Abstract
Objective
Abdominal aortic aneurysm (AAA) is a complex vascular disease characterized by matrix degradation and inflammation and is a major cause of mortality in older men. Specific interventions that prevent AAA progression remain to be identified. In this study, we tested the hypothesis that Group X secretory phospholipase A2 (GX sPLA2), an enzyme implicated in inflammatory processes, mediates AAA.
Methods and Results
GX sPLA2 was detected by immunostaining in human aneurysmal tissue and in angiotensin II (Ang II)-induced AAAs in apolipoprotein E-deficient (apoE−/−) mice. GX sPLA2 mRNA was increased significantly (11-fold) in abdominal aortas of apoE−/− mice in response to Ang II infusion. To define the role of GX sPLA2 in experimental AAAs, apoE−/− and apoE−/− × GX sPLA2−/− (GX DKO) mice were infused with Ang II for either 10 (n=7) or 28 (n=24–26) days. Deficiency of GX sPLA2 significantly reduced the incidence and severity of AAAs, as assessed by ultrasound measurements in vivo of aortic lumens and by computer-assisted morphometric analyses ex vivo of external diameter. Results from gene expression profiling indicated that the expression of specific matrix metalloproteinases and inflammatory mediators was blunted in aortas from GX DKO mice compared to apoE−/− mice after 10-day Ang II infusion. Ang II induction of cyclooxygenase-2, interleukin-6, matrix metalloproteinase (MMP)-2, MMP-13 and MMP-14 was reduced significantly in GX DKO mice compared to apoE−/− mice.
Conclusion
GX sPLA2 promotes Ang II-induced pathological responses leading to AAA formation.
Keywords: aneurysm, atherosclerosis, inflammation, interleukins, metalloproteinases
Introduction
Abdominal aortic aneurysm (AAA), defined as a permanent dilation, has been estimated to be responsible for ~1% of all deaths in 65–85-year old men in developed countries1. Although most patients with AAAs are asymptomatic, the risk of death due to rupture increases greatly as AAAs expand. Currently, the treatment for AAA is limited to surgical intervention due to a lack of other therapies with proven benefit. The pathophysiological processes leading to AAA formation and rupture are likely complex, involving chronic inflammation and excessive extracellular matrix breakdown that leads to medial degradation and weakening of the aortic wall. The mechanisms that initiate and advance these events are still poorly understood.
Secretory phospholipase A2’s (sPLA2) comprise a family of enzymes that hydrolyze glycerophospholipids at the sn-2 position to generate free fatty acids and lysophospholipids. These enzymes have been suggested to contribute to inflammatory processes through the generation of lipid mediators that act as second messengers in signal transduction. By liberating arachidonic acid, sPLA2’s have the potential to promote synthesis of a variety of bioactive lipid mediators, including prostaglandins and thromboxanes, which have potent and pleiotropic activities. Among the ten mammalian sPLA2’s Group X (GX) sPLA2 is the most potent in hydrolyzing phosphatidylcholine-containing substrates, including mammalian cell membranes and lipoprotein particles2. Accumulating experimental data suggest that GX sPLA2 may be a useful therapeutic target for treating inflammatory diseases. For example, mice deficient in GX sPLA2 exhibit reduced myocardial ischemia/reperfusion injury associated with an attenuation of neutrophil activity in ischemic myocardium3. GX sPLA2 has also been implicated in the pathogenesis of allergen-induced inflammation in the lung4. Although direct evidence that GX sPLA2 participates in atherosclerotic processes in vivo is currently lacking, this enzyme has been detected in mouse and human atherosclerotic lesions and has atherogenic properties in vitro5, 6.
In this study, we tested the hypothesis that GX sPLA2 plays a role in the pathogenesis of AAA. Our data show that deficiency of GX sPLA2 leads to significantly reduced induction of inflammatory mediators, metalloproteinases, and AAA formation in apoE−/− mice in response to Ang II infusion.
Materials and Methods
Experimental animals
Targeted deletion of the GX sPLA2 gene was performed by InGenious Targeting Laboratory (Stony Brook, NY) using embryonic stem cells from C57BL/6 mice. GX sPLA2−/− mice were crossed with apoE−/− mice (C57BL/6 background, N10; the Jackson Laboratory), and the resulting offspring were bred to generate apoE−/− mice that were either GX sPLA2+/+ or GX sPLA2−/− (GX DKO). All mice were housed in microisolator cages with normal rodent diet and water provided ad libitum. Ang II (1,000 ng kg-1 min-1; Sigma) or saline was administered subcutaneously into male mice (8–10 weeks old) for 10 or 28 days via Alzet osmotic minipumps (model 2004) using established techniques7. Studies were performed with the approval of the University of Kentucky Institutional Animal Care and Use Committees.
Blood Pressure Measurements
Mean systolic blood pressures were measured in conscious mice using a computerized tail-cuff method (BP-2000 Visitech Systems). Mice were acclimatized to the system for 1 week prior to the initiation of studies and systolic blood pressure was measured 5 days per week until study termination.
Lipid and Lipoprotein measurements
Plasma concentrations of total cholesterol were determined using enzymatic assays (Wako Chemicals, Inc). Lipoprotein cholesterol distribution was determined in plasma samples (50 μl) resolved using a Superose 6 column.
Immunostaining
The generation of rabbit anti-human GX sPLA2, which cross-reacts with mouse GX sPLA2, has been described8. Mouse anti-human macrophage antibody [3A5] was purchased from Abcam. Anti-mouse macrophage antiserum was obtained from Accurate (AI-AD31240). Human pathological tissue was obtained from archived samples that were collected following a protocol approved by the Washington University School of Medicine Human Research Subjects Committee. Formalin-fixed human aortic tissues were embedded in paraffin for sectioning. After deparaffinization, tissue sections were incubated in an antigen retrieval solution (Dako). Immunostaining of mouse AAAs was performed on acetone-fixed, frozen 10 μm thick sections.
Quantification of AAAs and Atherosclerotic Lesions
AAAs were assessed in anesthetized mice by high-frequency ultrasonography using a Visualsonics Vevo 660 high-resolution system. Maximal luminal diameters of suprarenal abdominal aortas were determined 1 day prior to the initiation of Ang II infusion and at termination. AAAs were also quantified ex vivo by measuring the maximal width of suprarenal aortas. For atherosclerosis quantification, the entire aorta was cleaned of adventitial tissue, longitudinally cut, and tissues were pinned en face to expose intimal surfaces. Tissues were visualized using a dissecting microscope, which was equipped with a Nikon digital camera that captured an image directly into an analysis program. Aortic arches were defined as the region from the ascending arch to 3 mm distal to the subclavian artery. Atherosclerotic lesions on the intimal surface of the aortic arch were easily distinguishable as bright white areas compared with the thin and translucent aorta. Areas of intima covered by atherosclerosis were delineated by two independent researchers who were blinded to the study, and quantified using Image Pro software. For analysis of lesion area in aortic roots, tissues that were frozen in OCT were serially cut in 10 μm thick sections from the aortic sinus (where the aortic valve leaflets appear) to the distal region of the root, covering a length of approximately 800 μm. Atherosclerotic lesion area was delineated visually using Oil red O staining and quantified using Image Pro software (Media Cybernetics).
RNA Isolation and Quantitative RT-PCR
Abdominal aortas were cleaned of adhering fat tissues, placed in RNAlater (Ambion), and then homogenized in RNeasy Fibrous Mini Kit solution (Qiagen). For gene expression profiling, 0.5 μg of aortic RNA was reverse transcribed using the High Capacity Reverse Transcriptase system (Applied Biosystems). The expression of 39 genes implicated in vascular pathology was assessed using a custom SABiosciences™ RT-PCR array per the manufacturer’s protocol. Quantification of mRNA was performed using the ΔΔCT method and normalized to 18S RNA. For real time RT-PCR, 0.2–0.5 ug RNA was reverse transcribed using the Reverse Transcription System (Promega). Real-time RT-PCR was performed using Power SYBR® Green Master Mix (Applied Biosystems) on a DNA Engine Opticon 2 System (MJ Research). Quantification was done using the standard curve method and normalized with 18S. Sequences of PCR primers are provided in Supplemental Table 1.
Gelatin Zymography
Abdominal aortas were extracted, cleaned of adventitial tissue, and homogenized in 0.1 ml lysis buffer (Cell Signaling); 10 μg protein was electrophoresed on a 7.5% SDS-polyacrylamide gel containing 2 mg/ml gelatin. Gels were renatured in 50 mM Tris-HCl containing 100 mM NaCl and 2.5% Triton X-100 and then incubated in 50 mM Tris-HCl containing 5 mM CaCl2 prior to staining with Coomassie Brilliant Blue.
Statistical Analyses
For comparing two groups on a continuous response variable, a two-sample Student’s t-test was used after verifying that data met constraints of normality and equivalence of variance to permit parametric analysis. A one-way ANOVA followed by Tukey’s multiple comparison test was used to compare more than two groups on a continuous response variable. Percent incidence of AAAs was analyzed by Fisher’s exact test. P values <0.05 were considered to be statistically significant. All data are represented as means ± SE.
Experimental Results
GX sPLA2 is present in human and mouse AAAs
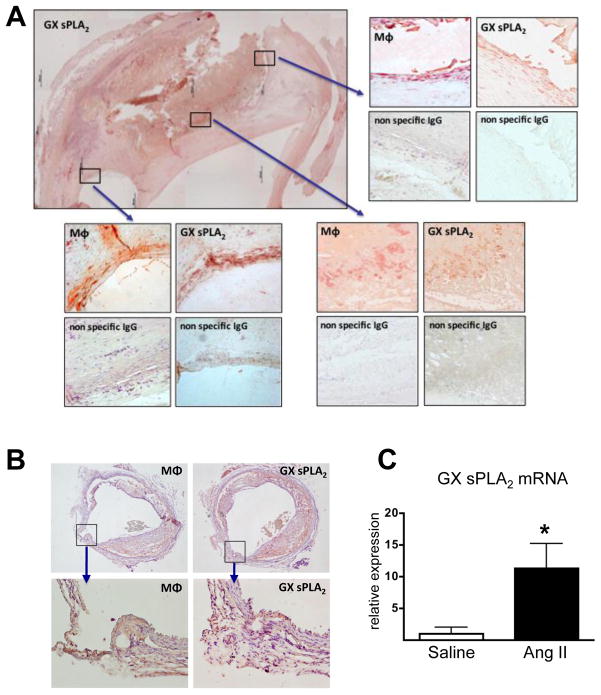
Previous studies have demonstrated the presence of GX sPLA2 in human and mouse atherosclerotic lesions5, 6. Here we show this enzyme is also associated with human AAA (Figure 1A). Positive immunostaining for GX sPLA2 was most pronounced in regions containing macrophages. Diffuse immunostaining was also detected in acellular regions.
Figure 1. GX sPLA2 is present in mouse and human AAA.
(A) Sections from aortic tissue removed during surgical repair of AAA were immunostained with 3A5 to detect macrophages, anti-human GX sPLA2, or appropriate control antibodies, as indicated. The upper-left image is a composite of 24 pictures taken at 4× magnification; boxed areas indicate the regions shown at higher (10×) magnification. (B) Sections from the abdominal aorta of an apoE−/− mouse infused with 1,000 ng · kg−1 · min−1 Ang II for 28 days were immunostained with antisera specific for mouse macrophages or anti-mouse GX sPLA2, as indicated (upper panels show cross-section of entire aorta, magnification = 4×; lower panels show region encompassing a break in the elastic lamellae, magnification = 20×). (B) Total RNA was extracted from abdominal aortas of apoE−/− mice infused with saline or 1,000 ng · kg−1 · min−1 Ang II for 10 days and analyzed by real-time RT-PCR. Values shown are mean ± SE; *, p < 0.05.
Chronic infusion of Ang II (1,000 ng · kg−1 · min−1) in apoE−/− mice leads to AAA that mimics many features of the human disease 9, 10. Immunostaining using an isoform-specific antibody detected GX sPLA2 in AAA of Ang II-infused mice that was present as diffuse staining in both cellular and acellular regions of remodeled tissues (Figure 1B). GX sPLA2 mRNA was also detected in abdominal aortas from apoE−/− mice after 10 days of Ang II infusion, with abundance increased ~11-fold compared to saline-infused mice (Figure 1C).
Deficiency of GX sPLA2 reduces AAA and atherosclerotic lesion area in AngII-infused apoE−/− mice
To determine whether GX sPLA2 plays a role in pathological processes leading to experimental AAAs, apoE−/− and GX DKO mice were infused with either saline or Ang II for 28 days. Body weights, plasma total cholesterol concentrations, and mean systolic blood pressures before and after Ang II infusion are presented in Supplemental Table 2. The hypertensive response to Ang II was similar in apoE−/− and GX DKO mice. No significant differences in lipoprotein cholesterol distributions were detected in the two strains of mice either before or after Ang II infusion (data not shown).
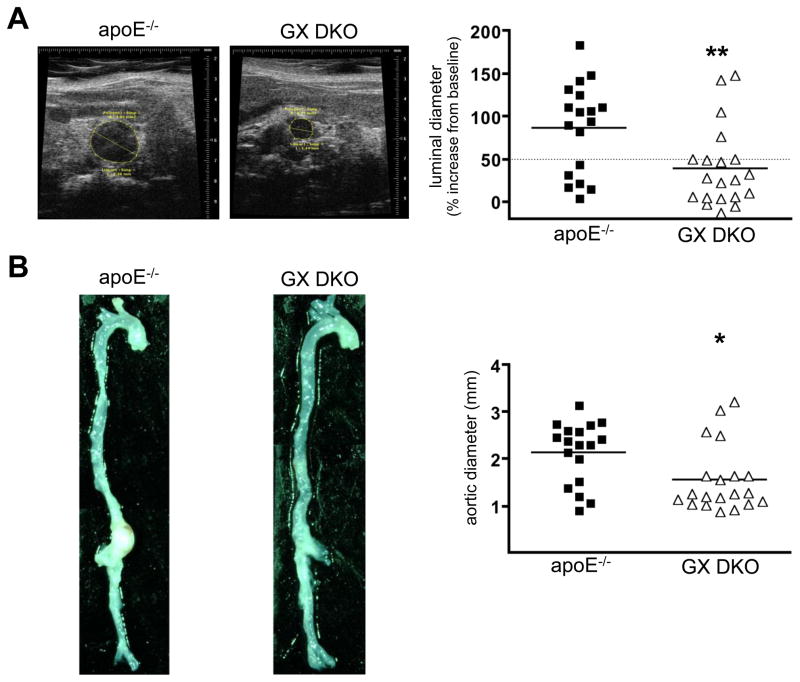
The incidence of death due to aortic rupture during Ang II infusion was not significantly different in the two strains (apoE−/− mice: 25% versus GX DKO mice: 23%). AAA was assessed in the surviving mice by in vivo ultrasound and by computer-assisted morphometric analysis ex vivo to determine the maximal luminal and external diameters of abdominal aortas, respectively (n = 18–20). AngII-induced abdominal aorta expansion was significantly less in GX DKO mice (38.9 ± 10.4% increase in lumen diameter) compared to apoE−/− mice (86.0 ± 12.5% increase in lumen diameter) after 28-day Ang II infusion (Figure 2A). Consistent with this finding, ex vivo determinations of AAA showed significantly smaller aortic diameters in GX DKO mice (1.55 ± 0.16 mm) compared to apoE−/− mice (2.14 ± 0.15 mm; Figure 2B). The overall incidence of AAA was calculated as the sum of the percent of total mice that died from aortic rupture (6 out of 24 apoE−/− mice; 6 out of 26 GX DKO mice) plus the percent of total mice that developed AAA, defined in surviving mice as a greater than 50% dilation of abdominal aorta lumen (12 out of 24 apoE−/− mice; 4 out of 26 GX DKO mice). Thus, the overall incidence of AAA was significantly higher in Ang II-infused apoE−/− mice (75%) compared to DKO mice (38.5%; p ≤ 0.05).
Figure 2. GX sPLA2 deficiency protects apoE−/− mice from Ang II-induced AAAs.
Male apoE−/− and GX DKO mice were infused with 1,000 ng kg−1 min−1 Ang II for 28 days. AAAs were assessed by (A) in vivo ultrasound imaging before and after Ang II infusion to determine the percent increase in luminal diameter of abdominal aortas; and (B) by computer-assisted morphometric analyses ex vivo. The images shown are representative aortas after 28-day infusion. Each symbol represents one animal; bars represent means. *, p < 0.05; **, p < 0.01.
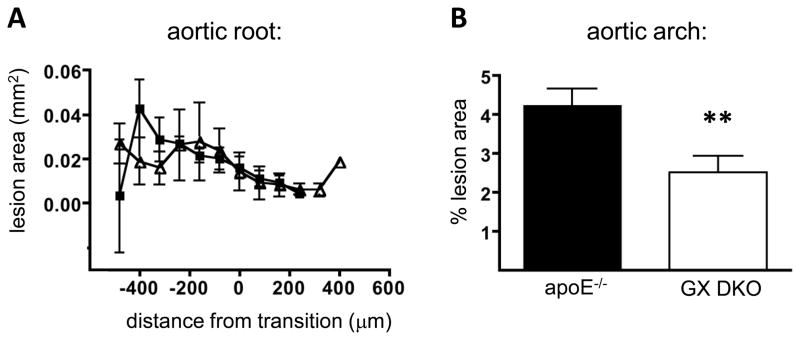
Atherosclerotic lipid deposition was quantified in serial sections throughout the aortic root and by en face analysis of the luminal surface of aortic arches. GX sPLA2 deficiency had no effect on atherosclerotic lesion size in aortic roots of AngII-infused mice (Figure 3A). However, there was a modest, but significant, 41% reduction in mean atherosclerotic lesion area in aortic arches of GX DKO mice compared to apoE−/− mice (Figure 3B). Atherosclerosis was not quantified in thoracic aortas, as very few discernible lesions were detected in this region. The focus of the current study was the effects on AAA, and thus abdominal aortas were reserved for the analysis of aneurysms. Furthermore, for Ang II-infused apoE−/− mice, the extensive remodeling of tissue in the abdominal aortic region precluded en face analyses.
Figure 3. GX sPLA2 deficiency reduces atherosclerosis in Ang II-infused apoE−/− mice.
Male apoE−/− and GX DKO mice were infused with 1,000 ng kg−1 min−1 Ang II for 28 days. (A) Atherosclerotic lesion area in the aortic root. Values are mean lesion areas (± SE) per section for sections ~64 μm apart (n = 4). The transition zone between the aortic sinus and the ascending aorta, de ned by disappearance of the valve cusps, is 0 on the x-axis. (B) Atherosclerotic lesion area quantified on the luminal surface of the aortic arch. Values are mean % lesion area (± SE; n = 17–18). **, p < 0.01.
Inflammatory responses to Ang II are blunted in abdominal aortas of GX DKO mice
To investigate the mechanisms by which GX sPLA2 deficiency protected against Ang II-induced AAAs and atherosclerosis, we quantified the expression of a number of mediators implicated in cardiovascular pathology in apoE−/− and GX DKO mice infused with either saline (n = 4) or Ang II (n = 8) for 10 days. The duration of Ang II infusion was chosen based on our finding that GX sPLA2 is upregulated in aortas of apoE−/− mice at 10 days (Figure 1C). Moreover, previous studies to define the temporal characteristics of AAA formation in apoE−/− mice identified a marked inflammatory infiltration into the abdominal aorta after 10-day Ang II infusion11. There were no differences in baseline measures of luminal diameter between apoE−/− and DKO mice, and saline infusion did not significantly alter this parameter in either strain (Table 1). One mouse from each group died during the short-term Ang II infusion due to abdominal aortic rupture. Ang II infusion in apoE−/− mice resulted in a significant (76.6 ± 7.7%) increase in luminal diameters compared to baseline values. GX DKO mice had only a modest and non-significant (26.3 ± 8.6%) expansion of abdominal aortas after 10-day infusions.
Table 1.
Incidence of AAA formation in mice after 10-day Ang II infusion
| Rupture incidence | luminal diameter* | |||
|---|---|---|---|---|
| Baseline (mm) | % change (saline) | % change (Ang II) | ||
| ApoE−/− | 12.5% | 1.1 ± 0.04 | −2.9 ± 2.0% | +76.6 ± 7.7% |
| GX DKO | 12.5% | 1.1 ± 0.02 | −9.1 ± 5.1% | +26.3 ± 8.6%† |
Data are mean ± SE; n = 4 for saline infusions and n = 8 for Ang II infusions
p = 0.001 compared to Ang II-infused apoE−/− mice.
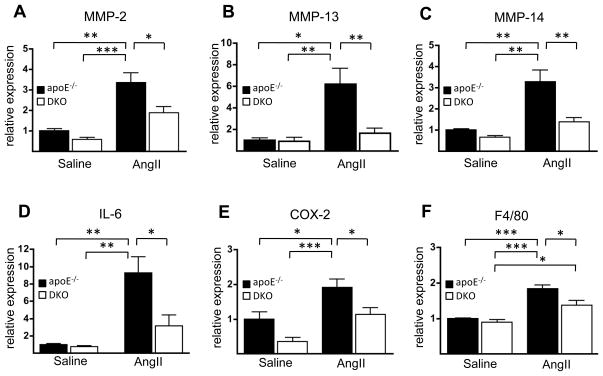
Gene expressions in abdominal aortas were analyzed using a SABiosciences™ RT-PCR array. The custom array included adhesion molecules, MMPs, inflammatory cytokines, chemokines, and chemokine receptors. Of the 39 genes analyzed, 10-day Ang II infusion resulted in a more than 2-fold increase in the expression of 18 genes in apoE−/− mice, with the upregulation of MMP-2, MMP-13, IL-1b, IL-6, COX-2, PAI-1, F4/80, MCP-1, and CCCR2 being statistically significant (shaded values, Supplemental Table 3). Based on the array data, the effect of Ang II to induce the expression of each of these genes appeared to be blunted in GX DKO mice. To confirm the finding that the decreased incidence of AAA in GX DKO mice was associated with an altered inflammatory response to Ang II, we carried out real time RT-PCR to quantify aortic expression of selected genes (Figure 4). MMP-2, MMP-13, MMP-14, IL-6, and COX-2 expression were all significantly increased in apoE−/− mice, but not GX DKO mice. Analysis by gelatin zymography confirmed that MMP-2 latent and active forms were increased in Ang II-infused apoE−/− mice compared to GX DKO mice (Supplemental Figure 1). F4/80 expression was significantly higher in both strains after Ang II infusion compared to baseline values. The expression of other mediators implicated in AAA, namely ICAM, MMP-9, TNF-α, IL-1β and IFN-γ, was not altered in either apoE−/− or GX DKO mice at this time point in agreement with the array data (Supplemental Figure 2). We were not able to detect 92 kDa proMMP-9 in either strain of mice before or after Ang II infusion by gel zymography (Supplemental Figure 1). Importantly, GX sPLA2 deficiency resulted in significantly reduced expression of all genes that were significantly upregulated in apoE−/− mice in response to 10-day Ang II infusion (Figure 4).
Figure 4. Induction of inflammatory genes by Ang II is blunted in abdominal aortas from GX DKO mice.
(A-F) Total RNA was extracted from abdominal aortas of apoE−/− and GX DKO mice infused with either saline or 1,000 ng · kg−1 · min−1 Ang II for 10 d and analyzed by semi-quantitative RT-PCR. Values shown are mean ± SE; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
Understanding the mechanisms underlying AAA formation is critical for the development of non-interventional therapies that inhibit their progression. In this study we took advantage of a widely used rodent model that involves infusion of Ang II into apoE−/− mice to test the hypothesis that GX sPLA2 mediates AAA. Aneurysms induced by Ang II exhibit many features of the human disease, including medial degeneration, inflammation, thrombus formation, and rupture of the abdominal aorta9. In addition, similar to humans, male apoE−/− mice exhibit increased susceptibility to Ang II-induced AAAs compared to female mice. A recent study analyzing whole genome expression in relation to Ang II-induced AAA formation highlighted the importance of inflammatory chemokines and cytokines as well as proteolytic enzymes, indicating that pathways implicated in human AAA are also important in this animal model10. Polymorphisms in genes encoding the Ang II type I receptor and angiotensin-converting enzyme are associated with human AAA, providing further support for the relevance of the Ang II infusion model12, 13. In the current study we determined that deficiency of GX sPLA2 protects apoE−/− mice from Ang II-induced AAAs. We also present novel evidence that GX sPLA2 is present in human aortic tissue removed during surgical AAA repair. The possibility that GX sPLA2 may provide a new target for treating AAA is substantiated by the recent report that the sPLA2 inhibitor varespladib significantly reduces experimental AAAs14.
Aortic expansion induced by Ang II infusion was significantly reduced in mice deficient in GX sPLA2. The incidence of abdominal aortic rupture was not different in apoE−/− and GX DKO mice, suggesting that GX sPLA2 may play a role in the progression rather than the initiation of the disease. One of the earliest observable events that occurs in apoE−/− mice after Ang II infusion is the accumulation of macrophages in the media11. While a variety of inflammatory cell types have been identified in human aneurysms, macrophages likely play a predominant role. Consistent with macrophage infiltration, we observed a marked increase in F4/80 mRNA in abdominal aortas of both apoE−/− and DKO mice after 10-day Ang II infusion that was coincident with a significant increase in GX sPLA2. Murine macrophages are known to express this enzyme15, and immunohistochemical analysis of human and mouse AAA detected immunopositive staining for GX sPLA2 and macrophages in the same regions. Thus, it seems likely that macrophages are a source of GX sPLA2 in both human and Ang II-induced AAAs. The finding that F4/80 expression in GX DKO mice after Ang II infusion was significantly higher compared to saline infused mice suggests that GX sPLA2 does not play a major role in the recruitment/infiltration of macrophages. However, this interpretation requires further study, given a recent report that aneurysmal tissue of Ang II-infused C57BL/6 mice is enriched in macrophages lacking F4/8016.
Interventions that disrupt medial SMC apoptosis, macrophage infiltration, MMPs, oxidative stress, prostanoid generation, or proinflammatory cytokines have all been shown to reduce Ang II-induced AAA, demonstrating the complex nature of this disease. In the current study, the upregulation of factors known to promote inflammation and matrix degradation was significantly reduced in GX DKO compared to apoE−/− mice after 10-day Ang II infusion. For example, whereas IL-6 was significantly upregulated almost 10-fold in aortas of apoE−/− mice, its expression was not significantly altered in GX DKO mice in response to Ang II. GX sPLA2 has been shown previously to enhance macrophage IL-6 expression in response to inflammatory stimuli in vitro17. Interestingly, deficiency of IL-6 leads to decreased monocyte recruitment and activation and protection from suprarenal aortic rupture induced by Ang II, consistent with an important role for this cytokine in aortic inflammation and destabilization16. Human aortic aneurysms are known to secrete IL-6 and circulating IL-6 concentrations are associated with AAA diameter and symptoms18.
The absence of a significant induction in the aortic expression of MMP-2, MMP-13, and MMP-14 is likely relevant to the diminished AAA progression in Ang II-infused GX DKO mice. The integrity of the arterial wall is a function of the elastin and collagen content. Since specific MMPs can degrade collagen and elastin, derangements in MMP activity could lead to vessel dilation and/or rupture. MMP activity is regulated at multiple levels, including transcription, zymogen activation, and proteolytic inactivation. A preponderance of evidence in animal models and in humans supports a role for specific MMPs in AAA initiation and progression19. In situ studies have demonstrated a significant increase in MMP-2 in tissue from human AAAs, where it is thought to act as the dominant enzyme in the formative stage of the disease20. While MMP-9 is probably the most extensively studied in the pathology of AAAs, it is thought to be the dominant enzyme in the later stages of AAA formation21, which may account for why MMP-9, unlike MMP-2, was not significantly induced in apoE−/− mice after short-term (10-day) infusion.
The induction of COX-2 was also blunted in GX DKO mice, consistent with in vitro findings that upregulation of COX-2 by inflammatory stimuli is enhanced in RAW264.7 macrophages when GX sPLA2 is overexpressed17. Genetic deletion or pharmacological inhibition of COX-2, or deletion of microsomal prostaglandin E synthase 1, protects against Ang II-induced AAA22–24, implicating a role for prostaglandins in AAA formation. The potent ability of GX sPLA2 to hydrolyze phospholipids and hence release arachidonic acid could provide an additional mechanism to augment COX-2-dependent prostanoid production. COX-2 and PGE2 are present in human aneurysmal tissue, and there is evidence to suggest that prostaglandins may promote AAA progression in humans25.
Our data demonstrate that GX sPLA2 deficiency has no effect on the hypertensive response to Ang II. This result may not have been predicted, given data that arachidonic acid metabolites modulate pressor responses to Ang II26. AAA formation in this model is unlikely to be due to Ang II-induced hypertension, since a number of interventions have been shown to have an inhibitory effect on AAA without significantly altering blood pressure. Furthermore, administration of hydralazine to reduce Ang II-induced hypertension does not affect the generation of AAA27.
In addition to protecting against Ang II-induced AAA, GX sPLA2 deficiency also resulted in a significant, albeit modest, reduction in atherosclerotic lipid deposition in aortic arches. Atherosclerotic lesion area in aortic roots was not altered, however. Although the mechanistic basis for this regional difference in atherosclerosis is not clear, deficiency of another member of the sPLA2 family, GV sPLA2, was similarly shown to reduce atherosclerosis in aortic arches/thoracic aortas, but not aortic roots of LDL receptor-deficient mice fed a high fat diet28. GX sPLA2 is present in human and mouse atherosclerotic lesions, where it has been suggested to promote atherosclerotic processes through its ability to hydrolyze lipoprotein particles5, 29. Although GX sPLA2 and other sPLA2’s have been investigated as potential targets in the prevention of atherosclerotic cardiovascular disease30, our data provides the first experimental evidence this enzyme contributes to atherogenic processes in vivo.
Although GX sPLA2 is generally considered to induce inflammatory responses, this enzyme has also been suggested to have anti-inflammatory activity17. Nevertheless, studies in gene-targeted mice support the conclusion that GX sPLA2 promotes pathogenic inflammatory processes. In the setting of acute and chronic asthma, GX sPLA2 deficiency results in a significant reduction in inflammatory cell infiltration, smooth muscle cell layer thickening and subepithelial fibrosis 4. Myocardial ischemia/reperfusion injury is also reduced in GX sPLA2−/− mice, at least partly due to a suppression of neutrophil activation3. Interestingly, sPLA2s are known to exhibit pro-inflammatory properties independent of their catalytic function. For example, catalytically inactive GX sPLA2 was reported to be equally effective in enhancing LPS-induced cytokine production in human lung macrophages as wild-type GX sPLA231. Whether GX sPLA2 mediates AAA through a non-enzymatic mechanism or through its potent hydrolytic activity requires further study.
In summary, our results demonstrate that the incidence and severity of Ang II-induced AAAs is significantly reduced in apoE−/− mice that are also deficient in GX sPLA2. We provide evidence that GX sPLA2 augments pathogenic responses that occur in aortas of apoE−/− mice infused with Ang II. Our data that GX sPLA2 is present in human aneurysmal tissue suggests it may provide a useful target for the treatment of AAA.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by NIH grant PO1HL080100 (to NW and AD) and United States Department of Agriculture Fellowship Grant n2005-38420-15825 (to MZ).
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 2.Singer AG, Ghomashchi F, Le Clavez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, Gelb MH. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipase A2 J. Biol. Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 3.Fujioka D, Saito Y, Kobayashi T, Yano T, Tezuka H, Ishimoto Y, Suzuki N, Yokota Y, Nakamura T, Obata JE, Kanazawa M, Kawabata K, Hanasaki K, Kugiyama K. Reduction in myocardial ischemia/reperfusion injury in group X secretory phospholipase A2-deficient mice. Circulation. 2008;117:2977–2985. doi: 10.1161/CIRCULATIONAHA.107.743997. [DOI] [PubMed] [Google Scholar]
- 4.Henderson WR, Jr, Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GK, Nevalainen T, Rudensky AY, Gelb MH. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J Exp Med. 2007;204:865–877. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanasaki K, Yamada K, Yamamoto S, Ishimoto Y, Saiga A, Ono T, Ikeda M, Notoya M, Kamitani S, Arita H. Potent modification of low density lipoprotein by group X secretory phospholipase A2 is linked to macrophage foam cell formation. J Biol Chem. 2002;277:29116–29124. doi: 10.1074/jbc.M202867200. [DOI] [PubMed] [Google Scholar]
- 6.Karabina SA, Brocheriou I, Le Naour G, Agrapart M, Durand H, Gelb M, Lambeau G, Ninio E. Atherogenic properties of LDL particles modified by human group X secreted phospholipase A2 on human endothelial cell function. FASEB J. 2006;20:2547–2549. doi: 10.1096/fj.06-6018fje. [DOI] [PubMed] [Google Scholar]
- 7.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann NY Acad Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 8.Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, Reithmeier R, Lindsay TF, Lichtenberger C, Reinisch W, Lambeau G, Arm J, Tischfield J, Gelb MH, Rubin BB. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J Biol Chem. 2002;277:5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- 9.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 10.Rush C, Nyara M, Moxon JV, Trollope A, Cullen B, Golledge J. Whole genome expression analysis within the angiotensin II-apolipoprotein E deficient mouse model of abdominal aortic aneurysm. BMC Genomics. 2009;10:298. doi: 10.1186/1471-2164-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saraff K, Babamusta F, Cassis L, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 12.Jones GT, Thompson AR, van Bockxmeer FM, Hafez H, Cooper JA, Golledge J, Humphries SE, Norman PE, van Rij AM. Angiotensin II type 1 receptor 1166C polymorphism is associated with abdominal aortic aneurysm in three independent cohorts. Arterioscler Thromb Vasc Biol. 2008;28:764–770. doi: 10.1161/ATVBAHA.107.155564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatini C, Pratesi G, Sofi F, Gensini F, Sticchi E, Lari B, Pulli R, Dorigo W, Azas L, Pratesi C, Gensini GF, Abbate R. ACE DD genotype: a predisposing factor for abdominal aortic aneurysm. Euro J Vasc Endovasc Surg. 2005;29:227–232. doi: 10.1016/j.ejvs.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Fraser H, Hislop C, Christie RM, Rick HL, Reidy CA, Chouinard ML, Eacho PI, Gould KE, Trias J. Varespladib (A-002), a Secretory Phospholipase A2 Inhibitor, Reduces Atherosclerosis and Aneurysm Formation in ApoE−/− Mice. J Cardiovasc Pharmacol. 2009 doi: 10.1097/FJC.0b013e318195bfbc. [DOI] [PubMed] [Google Scholar]
- 15.Morioka Y, Saiga A, Yokota Y, Suzuki N, Ikeda M, Ono T, Nakano K, Fujii N, Ishizaki J, Arita H, Hanasaki K. Mouse group X secretory phospholipase A2 induces a potent release of arachidonic acid from spleen cells and acts as a ligand for the phospholipase A2 receptor. Arch Biochem Biophys. 2000;381:31–42. doi: 10.1006/abbi.2000.1977. [DOI] [PubMed] [Google Scholar]
- 16.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curfs DM, Ghesquiere SA, Vergouwe MN, van der Made I, Gijbels MJ, Greaves DR, Verbeek JS, Hofker MH, de Winther MP. Macrophage secretory phospholipase A2 group X enhances anti-inflammatory responses, promotes lipid accumulation, and contributes to aberrant lung pathology. J Biol Chem. 2008;283:21640–21648. doi: 10.1074/jbc.M710584200. [DOI] [PubMed] [Google Scholar]
- 18.Treska V, Topolcan O, Pecen L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin Chem Lab Med. 2000;38:1161–1164. doi: 10.1515/CCLM.2000.178. [DOI] [PubMed] [Google Scholar]
- 19.Rizas KD, Ippagunta N, Tilson MD., 3rd Immune cells and molecular mediators in the pathogenesis of the abdominal aortic aneurysm. Cardiol Rev. 2009;17:201–210. doi: 10.1097/CRD.0b013e3181b04698. [DOI] [PubMed] [Google Scholar]
- 20.Thompson M, Cockerill G. Matrix metalloproteinase-2: the forgotten enzyme in aneurysm pathogenesis. Ann NY Acad Sci. 2006;1085:170–174. doi: 10.1196/annals.1383.034. [DOI] [PubMed] [Google Scholar]
- 21.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15:1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 22.King VL, Trivedi DB, Gitlin JM, Loftin CD. Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice. Arterioscler Thromb Vasc Biol. 2006;26:1137–1143. doi: 10.1161/01.ATV.0000216119.79008.ac. [DOI] [PubMed] [Google Scholar]
- 23.Gitlin JM, Trivedi DB, Langenbach R, Loftin CD. Genetic deficiency of cyclooxygenase-2 attenuates abdominal aortic aneurysm formation in mice. Cardiovasc Res. 2007;73:227–236. doi: 10.1016/j.cardiores.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Lee E, Song W, Ricciotti E, Rader DJ, Lawson JA, Pure E, FitzGerald GA. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation. 2008;117:1302–1309. doi: 10.1161/CIRCULATIONAHA.107.731398. [DOI] [PubMed] [Google Scholar]
- 25.Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, Powell JT. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation. 1999;100:48–54. doi: 10.1161/01.cir.100.1.48. [DOI] [PubMed] [Google Scholar]
- 26.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bostrom MA, Boyanovsky BB, Jordan CT, Wadsworth MP, Taatjes DJ, de Beer FC, Webb NR. Group V secretory phospholipase A2 promotes atherosclerosis: evidence from genetically altered mice. Arterioscler Thromb Vasc Biol. 2007;27:600–606. doi: 10.1161/01.ATV.0000257133.60884.44. [DOI] [PubMed] [Google Scholar]
- 29.Ishimoto Y, Yamada K, Yamamoto S, Ono T, Notoya M, Hanasaki K. Group V and X secretory phospholipase A2s-induced modification of high-density lipoprotein linked to the reduction of its antiatherogenic functions. Biochim Biophys Acta. 2003;1642:129–138. doi: 10.1016/s0167-4889(03)00120-4. [DOI] [PubMed] [Google Scholar]
- 30.Rosenson RS. Future role for selective phospholipase A2 inhibitors in the prevention of atherosclerotic cardiovascular disease. Cardiovasc Drugs Ther. 2009;23:93–101. doi: 10.1007/s10557-008-6148-1. [DOI] [PubMed] [Google Scholar]
- 31.Granata F, Petraroli A, Boilard E, Bezzine S, Bollinger J, Del Vecchio L, Gelb MH, Lambeau G, Marone G, Triggiani M. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M-type receptor. J Immunol. 2005;174:464–474. doi: 10.4049/jimmunol.174.1.464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.