.00 | |
| CHLA-258 | 1.82 ± 0.39 | 1.82 | 3.12 ± 0.53 | 3.49 | 0.03 ± 0.01 | 0.05 | 0.77 ± 0.20 | >100 | |
| TC-71 | 0.34 ± 0.02 | 0.34 | 3.22 ± 0.25 | 3.22 | 0.15 ± 0.01 | 0.15 | NA | >100 | |
| Neuroblastoma | NB-1643 | 0.43 ± 0.03 | 0.49 | 4.70 ± 1.07 | 3.79 | 0.10 ± 0.02 | 0.09 | 0.53 ± 0.24 | >100 |
| NB-EBc1 | 4.92 ± 0.50 | 4.93 | 1.23 ± 0.08 | 1.20 | 0.11 ± 0.01 | 0.10 | 1.18 ± 0.27 | >100 | |
| CHLA-90 | 0.65 ± 0.07 | 0.80 | 7.57 ± 0.98 | 11.16 | 0.45 ± 0.18 | 0.62 | 0.42 ± 0.04 | 2.25 | |
| CHLA-136 | 6.56 ± 1.91 | 6.58 | 3.87 ± 0.48 | 4.50 | 0.34 ± 0.02 | 0.36 | NA | >100 | |
| Leukemia | NALM-6 | 0.71 ± 0.14 | 0.73 | 1.72 ± 0.46 | 1.84 | 0.04 ± 0.00 | 0.04 | 1.71 ± 0.43 | >100 |
| COG-LL-317 | 0.29 ± 0.39 | 0.29 | 1.41 ± 0.14 | >100 | 0.01 ± 0.00 | 0.01 | 0.36 ± 0.06 | 0.59 | |
| RS4-11 | 0.32 ± 0.05 | 0.41 | 1.31 ± 0.14 | 1.31 | 0.03 ± 0.00 | 0.03 | 3.46 ± 3.63 | >100 | |
| MOLT-4 | 0.26 ± 0.01 | 0.27 | 1.47 ± 0.27 | 1.47 | 0.01 ± 0.00 | 0.01 | 0.70 ± 0.05 | 1.23 | |
| CCRF-CEM | 1.57 ± 0.21 | 1.68 | 2.13 ± 0.10 | 2.13 | 0.03 ± 0.00 | 0.03 | 0.35 ± 0.02 | 0.65 | |
| KASUMI-1 | 0.47 ± 0.04 | 0.49 | 4.08 ± 0.76 | 4.08 | 0.17 ± 0.05 | 0.22 | 1.19 ± 0.30 | >100 | |
| Lymphoma | KARPAS-299 | 0.25 ± 0.02 | 0.27 | 2.25 ± 0.43 | 2.18 | 0.10 ± 0.01 | 0.10 | 0.73 ± 0.24 | 1.42 |
| RAMOS | 0.31 ± 0.01 | 0.32 | 4.76 ± 0.61 | 4.65 | 0.03 ± 0.01 | 0.03 | 0.22 ± 0.02 | 0.27 | |
| Median | 0.51 | 0.54 | 3.12 | 3.49 | 0.12 | 0.13 | 0.74 | N/D | |
| Mean | 1.13 | 1.25 | 4.06 | 8.79 | 0.98 | 1.00 | 1.02 | N/D | |
Not analyzed because Ymin > 75%; Relative IC50 (R-IC50): value ± SD of estimation; Absolute IC50 (A-IC50) values are calculated using R-IC50 values as described under methods.
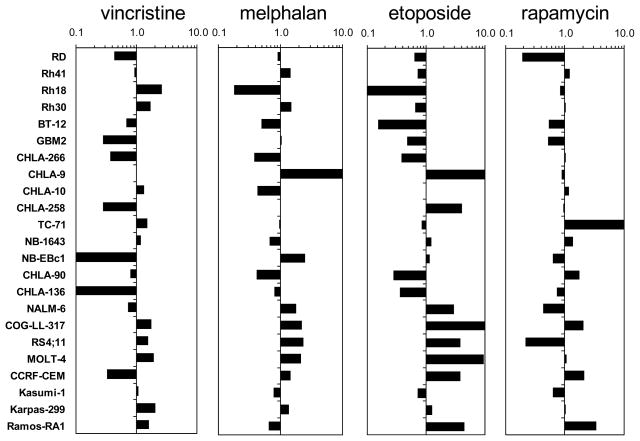
Of the 3 cytotoxic drugs (vincristine, melphalan, etoposide), vincristine was the most potent against the 23 cell lines tested, with a median relative IC50 of 0.51 nM compared to 3.2 μM and 0.12 μM for melphalan and etoposide, respectively (Table II). In Fig. 3, the relative sensitivity to the drugs is represented as the ratio of the relative IC50 for each cell line and each drug to the median relative IC50 with <1.00 (left) denoting resistance and >1.00 (right) denoting sensitivity relative to the rest of the panel. It is apparent from the figures that the relative sensitivity within panels was similar for the three cytotoxic agents. For melphalan, and etoposide, the median relative IC50 values for the five ALL cell lines were significantly lower than those of the remaining cell lines (p=0.01 for ALL vs other cell lines for melphalan and p=0.007 for etoposide) (Supplemental Table II). By contrast, relative IC50 values for rapamycin ranged from 0.22 to 3.93 nM, and the relative IC50 value of the ALL cell lines was not significantly different from the relative IC50 values of the remaining cell lines. For selected cell lines, there were different patterns of activity to the 3 cytotoxic agents, as illustrated by CHLA-9 being highly sensitive to etoposide and melphalan, but only moderately sensitive to vincristine and by NB-EBc1, which was relatively insensitive to vincristine but relatively sensitive to melphalan.
Fig. 3.
Relative sensitivity to vincristine, melphalan, etoposide, and rapamycin is represented as a ratio of the median relative IC50 of the panel to the individual relative IC50 for each line, with <1.00 (left) denoting resistance and >1.00 (right) denoting sensitivity relative to the rest of the panel. This method of defining sensitivity is similar to the “mean graph” used by the NCI in other studies [46], and was employed here as part of our effort to determine the best method of evaluating and calibrating the PPTP in vitro models.
The median Ymin values varied by agent, being lowest for melphalan and etoposide (0.5%) and, as expected, highest for rapamycin (51%). For each of the three cytotoxic agents, the median Ymin values were ≤ 0.1% for the ALL cell lines, and the ALL panel had significantly lower Ymin values compared to the other cell lines (Supplemental Table II). Dose-response curves show a plateau effect at concentrations > 10 nM for each cell line for rapamycin (Fig. 2d), an agent that acts primarily by inducing cell cycle arrest rather than apoptosis [33–35]. Plateau effects were also observed for vincristine for some cell lines (e.g., RD, Rh41, Rh81, Rh30) (Supplemental Table III). That a true plateau effect is present for these cell lines and for all of the cell lines for rapamycin is evidenced by the comparability of the observed Ymin values and the modeled Ymin values (Supplemental Table III). A plateau effect was rarely observed for melphalan (Fig. 2b).
Doubling Time and Relative Input/Output
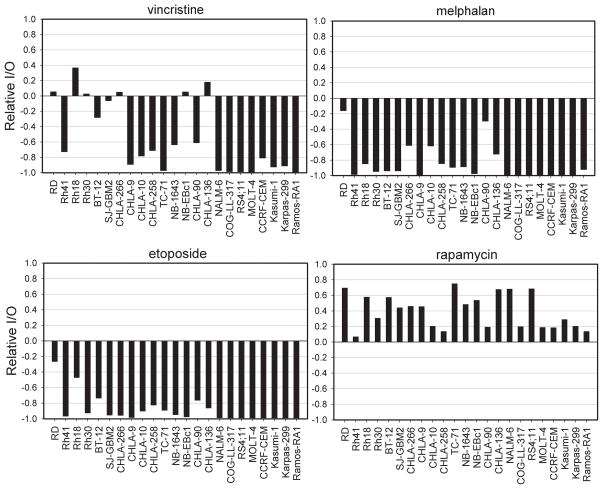
Because of the characteristics of the DIMSCAN assay, initial cell number must be inferred from the doubling times of the cell lines. Using the doubling times for each cell line, the fold-increase of cellular fluorescence at 96 hours after plating and the predicted Ymin at 96 hours for a completely effective cytostatic agent were estimated, as shown in Table III. This predicted Ymin at 96 hours is essentially a measure of the starting number of cells for each testing experiment. Relative I/O was estimated for each drug as described under Materials and Methods and are shown in Fig. 4. Values close to 0 are consistent with cytostasis, values close to 1 indicate no treatment effect, and values close to -1 indicate complete cytotoxicity. Note that because of the rapid growth rate for Ramos and TC-71, their predicted Ymin values for a completely effective cytostatic agent are approximately 1%, making it more difficult to use the Relative I/O values as indicators of cytotoxic activity for these two cell lines.
Table III.
Doubling times, fold-increase at 96 hours and predicted survival fraction for cytostatic agents (Predicted Ymin).
| Cell Line | Doubling time (h) | Fold-Increase at 96 Hrs | Predicted Ymin (%)* | Observed Ymin (%) | Modeled Ymin (%)** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VCR | LPAM | ETO | RM | VCR | LPAM | ETO | RM | ||||
| RD | 23 | 18.3 | 5.5 | 10.7 | 4.6 | 4.0 | 71.2 | 10.0 | 12.3 | 16.1 | 63.0 |
| Rh41 | 44 | 4.5 | 22.2 | 6.0 | 0.3 | 0.7 | 27.4 | 5.7 | 1.0 | 3.0 | 28.6 |
| Rh18 | 83 | 2.2 | 44.6 | 64.9 | 6.8 | 23.6 | 76.6 | 67.2 | 0.0 | 0.0 | 80.6 |
| Rh30 | 37 | 6 | 16.6 | 18.8 | 0.8 | 1.2 | 42.2 | 23.1 | 2.0 | 2.2 | 42.7 |
| BT-12 | 27 | 12.1 | 8.2 | 5.9 | 0.5 | 2.2 | 60.8 | 8.1 | 1.0 | 2.6 | 60.2 |
| SJ-GBM2 | 29 | 10.1 | 9.9 | 9.3 | 0.6 | 0.4 | 49.6 | 6.3 | 1.2 | 1.2 | 50.7 |
| CHLA-266 | 50 | 3.8 | 26.1 | 29.7 | 10.1 | 1.1 | 60.0 | 27.7 | 10.7 | 0.0 | 60.3 |
| CHLA-9 | 20 | 27.4 | 3.6 | 0.4 | 0.0 | 0.1 | 47.6 | 0.4 | 4.9 | 0.6 | 50.4 |
| CHLA-10 | 24 | 15.9 | 6.3 | 1.4 | 2.4 | 0.6 | 25.4 | 1.3 | 1.9 | 1.0 | 26.3 |
| CHLA-258 | 71 | 2.6 | 39 | 11.2 | 6.0 | 6.8 | 47.4 | 2.7 | 2.9 | 15.1 | 51.4 |
| TC-71 | 15 | 77.4 | 1.3 | 0.0 | 0.1 | 0.1 | 75.4 | 2.4 | 0.0 | 3.0 | 79.7 |
| NB-1643 | 43 | 4.7 | 21.1 | 7.6 | 2.3 | 1.1 | 59.3 | 6.9 | 0.2 | 0.1 | 61.1 |
| NB-Ebc1 | 45 | 4.4 | 22.8 | 26.9 | 0.5 | 0.5 | 64.2 | 0.0 | 0.7 | 0.4 | 65.9 |
| CHLA-90 | 52 | 3.6 | 27.9 | 10.8 | 19.6 | 6.6 | 41.7 | 10.8 | 23.8 | 10.1 | 43.0 |
| CHLA-136 | 53 | 3.5 | 28.7 | 41.5 | 7.9 | 3.9 | 76.8 | 1.5 | 6.2 | 3.2 | 75.9 |
| NALM-6 | 19 | 34.5 | 2.9 | 0.1 | 0.0 | 0.0 | 69.1 | 0.1 | 0.0 | 0.2 | 72.0 |
| COG-LL-317 | 21 | 22.4 | 4.5 | 0.1 | 0.0 | 0.0 | 23.5 | 0.1 | 0.0 | 0.0 | 23.4 |
| RS4;11 | 35 | 6.6 | 15.1 | 0.1 | 0.0 | 0.0 | 73.2 | 12.4 | 1.2 | 0.0 | 77.0 |
| MOLT-4 | 29 | 10 | 10 | 0.0 | 0.0 | 0.0 | 27.0 | 0.0 | 0.0 | 0.6 | 28.5 |
| CEM | 24 | 15.9 | 6.3 | 1.2 | 0.1 | 0.0 | 23.6 | 0.0 | 0.9 | 0.2 | 23.8 |
| Kasumi-1 | 53 | 3.5 | 28.7 | 2.1 | 0.2 | 0.2 | 49.3 | 1.3 | 0.0 | 2.6 | 51.1 |
| Karpas-299 | 26 | 12.9 | 7.8 | 0.7 | 0.0 | 0.0 | 26.5 | 2.2 | 1.1 | 3.1 | 23.6 |
| Ramos-RA1 | 14 | 105.2 | 1 | 0.0 | 0.1 | 0.0 | 14.4 | 1.9 | 0.9 | 1.3 | 14.7 |
LPAM: melphalan;
The survival fraction calculated for each cell line in the case of the drug being cytostatic;
In the cases that Modeled Ymin (minimal survival fraction at the highest concentration tested) estimated using Kaleidagraph is less than 0, we used 0.001 to determine Relative I/O for Fig. 4
Fig. 4.
Relative I/O of each cell line in response to vincristine, melphalan, etoposide, and rapamycin. Values close to 0 are consistent with cytostasis, values close to 1 indicate no treatment effect, and values close to -1 indicate primarily cytotoxicity. In using results obtained for the Relative I/O metric, we categorize results, for example, a value of < −0.8 would indicate a strong cytotoxic effect, while a value of > 0.8 would indicate no treatment effect. Values between −0.2 and +0.2 likely indicate a primarily cytostatic effect. Other values could be assigned to intermediate categories (for example, modest cytostasis for 0.2 to 0.8 or moderate cytotoxicity for −0.2 to −0.8).
Visualization of the Relative I/O values as shown in Fig. 4 provides additional insights about the activity of agents against selected cell lines. For example, at the highest concentration of vincristine tested the survival fractions relative to control for RD and Rh30 were 10.7% and 18.8%, respectively. The Relative I/O’s for these two cell lines were between 0 and 0.1, consistent with vincristine having a primarily cytostatic effect for these cell lines. Most other cell lines show some level of cytotoxic response (Relative I/O < 0) for each of the cytotoxic agents, with the effect being most pronounced and consistently present for the five ALL cell lines. Rapamycin, an agent known to act primarily through cell cycle arrest, shows a clearly different Relative I/O profile from the three cytotoxic agents, with all values being > 0. Using absolute IC50 criteria, rapamycin could be considered as inactive for CHLA-258 because an absolute IC50 is not achieved. However, when the starting cell number is taken into account, it is apparent that the response of CHLA-258 to rapamycin is consistent with a cytostatic effect and is very similar to that of CHLA-10, with both having Relative I/O values between 0 and 0.1. As expected, the Relative I/O data indicate that the activity of melphalan and etoposide is cytotoxic in the majority of the cell lines tested, and the two drugs showed a similar pattern for Relative I/O (Fig. 4).
DISCUSSION
The need for preclinical testing to better prioritize early phase trials in pediatric oncology has become increasingly clear in recent decades [36]. The predictive capabilities for clinical activity in oncology of both in vivo models (either xenografted or genetic models) and for cell culture (in vitro) models remains unclear. The robust testing conducted within the PPTP should provide data to address the contribution of a systematic testing approach using panels of xenografts and an in vitro cell line panel. Cost and logistics limit the range of combinations and concentrations of drugs that can be tested with in vivo models, while cell culture (in vitro) models employing microwell formats can test a range of concentrations and also can test drug combinations quickly and cost-effectively, complementing in vivo testing using xenografts. A range of drug concentrations reflective of exposures in patients can be employed in vitro.
The PPTP in vitro panel includes cell lines representative of most of the common pediatric cancers. Our aims are to use this cell line panel to complement in vivo testing panels and to rapidly test a wide variety of drugs (on average, 2 per month against 23 cell lines), thus creating a centralized data set indicating the range of sensitivity to single agents and also combinations of drugs. The eventual goal of the PPTP is to generate both in vitro and in vivo data in pediatric cancer models for new drugs or drug combinations that will inform the design and prioritization of early phase pediatric clinical trials. This paper describes the methods of assay and analysis for PPTP in vitro cytotoxicity testing.
Our in vitro testing seeks to build upon the experience of previous preclinical screening models, such as the NCI 60 cell line panel [37]. As data are accumulated, and new cell lines become available, further expansion and/or substitution of the lines in the panel may improve the predictive capability of the PPTP panel of lines. To address the impact of resistance acquired to current therapies on the response to new agents it would be ideal to include cell lines established before and after therapy. For example, our Ewing cell lines include one from diagnosis, 2 post-treatment, and 1 post-bone marrow transplant, with one pair (CHLA-9 and CHLA-10) from the same patient, primary tumor before therapy and a nodal metastasis after therapy. By contrast, our leukemia lines were all established post-treatment. Moreover, most leukemia cell lines have been in culture for hundreds of generations, possibly leading to the selection of sub-populations of cells that might alter drug sensitivity. Future additions to the panel of cell lines cultured under conditions that provide minimal selection pressure in vitro and utilization of cell lines at fairly low passage number may increase the predictive value of the in vitro testing panel. Such additions could include cell lines established at relapse after currently employed standard therapies, likely improving the ability to test for the ability to overcome drug resistance to modern therapies. Also, the small numbers of cell lines included for any given disease type in the panel limits the power of the panel to predict activity by disease type. Given that a minimum of 10 to 12 patients are usually evaluated in phase 2 trials before a decision is made on the activity status of a drug, a larger number of cell lines for each tumor type would enhance the potential for the in vitro response rates to correlate with clinical response rates. Due to the limitations in resources and time constraints the types of pediatric cancer tested for in vitro cytotoxicity testing are primarily from the six most common types of cancers found in children. However, less common pediatric cancers, including medulloblastoma, Wilms tumor, osteosarcoma, ependymoma are included as xenograft models of the NCI PPTP testing panel [1, 2].
The in vitro assay that we use for the PPTP employs a semi-automated cytotoxicity testing system (DIMSCAN) that measures survival rates up to a 4-log range [7]. We have also incorporated cell line doubling times into data analyses to provide an assessment of whether a drug’s activity is most consistent with a primarily cytotoxic or cytostatic effect. The DIMSCAN system can process largenumbers of samples rapidly and conveniently, and the data produced are comparable withresults obtained by other in vitro cytotoxicity assays for single agent activity testing. However, the wide dynamic range (3–4 logs) of DIMSCAN should enable readily identifying in vitro responses that may predict in vivo activity [38], and for combination cytotoxicity testing, the wide dynamic range enables more readily identifying drug synergism [7].
In vitro testing data are commonly analyzed by curve fitting to the Hill equation. Assuming a control value T/C of 100%, three parameters define the sigmoidal curves derived using the Hill equation: a) Ymin, which is the plateau value obtained at high concentrations; b) relative IC50, which is the concentration of agent that induces 50% of the maximal response; and c) Hill slope, which reflects the steepness of the transition from no effect to maximal effect. The first two parameters are the primary ones utilized for comparing anticancer agents, and are the ones on which the PPTP focuses. Comparisons of the relative IC50 values can assess the relative potency of agents across the PPTP cell lines. Relative IC50 values are particularly useful in confirming that observed in vitro effects are occurring within concentration ranges that are relevant to the clinical setting. In our assay system using T/C% values at 96 hours of drug exposure, the absolute IC50 can provide misleading estimates of the potency of an agent that acts primarily by growth inhibition, as it is dependent upon both the potency of the drug in causing its biological effect as well as upon the proliferation rate and the extent to which proliferation is blocked at the higher plateau concentrations tested.
The Ymin values are important because they provide an assessment of whether an agent’s activity profile is most consistent with a clearly cytotoxic effect or with a primarily cytostatic effect. The Relative I/O value provides a comparison of the estimated starting cell number to the final cell number (measured indirectly as T/C%) at maximum test concentrations. A Relative I/O of −1.0 indicates a cytotoxic effect, a Relative I/O of 0 suggests a primarily cytostatic effect, and a Relative I/O of 1.0 is indicative of minimal treatment effect. For the four agents described in this report, the Relative I/O values demonstrate strong cytotoxic activity for melphalan and etoposide against most PPTP cell lines, while they show that rapamycin has activity consistent with variably effective cytostasis for all of the PPTP cell lines. Vincristine primarily shows cytotoxic activity (particularly against the leukemia and lymphoma cell lines), although for some rhabdomyosarcoma cell lines (e.g., RD, Rh18, and Rh30) the effect is consistent with cytostasis. It is important to acknowledge that the terms cytostatic and cytotoxic are applied in an operational way, and that they do not necessarily represent the underlying biological response that leads to the Relative I/O, but do provide an indication of drug mechanism for the PPTP cell line panel.
The PPTP has reported in vitro testing results for several molecularly targeted kinase inhibitors, and their pattern of response is noteworthy for how it differs from that of the three cytotoxic agents and from rapamycin described herein. Agents such as sunitinib [39], sorafenib [40], and dasatinib [41] showed little activity against most of the PPTP cell lines at concentrations achievable in the clinic, at which the agents show their specific kinase inhibitory activity. The one cell line consistently showing sensitivity to these kinase inhibitors was Kasumi-1, which has an activating KIT mutation [42, 43]. This pattern of selective sensitivity of a small percentage of tested cell lines to a kinase inhibitor is indicative of the inhibitor being effective against a kinase that is activated by genomic alterations in the sensitive cell lines [44], At higher concentrations at which non-specific kinase inhibitory effects can occur, many cell lines may show sensitivity [40, 45], although there is little clinical relevance to these “off target” effects observed at drug exposures that exceed those achievable in the clinic.
The current in vitro cell line panel likely only represents a portion of the different biological phenotypes found within each cancer type. Due to the limited number of lines employed, conclusions about the activity of various targeted agents must take into account the nature and presence of the specific molecular target. This can be addressed to some degree as genome-wide expression profiling is available for PPTP cell lines and xenografts, enabling a comparison of response and target RNA expression. Another approach to testing of targeted agents would be testing selected agents in an expanded panel of cell lines expressing the known specific target.
In summary, we described here the in vitro cytotoxicity testing component of the NCI PPTP which consists of a robust cytotoxicity assay testing a panel of 23 cell lines spanning the major disease types of pediatric oncology and a computational method incorporating doubling times of cell lines. As the PPTP continues to obtain laboratory data on new anticancer agents that may indicate potential activity in pediatric clinical trials, we will refer to our experience with these three well-studied drugs reported in this paper to guide our analysis.
Supplementary Material
Acknowledgments
National Cancer Institute, NO1-CM-42216, CA21765, CA108786, CA82830
We would like to thank Nancy Yen, Maya Willie, and Leticia J. Campbell for cell line expansion and DIMSCAN assays and Heather Feaman and Daniel Cabral for DNA genotyping.
Footnotes
Conflict of Interest Statement
C Patrick Reynolds, MD PhD is a co-inventor on patents related to the DIMSCAN system that have been licensed by Children’s Hospital Los Angeles to BioImaging, Solutions Inc.
References
- 1.Houghton PJ, Adamson PC, Blaney S, et al. Testing of new agents in childhood cancer preclinical models: meeting summary. Clin Cancer Res. 2002;8:3646–3657. [PubMed] [Google Scholar]
- 2.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 3.Batra S, Reynolds CP, Maurer BJ. Fenretinide cytotoxicity for Ewing’s sarcoma and primitive neuroectodermal tumor cell lines is decreased by hypoxia and synergistically enhanced by ceramide modulators. Cancer Res. 2004;64:5415–5424. doi: 10.1158/0008-5472.CAN-04-0377. [DOI] [PubMed] [Google Scholar]
- 4.Keshelava N, Zuo JJ, Chen P, et al. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61:6185–6193. [PubMed] [Google Scholar]
- 5.Neale G, Su X, Morton CL, et al. Molecular characterization of the pediatric preclinical testing panel. Clin Cancer Res. 2008;14:4572–4583. doi: 10.1158/1078-0432.CCR-07-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whiteford CC, Bilke S, Greer BT, et al. Credentialing preclinical pediatric xenograft models using gene expression and tissue microarray analysis. Cancer Res. 2007;67:32–40. doi: 10.1158/0008-5472.CAN-06-0610. [DOI] [PubMed] [Google Scholar]
- 7.Frgala T, Kalous O, Proffitt RT, Reynolds CP. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007;6:886–897. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 8.Kurmasheva R, Morton C, Houghton PJ. Developing new agents for the treatment of childhood cancer. Curr Opin Investig Drugs. 2005;6:1215–1227. [PubMed] [Google Scholar]
- 9.McAllister RM, Melnyk J, Finkelstein JZ, Adams EC, Jr, Gardner MB. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969;24:520–526. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Petak I, Douglas L, Tillman DM, Vernes R, Houghton JA. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin Cancer Res. 2000;6:4119–4127. [PubMed] [Google Scholar]
- 11.Hazelton BJ, Houghton JA, Parham DM, et al. Characterization of cell lines derived from xenografts of childhood rhabdomyosarcoma. Cancer Res. 1987;47:4501–4507. [PubMed] [Google Scholar]
- 12.Rodriguez-Perales S, Martinez-Ramirez A, de Andres SA, et al. Molecular cytogenetic characterization of rhabdomyosarcoma cell lines. Cancer Genet Cytogenet. 2004;148:35–43. doi: 10.1016/s0165-4608(03)00216-4. [DOI] [PubMed] [Google Scholar]
- 13.Leggas M, Stewart CF, Woo MH, et al. Relation between Irofulven (MGI-114) systemic exposure and tumor response in human solid tumor xenografts. Clin Cancer Res. 2002;8:3000–3007. [PubMed] [Google Scholar]
- 14.Houghton PJ, Cheshire PJ, Hallman JD, et al. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol. 1995;36:393–403. doi: 10.1007/BF00686188. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Reynolds CP. Novel cell lines from pediatric brain tumors showed striking multi-drug resistance. Proc Amer Assoc Cancer Res. 2006;47:4348. (Abstract) [Google Scholar]
- 16.Yee D, Favoni RE, Lebovic GS, et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine growth factor. J Clin Invest. 1990;86:1806–1814. doi: 10.1172/JCI114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson J, Zamboni WC, Cheshire PJ, et al. Efficacy of systemic administration of irinotecan against neuroblastoma xenografts. Clin Cancer Res. 1997;3:423–431. [PubMed] [Google Scholar]
- 18.Keshelava N, Seeger RC, Groshen S, Reynolds CP. Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res. 1998;58:5396–5405. [PubMed] [Google Scholar]
- 19.Keshelava N, Groshen S, Reynolds CP. Cross-resistance of topoisomerase I and II inhibitors in neuroblastoma cell lines. Cancer Chemother Pharmacol. 2000;45:1–8. doi: 10.1007/PL00006736. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz R, Hozier J, LeBien T, et al. Characterization of a leukemic cell line of the pre-B phenotype. Int J Cancer. 1979;23:174–180. doi: 10.1002/ijc.2910230206. [DOI] [PubMed] [Google Scholar]
- 21.Stong RC, Korsmeyer SJ, Parkin JL, Arthur DC, Kersey JH. Human acute leukemia cell line with the t(4;11) chromosomal rearrangement exhibits B lineage and monocytic characteristics. Blood. 1985;65:21–31. [PubMed] [Google Scholar]
- 22.Sheard MA, Kang MH, Cabral D, et al. Bone marrow-level oxygen tension enables enhanced and sustained growth of 3 new pediatric acute lymphoblastic leukemia cell lines. Proc Amer Assoc Cancer Res. 2007;48:1808. (Abstract) [Google Scholar]
- 23.Minowada J, Onuma T, Moore GE. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972;49:891–895. [PubMed] [Google Scholar]
- 24.Foley GE, Lazarus H, Fabber S, Uzman BG, Boone BA, McCarthy RE. Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer. 1965;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25.Asou H, Tashiro S, Hamamoto K, Otsuji A, Kita K, Kamada N. Establishment of a human acute myeloid leukemia cell line (Kasumi-1) with 8;21 chromosome translocation. Blood. 1991;77:2031–2036. [PubMed] [Google Scholar]
- 26.Fischer P, Nacheva E, Mason DY, et al. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin’s lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor beta-chain gene. Blood. 1988;72:234–240. [PubMed] [Google Scholar]
- 27.Benjamin D, Magrath IT, Maguire R, Janus C, Todd HD, Parsons RG. Immunoglobulin secretion by cell lines derived from African and American undifferentiated lymphomas of Burkitt’s and non-Burkitt’s type. J Immunol. 1982;129:1336–1342. [PubMed] [Google Scholar]
- 28.Klein G, Giovanella B, Westman A, Stehlin JS, Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5:319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- 29.Grigoryan R, Keshelava N, Anderson C, Reynolds CP. In vitro testing of chemosensitivity in physiological hypoxia. Methods Mol Med. 2005;110:87–100. doi: 10.1385/1-59259-869-2:087. [DOI] [PubMed] [Google Scholar]
- 30.Keshelava N, Frgala T, Krejsa J, Kalous O, Reynolds CP. DIMSCAN: a microcomputer fluorescence-based cytotoxicity assay for preclinical testing of combination chemotherapy. Methods Mol Med. 2005;110:139–153. doi: 10.1385/1-59259-869-2:139. [DOI] [PubMed] [Google Scholar]
- 31.Masters JR, Thomson JA, ly-Burns B, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. PNAS. 2001;98:8012–8017. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parson W, Kirchebner R, Muhlmann R, et al. Cancer cell line identification by short tandem repeat profiling: power and limitations. FASEB J. 2005;19:434–436. doi: 10.1096/fj.04-3062fje. [DOI] [PubMed] [Google Scholar]
- 33.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci U S A. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Law BK, Chytil A, Dumont N, et al. Rapamycin potentiates transforming growth factor beta-induced growth arrest in nontransformed, oncogene-transformed, and human cancer cells. Mol Cell Biol. 2002;22:8184–8198. doi: 10.1128/MCB.22.23.8184-8198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reaman GH. Pediatric cancer research from past successes through collaboration to future transdisciplinary research. J Pediatr Onc Nursing. 2004;21:123–127. doi: 10.1177/1043454204264406. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JI, Decker S, Zaharevitz D, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison S. Perspective on the history of tumor models. In: Teicher BA, editor. Tumor models in cancer research. Humana PRess; Totowa (NJ): 2002. pp. 3–19. [Google Scholar]
- 39.Maris JM, Courtright J, Houghton PJ, et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51:42–48. doi: 10.1002/pbc.21535. [DOI] [PubMed] [Google Scholar]
- 40.Kolb EA, Gorlick R, Houghton PJ, et al. Pediatric Preclinical Testing Program (PPTP) evaluation of the multi-targeted kinase inhibitor sorafenib. Proc Amer Assoc Cancer Res. 2009;50:3196. (Abstract) [Google Scholar]
- 41.Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing of dasatinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:1198–1206. doi: 10.1002/pbc.21368. [DOI] [PubMed] [Google Scholar]
- 42.Larizza L, Magnani I, Beghini A. The Kasumi-1 cell line: a t(8;21)-kit mutant model for acute myeloid leukemia. Leuk Lymphoma. 2005;46:247–255. doi: 10.1080/10428190400007565. [DOI] [PubMed] [Google Scholar]
- 43.Beghini A, Magnani I, Ripamonti CB, Larizza L. Amplification of a novel c-Kit activating mutation Asn(822)-Lys in the Kasumi-1 cell line: a t(8;21)-Kit mutant model for acute myeloid leukemia. Hematol J. 2002;3:157–163. doi: 10.1038/sj.thj.6200168. [DOI] [PubMed] [Google Scholar]
- 44.McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68:3389–3395. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 45.Gorlick R, Kolb EA, Houghton PJ, et al. Initial testing (stage 1) of lapatinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2009;53:594–598. doi: 10.1002/pbc.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monga M, Sausville EA. Developmental therapeutics program at the NCI: molecular target and drug discovery process. Leukemia. 2002;16:520–526. doi: 10.1038/sj.leu.2402464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.