Abstract
It has been hypothesized that oncogenesis and neurodegeneration may share common mechanistic foundations. Recent evidence now reveals a number of genes in which alteration leads to either carcinogenesis or neurodegeneration, depending on cellular context. Pathways that have emerged as having critical roles in both cancer and neurodegenerative disease include those involving genes such as PARK2, ATM, PTEN, PTPRD, and mTOR. A number of mechanisms have been implicated, and commonly affected cellular processes include cell cycle regulation, DNA repair, and response to oxidative stress. For example, we have recently shown that the E3 ubiquitin ligase PARK2 is mutated or deleted in many different human malignancies and helps drive loss on chromosome 6q25.2–27, a genomic region frequently deleted in cancers. Mutation in PARK2 is also the most common cause of juvenile Parkinson’s disease. Mutations in PARK2 result in an upregulation of its substrate cyclin E, resulting in dysregulated entry into the cell cycle. In neurons, this process results in cell death, but in cycling cells, the result is a growth advantage. Thus, depending on whether the cell affected is a dividing cell or a post-mitotic neuron, responses to these alterations may differ, ultimately leading to varying disease phenotypes. Here, we review the substantial data implicating specific genes in both cancer and neurodegenerative disease.
Keywords: tumor suppressor, neurodegeneration, cancer, PARK2
Introduction
Although neurons are generally considered to be post-mitotic cells that have terminally differentiated and are non-replicating, specific components of the cell cycle machinery may be reactivated in some neurons in response to certain stimuli, such as growth factors (Park et al., 1998), excitotoxicity (Giardina and Beart, 2002; Verdaguer et al., 2002) and DNA damage (Kruman et al., 2004). However, reactivation of the cell cycle in neurons generally eventuates in apoptosis. Neurons are not mitotically competent, because of the absence of requisite proteins such as certain cyclin-dependent kinases (CDK) and an inadequate subcellular structure for cell division (Nouspikel and Hanawalt, 2003; Ackman et al., 2007), and activation of the core cell cycle machinery in these cells often results in an abortive cell cycle and cell death. Moreover, neurons are unable to use synthesis-related mechanisms of DNA repair such as mismatch repair or nucleotide excision repair, thereby rendering these cells more sensitive to DNA damage, which can trigger apoptosis (Staropoli, 2008). Although the eventual outcome of cell cycle activation or DNA damage in neurons may be apoptosis, normally cycling cells may instead respond with proliferation and possibly tumorigenesis. Ultimately, these dichotomous pathways may manifest as either cancer or neurodegenerative disorders, depending on the cell affected. Disorders such as Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease, and ataxia-telangiectasia (AT) provide windows into several genetic determinants of cancer and neurodegenerative diseases. Genes in which dysregulation is capable of producing both outcomes include PARK2, ATM, PTEN, mTOR, APP, and PTPRD.
PARK2: cell cycle dysregulation
Modulation of the cell cycle requires a fine balance between a number of activating, inhibitory, and checkpoint proteins which together control progression through the phases of the cell cycle (G1, S, G2, mitosis), quiescence and re-entry (Deshpande et al., 2005). To achieve tight temporal control, the cyclin and CDK complexes that drive cell cycle progression are post-translationally regulated by ubiquitin-mediated degradation. The accumulation of CDK5 and cyclin E in the brains of PD patients first suggested that dysfunction in ubiquitination of cell cycle proteins might be related to the development of PD (Brion and Couck, 1995; Staropoli et al., 2003).
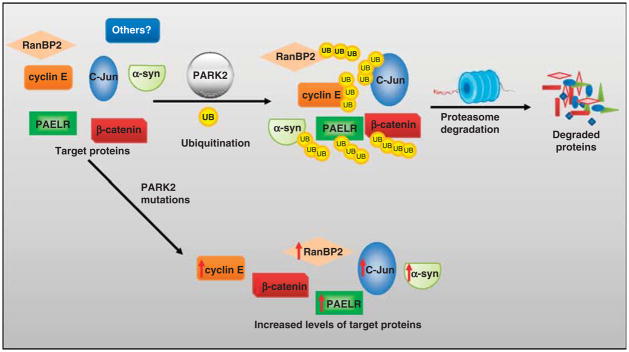
PD is one of the most frequent neurodegenerative disorders, with a prevalence of nearly 2% in those over 65 years of age. The typical motor signs of PD—resting tremor, rigidity, and bradykinesia—are the manifestation of massive loss of dopaminergic neurons within the pars compacta of the substantia nigra (Lucking et al., 2000). Mutation in the gene PARK2 is the most frequent cause of autosomal recessive early-onset PD, accounting for nearly 50% of early-onset cases. In this form of PD, germline PARK2 mutations cause loss of dopaminergic neurons within the substantia nigra (Fearnley and Lees, 1991; Kitada et al., 1998; Abbas et al., 1999; Lucking et al., 2000; Shimura et al., 2000). PARK2 is an E3 ubiquitin ligase with two really interesting new gene (RING) finger domains, acting within a multiprotein Skp1-Cullin1-F-box ligase complex (Shimura et al., 2000, 2001; Tanaka et al., 2001). This complex binds to UBCH7 and UBCH8 and thereby promotes mono-and poly-ubiquitination of target proteins, followed by proteasome-mediated degradation (Shimura et al., 2001; Corti et al., 2005; Sriram et al., 2005; Hampe et al., 2006). In neuronal systems, substrates for PARK2 include CDCrel-1 and 2a, synphilin-1, Pael-R, synaptotagmin XI, RanBP2, p38/AIMP2, FUSE-binding protein 1, Hsp70 and ataxin-2 (Huynh et al., 2007; Moore et al., 2008) (Figure 1). Which of these targets are the most physiologically significant for the disease state is unclear. A particularly interesting target protein of PARK2-mediated ubiquitination and degradation is cyclin E, the primary cyclin that drives S-phase progression. CDK2/cyclin E phosphorylates the tumor suppressor retinoblastoma, releasing the transcription factor E2F-1 from inhibition. In cycling cells, E2F-1 upregulates proteins facilitating progression through S phase, but in post-mitotic neurons, E2F-1 triggers apoptosis through p53 and Bax (Harbour et al., 1999; Nguyen et al., 2002; Staropoli et al., 2003).
Figure 1.
Summary of PARK2 mechanism of action and selected known targets of ubiquitination. PARK2 is an E3 ubiquitin ligase that normally facilitates the ubiquitination and subsequent degradation of target proteins. Mutations in PARK2 result in a failure to ubiquitinate target proteins and results in increased levels of these targets. UB denotes a ubiquitin moiety. α-syn, alpha-synuclein; C-Jun, c-Jun N-terminal kinase; PAELR, parkin-associated endothelin receptor-like receptor; RanBP2, RAN-binding protein 2.
By facilitating ubiquitin proteasome-mediated degradation of cyclin E and thereby inhibiting pro-apoptotic signaling, PARK2 is able to carry out a number of neuroprotective functions. In vitro evidence has shown that PARK2 protects neurons from a number of insults, including partial proteasome inhibition (Petrucelli et al., 2002; Yang et al., 2007), nerve growth factor withdrawal (Darios et al., 2003), and kainate excitotoxicity (Staropoli et al., 2003). These stressors are all associated with cell cycle dysregulation. Proteasome inhibition leads to apoptosis through CDK2 and CDK6 activation (Rideout et al., 2003). Withdrawal of nerve growth factor induces apoptosis which can be prevented with CDK inhibitors and dominant-negative CDK4 and CDK6 (Park et al., 1997, 1998). Kainate excitotoxicity is associated with upregulation of cyclin D, cyclin E, CDK2, and E2F-1 (Giardina and Beart, 2002; Verdaguer et al., 2002). The involvement of cyclin E in these pro-apoptotic pathways is substantial and the ubiquitination of cyclin E by PARK2 is one means by which this protein may exert its neuroprotective effects.
The precise mechanism by which mutations in PARK2 lead to PD remains unclear. However, it has been shown that the PARK2 mutations identified in early-onset PD result in deficient E3 ligase activity (Imai et al., 2000; Shimura et al., 2000; Zhang et al., 2000), abrogate binding to putative substrates (Imai et al., 2000), or otherwise cause loss of function (Sriram et al., 2005), allowing accumulation of cyclin E (Verdaguer et al., 2002) and other substrates. Overexpression of PARK2 protects neurons from kainate excitotoxicity-mediated apoptosis, and PARK2 knockdown through siRNA causes accumulation of cyclin E and sensitivity to kainate excitotoxicity in neurons (Staropoli et al., 2003). Kainate excitotoxicity of dopaminergic neurons is believed to be akin to glutamate excitotoxicity, which has been implicated in neuronal loss seen in sporadic PD (Olanow and Tatton, 1999). The sensitivity to excitotoxicity from PARK2 knockdown appears to be strongest in dopaminergic neurons (Staropoli et al., 2003). Therefore, inactivating mutations of PARK2 prevent ubiquitin proteasome-mediated degradation of cyclin E, rendering dopaminergic neurons in the midbrain sensitive to excitotoxicity and susceptible to apoptosis, leading to neuronal cell death and PD.
In parallel with PARK2’s relationship with cyclin E, PARK2 has also been shown to regulate signaling through the Wnt/β-catenin pathway. In vitro evidence reveals that PARK2 regulates β-catenin levels by reducing the ability of Wnt to stabilize β-catenin. Excessive signaling through this pathway in neurons cultured with Wnt3a results in cell cycle re-entry followed by apoptosis. Neurons in the ventral midbrain overexpress β-catenin, and Wnt3a administration leads to dopaminergic neuronal death in Park2 null mice, but not in Park2 wild-type mice (Rawal et al., 2009). Together, these data show that PARK2 protects dopaminergic neurons from excessive Wnt/β-catenin signaling.
In non-neuronal cycling cells, the roles of PARK2 in regulating cyclin E suggest that it could be an important inhibitor of cell cycle progression. Alterations in PARK2 expression have been shown in hepatocellular carcinoma cell lines (Mazieres et al., 2005; Lee et al., 2006). Recent work by our group has identified this gene as a tumor suppressor or suppressor of cancer cell growth, and functional data are consistent with a role in cell cycle control (Veeriah et al., 2009b). Chromosome 6q25.2–27 is a well known genomic region that frequently undergoes loss in multiple human cancers (Cesari et al., 2003; Weir et al., 2007; Parsons et al., 2008; TCGA, 2008; Toma et al., 2008). Our array comparative genomic hybridization data showed that chromosomal losses of PARK2 occur in approximately 25% of glioblastoma multiforme and colon cancer tumors. Interestingly, by comparing array comparative genomic hybridization profiles across tumor types, we showed that PARK2 is most likely the primary gene driving loss at 6q25.2–27 in cancer (Veeriah et al., 2009b). When we sequenced PARK2 in a number of human cancers, we found that somatic mutations occurred in the same domains as the germline mutations that cause early-onset PD, clustering in the ubiquitin-like, RING finger, and in-between RING finger domains. Many alterations were heterozygous in nature, suggesting that PARK2 may act in a haploinsufficient manner, like FBXW7/hCDC4, another E3 ubiquitin ligase targeting cyclin E (Mao et al., 2004). Expression of PARK2 in PARK2-deficient human cancer cell lines inhibited growth, but did not inhibit growth in cell lines with intact PARK2 expression. PARK2 cDNA harboring cancer-specific mutations were unable to inhibit growth. Similar results were obtained in vivo using glioblastoma xenografts. The PARK2 mutants showed reduced E3 ligase function, and were found to have a decreased ability to bind, ubiquitinate, and degrade cyclin E. Knockdown of PARK2 with siRNA resulted in accumulation of cyclin E, and an increased proportion of cells in S and G2/M phases. Immunofluorescence staining after PARK2 knockdown showed an increased frequency of multipolar spindles and abnormal mitoses, and an increased number of nuclei with atypia or micronuclei (Veeriah et al., 2009b).
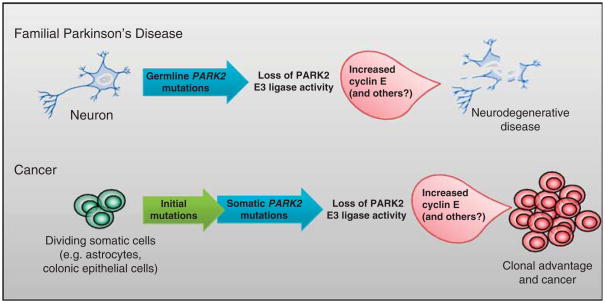
Together, these genetic and functional data show that PARK2 is a tumor suppressor, and that somatic mutations in PARK2 abrogate its ability to ubiquitinate cyclin E, promoting tumor growth (Figure 2). These data provide evidence for the hypothesis that a common factor—cyclin E-mediated cell cycle activation—drives both neurodegeneration and tumorigenesis in the setting of PARK2 mutation, depending on whether the mutation is somatic or germline. PARK2 mutation is known to cause autosomal recessive early-onset PD, but has not been definitively linked with cancer predisposition. Among general populations of PD patients, there is believed to be a marginal but significant increase in cancer risk (Moller et al., 1995; Olsen et al., 2005), but causes for this remain speculative, and it is unknown whether PARK2 may be responsible. PARK2 hypermethylation may also be contributory, and has been shown in hematologic malignancies (Agirre et al., 2006). Of note, cyclin E levels have been noted to be increased in a number of neurodegenerative diseases (Husseman et al., 2000) and in many human cancers (Donnellan and Chetty, 1999), lending further support to the possibility that cell cycle dysregulation may be a common link.
Figure 2.
Simplified model of PARK2 dysfunction in cancer and familial PD. In PD, mutational inactivation of PARK2 is sufficient to raise cyclin E levels, which helps bring about neurotoxicity. In cancer cells, PARK2 mutations occur in conjunction with other oncogenic alterations, resulting in increased tumor cell growth.
AT-mutated (ATM) and ATM-related protein: loss of cell cycle control and DNA repair
AT is a neurodegenerative disorder with a predisposition for cancer and radiation sensitivity (Lee and McKinnon, 2007). AT is characterized by a progressive loss of muscle coordination over the first few years of life, in addition to cutaneous and ocular telangiectasias (Chun and Gatti, 2004; Nowak-Wegrzyn et al., 2004). Patients develop immune deficiencies (decreased or absent IgA, IgE, and IgG), sterility, and cerebellar degeneration (Farina et al., 1994). Nearly 40% of AT-mutated (ATM) homozygotes will develop cancer, usually childhood leukemia or lymphoma. Solid tumors are less common, and include gastric adenocarcinoma, breast carcinoma, ovarian germ cell tumor, gonadoblastoma, and medulloblastoma (Ball and Xiao, 2005; Gumy-Pause et al., 2006; Mavrou et al., 2008).
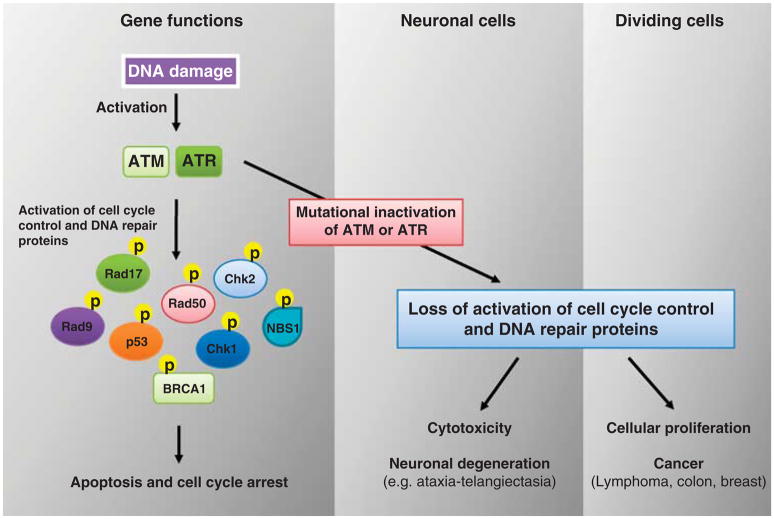
AT is caused by mutations in the gene ATM. Located on chromosome 11q23, more than 400 mutations have been described, encompassing all parts of the gene (Mavrou et al., 2008). Most ATM mutations are null mutations, leading to complete inactivation of the gene and absence of the ATM protein product, causing disease with nearly 100% penetrance (Prokopcova et al., 2007). Rare missense mutations with lesser penetrance have been reported (Milne, 2009). The ATM protein kinase is part of the phosphatidylinositol-3 kinase (PI3K) superfamily, and is integral to both cell cycle control and DNA repair pathways. In cases of double-strand DNA breaks, ATM auto-phosphorylates and activates a number of substrates. One target is the Rad50–Mre11–Nsb1 protein complex, which binds to and coordinates repair of double-strand breaks. In addition, ATM is a master controller of cell cycle checkpoints, facilitating either a halt to cellular proliferation or the induction of apoptosis (Bao et al., 2001; Falck et al., 2001; Mavrou et al., 2008; Staropoli, 2008). ATM exerts checkpoint control at the G1/S, S phase, and G2/M transitions. At the G1/S checkpoint, ATM phosphorylates p53, stabilizing the protein, and increasing its activity (Banin et al., 1998; Canman et al., 1998; Lavin and Kozlov, 2007). p53 then either inhibits the cell cycle by inducing expression of p21 and 14–3–3α, or induces Puma, Noxa, and Bax, causing apoptosis (Xiong et al., 1993; el-Deiry et al., 1993; Yu et al., 2003; Chipuk et al., 2004; Meulmeester et al., 2005). Similarly, ATM activation controls S phase, G2/M, and spindle checkpoints through activation of BRCA 1, FANCD2, CHK1, and CHK2 (Taniguchi et al., 2002). Through these and a wide variety of nearly 30 additional substrates, ATM has a central role in halting cell cycle progression or inducing apoptosis (Lavin and Kozlov, 2007) (Figure 3). Mutated ATM is unable to respond to double-strand DNA breaks with activation of DNA repair, or inhibition of the cell cycle, thereby predisposing cells to carcinogenesis.
Figure 3.
Model of differential effects of ATM and ATR mutation in neuronal vs dividing somatic cells. In neurons, ATM/ATR loss-of-function manifests as cell death and neurodegeneration. In normally dividing cells such as epithelial cells, the same mutations can result in the accumulation of additional mutations with each successive generation, eventually resulting in cancer. Yellow circles labeled with P denotes phosphorylation. Abbreviations: ATM, ataxia-telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; BRCA1, breast cancer 1; NBS1, nijmegen breakage syndrome 1 (nibrin); Rad17, RAD17 homolog.
ATM has been characterized as a susceptibility gene for several cancers. Epidemiologic studies have shown that heterozygous carriers of ATM mutations are at approximately twofold higher risk of developing breast cancer (Inskip et al., 1999; Thompson et al., 2005; Renwick et al., 2006), and even less penetrant missense mutations confer a significant increase in the risk of breast cancer (Gatti et al., 1999). Patients with homozygous and heterozygous ATM mutations are at significantly increased risk of various non-Hodgkin’s lymphomas (Morrell et al., 1986; Swift et al., 1986), and ATM deletions have been associated with up to 15% risk of developing non-Hodgkin’s lymphomas and B-cell chronic lymphocytic leukemia (Fegan et al., 1995).
In contrast to cancer risk, neurodegeneration resulting from ATM mutation is not as well understood. In AT, the precise mechanism for neurodegeneration has not been fully described. Germline disruption of ATM does not result in overt, immediate neurodegeneration. However, neural development is believed to be a process that is highly reliant on intact DNA repair systems. It has been hypothesized that ATM-deficient neurons, unable to repair double-strand breaks or undergo apoptosis, may incorporate DNA aberrations into the genome of neurons during development, triggering cell death at a later time (Orii et al., 2006; Lee and McKinnon, 2007; Subba Rao, 2007). It is unknown why granule and Purkinje cells of the cerebellum are particularly susceptible to these lesions.
In contrast to ATM, which is activated after double-strand DNA breaks, ATM-related protein (ATR) is activated by ultraviolet light-induced DNA damage and stalled DNA forks (Cliby et al., 1998). ATR promotes DNA repair in concert with the Rad9–Rad1–HUS1 and Rad17–RFC complexes (Rauen et al., 2000; Burtelow et al., 2001; Lindsey-Boltz et al., 2001; Roos-Mattjus et al., 2002). Similar to ATM, ATR exercises checkpoint control through Chk1 and Chk2, facilitates apoptosis through p53, and facilitates DNA repair through BRCA1 (Liang et al., 2009). ATR mutations have been identified in endometrial carcinomas, colon gastric cancers, and lymphomas (Lewis et al., 2005; Zighelboim et al., 2009). When in the germline, ATR mutations cause Seckel Syndrome, defined by growth retardation and microcephaly. As in AT, it is believed that neural development is highly dependent on intact DNA damage repair pathways, and that mutations in ATM or ATR lead to impaired neural development and degeneration of neurons (Alderton et al., 2004; O’Driscoll et al., 2006; Auclair et al., 2008).
Amyloid precursor protein: mitochondrial dysfunction
The APP gene encodes the amyloid precursor protein (APP). APP undergoes extensive post-translational modification, including initial cleavage by α-secretase and β-secretase, forming soluble APP (sAPP), and cleavage of the remaining 99 amino acid fragment by γ-secretase, producing amyloid-β (Aβ) (Nunan and Small, 2000). The 42 amino acids form of Aβ forms amyloid plaques that accumulate in brain regions affected by AD (Tabira et al., 2002). The diagnosis of AD requires the presence of extracellular deposits of Aβ and intracellular neurofibrillary tangles composed of tau protein. Several forms of familial early-onset AD have been linked to mutations in APP around the Aβ region, leading to increased production of Aβ42 (Anandatheerthavarada and Devi, 2007). The precise mechanism of neuronal cell death resulting from Aβ is attributable to mitochondrial dysfunction, through a number of mechanisms. Aβ inhibits key mitochondrial enzymes, notably cytochrome c oxidase, leading to the release of cytochrome c and resultant apoptosis. Furthermore, Aβ generates reactive oxygen and nitrogen species, and fragments mitochondrial DNA, all of which increase sensitivity to oxidative stress from free radical accumulation (Parker, 1991; Beal, 2005; Querfurth and LaFerla, 2010). APP gene overexpression is believed to result in accumulation of both APP and Aβ in the mitochondria, causing mitochondrial dysfunction, and eventually, neuronal death.
The Alzheimer’s-type neuropathology occurring in patients with Down syndrome has been attributed to trisomy 21 and accompanying upregulation of the APP gene on chromosome 21. The coexistent increased risk of hematologic malignancy in Down syndrome patients was an early suggestion that APP might predispose to cancer. In fact, there is a 10–20-fold increased risk of acute lymphoblastic leukemia and acute myeloid leukemia in Down syndrome children (Xavier et al., 2009). Although several genes, including APP, have been implicated in the risk of leukemia, APP is the most overexpressed gene in acute myeloid leukemia patients with complex karyotypes (Baldus et al., 2004). In solid tumors, APP has also been reported to be overexpressed in oral cavity, esophageal, pancreatic, neuroendocrine, thyroid, and colorectal cancers (Hansel et al., 2003; Ko et al., 2004; Arvidsson et al., 2008; Krause et al., 2008). APP is believed to be a growth factor, which has been shown to increase epithelial cell proliferation and migration, although the precise mechanism remains to be worked out (Schmitz et al., 2002). Several reports have suggested that this effect is attributable to the N-terminal cleavage product of APP, sAPP, which is formed after cleavage by α-secretase (Ko et al., 2004). After binding to an unknown receptor, sAPP is able to induce cellular proliferation, possibly through activation of the MAP kinase (Nishimura et al., 2003; Gakhar-Koppole et al., 2008), protein kinase C (Ishiguro et al., 1998), or PI3K/Akt/mammalian target of rapamycin (mTOR) pathways (Cheng et al., 2002). In fact, this is consistent with an overall, pro-growth, anti-apoptotic role for APP. In a situation of nerve growth factor withdrawal, Aβ production is upregulated, leading to neuronal apoptosis (Matrone et al., 2008). In this respect, APP activates both trophic (through sAPP) and apoptotic (through Aβ) pathways, and the predominance of each may be context dependent.
Beyond APP, mitochondrial dysfunction may also form a link between neurodegeneration and cancer through the actions of polo-like kinase 2 and α-synuclein. Mitochondrial dysfunction has been shown to induce high levels of polo-like kinase 2 expression, leading to phosphorylation of both α-synuclein, a hallmark of PD and Lewy body dementia, as well as PLK1, a promotor of cell proliferation that is overexpressed in several human cancers (Inglis et al., 2009; Matsumoto et al., 2009).
PTEN, PTEN-induced kinase 1, and DJ-1: sensitivity to oxidative stress
The gene phosphatase and tensin homolog (PTEN) encodes a protein that has a critical role in the PI3K/Akt/mTOR pathway. The primary role of the phosphatase protein encoded by PTEN is to dephosphorylate the inositol ring in phosphatidylinositol (3,4,5)-trisphosphate (PIP3), producing the biphosphate PIP2. In so doing, PTEN opposes the action of the PI3Ks and inhibits activation of the PI3K/Akt/mTOR signaling pathway, which ultimately promotes cell growth, survival, and metabolism (Wong et al., 2009). PTEN has recently been shown to be inhibited by P-REX2a (Fine et al., 2009). PTEN is a tumor suppressor, which has been found to be mutated in a large number of human cancers. When present in the germline, mutation of PTEN results in Cowden syndrome, as well as other PTEN-related hamartoma syndromes. Affected patients with Cowden syndrome develop benign hamartomas of the hair follicles (trichilemmomas), mucocutaneous surfaces, breasts, thyroid, and intestines (Eng, 2000). These patients also experience elevated risk of malignancies of the breast, thyroid, endometrium, and genitourinary tract. Somatic mutation or deletion of PTEN is a common event in high-grade glioblastoma (30–40% prevalence), melanoma (7–20%), prostate cancer (20–50%), and endometrial cancer (50%). In addition, PTEN mutations have been reported at lower prevalence in cancers of the bladder, lung, ovary, colon, and in lymphoma (Cairns et al., 1997; Gronbaek et al., 1998; Kim et al., 1998; Kohno et al., 1998; Sansal and Sellers, 2004).
PTEN itself has been hypothesized to have a direct role in neurodegeneration through mitochondria-dependent apoptosis in situations of oxidative stress. Although human data have not been reported, in rat hippocampal cells, oxidative stress leads to mitochondrial accumulation of PTEN, which is associated with Bax and cytochrome c, and activates caspase 3. Knockdown of PTEN inhibits caspase 3 activation and prevents neuronal apoptosis (Zhu et al., 2006).
There is evidence relating PTEN signaling to PD in humans, through two related genes: PTEN-induced kinase 1 (PINK1) and DJ-1. Both genes have a role in neuronal protection from mitochondrial damage in situations of oxidative stress, which are believed to be an important cause of neurodegenerative diseases such as PD. PINK1, which is transcriptionally activated by PTEN, has been associated with early-onset PD. PTEN deletion leads to downregulation of PINK1 (Inzelberg and Jankovic, 2007). A key role of PINK1 is to phosphorylate TRAP1, a mitochondrial molecular chaperone with anti-apoptotic function, preventing release of mitochondrial cytochrome c in cases of oxidative stress (Pridgeon et al., 2007). In dopaminergic neurons, inhibition of PINK1 leads to impaired mitochondrial function, as evidenced by loss of mitochondrial membrane potential and decreased mitochondrial ATP synthesis (Gegg et al., 2009). Through this mechanism, PINK1 deficiency causes sensitivity to oxidative stress, followed by neuronal death (Gispert et al., 2009). PINK1 also forms an E3 ligase complex with PARK2 (Xiong et al., 2009), and has recently been reported to recruit PARK2 to the mitochondria, where PARK2 has a role in facilitating mitochondrial autophagy. Through these mechanisms, PINK1 has an important neuroprotective role, loss of which causes neurodegeneration in PD (Vives-Bauza et al., 2009). Mutations in PINK1 have been reported in several kindreds with familial PD (Valente et al., 2004). Thus, loss of PTEN, commonly an oncogenic event, is also able to predispose to neurodegeneration through PINK1. It will be of interest to determine whether PINK1 is mutated in human cancers.
Dysregulation of PTEN and PD may also be related through DJ-1, a candidate oncogene that was originally identified as PARK7. In vitro, DJ-1 inhibits PTEN’s negative regulation of the PI3K/Akt/mTOR pathway, which has neuroprotective and pro-survival properties in situations of oxidative stress (Delgado-Esteban et al., 2007). Inhibition of DJ-1 with siRNA leads to cellular sensitivity to oxidative stress in Drosophila and mice brains (Kim et al., 2005). DJ-1 itself may be a sensor of reactive oxygen species (Shendelman et al., 2004). A small percentage of early-onset PD cases have been linked to loss-of-function mutations in DJ-1 (Bonifati et al., 2003). DJ-1 is also overexpressed in breast and lung cancers (Kim and Mak, 2006), consistent with its oncogenic role as an inhibitor of PTEN. Therefore, DJ-1 is both potentially oncogenic and neuroprotective. Together, DJ-1 and PINK1 show that PTEN, a tumor suppressor, is a ‘double-edged sword’ in the setting of central nervous system function–PTEN can mediate both neuroprotection and neurodegeneration. PTEN signaling is necessary for protection of neuronal cells from oxidative stress (through PINK1), but high levels of PTEN signaling (when DJ-1 function is decreased) cause hypersensitivity to oxidative stress through inhibition of the PI3K/Akt/mTOR pathway.
PTEN and Akt: regulators of tau and Aβ
In addition to its signaling role in oxidative stress, evidence has also linked PTEN to neurodegeneration in AD. Initially, Akt was believed to have pro-survival properties in neurons. In cell lines and mouse models, PI3K/Akt/mTOR pathway activation had been found to protect neurons from Aβ-induced neurotoxicity (Stein and Johnson, 2002; Wei et al., 2002). This was in line with the general function of the Akt pathway in promoting cell survival. This neuroprotective effect was believed to be mediated by glycogen synthase kinase 3. Activated Akt inhibits glycogen synthase kinase 3, preventing it from phosphorylating tau protein. Abnormally hyperphosphorylated tau is the primary component of the intraneuronal neurofibrillary tangles that, in addition to Aβ, help define AD pathologically (Lovestone and Reynolds, 1997). However, more recent evidence has contradicted the initial premise of Akt as a pro-survival signal in this setting. Instead of neuroprotection, Akt now appears to be associated with neurodegeneration in AD. Consistent evidence from several human studies indicates that AD brains contain higher levels of phosphorylated Akt than control brains, and that levels of p-Akt are correlated with the severity of neural degeneration on the Braak histologic staging system (Braak et al., 1996; Pei et al., 2003; Rickle et al., 2004, 2006). Further data have also shown significant loss of PTEN in the hippocampus of AD brains, indicating that PTEN loss and resultant constitutive Akt activation are present in AD (Griffin et al., 2005). Indeed, it is now appreciated that PTEN loss and Akt activation are associated with progressive neuronal loss (Marino et al., 2002; Chen et al., 2003), and that glycogen synthase kinase 3 is unlikely to have a role (Kerr et al., 2006).
There are currently two hypotheses for the role of PTEN and Akt in regulating phosphorylation of tau. The first is that Akt itself is a tau kinase, and activation of Akt predisposes toward accumulation of tau protein in neurons (Griffin et al., 2005). The second is that PTEN acts on tau through the MAP kinase pathway. PTEN, a lipid phosphatase, is known to also lead to the inhibition of ERK1/2, kinases that phosphorylate tau (Kerr et al., 2006). However, the in vivo significance of PTEN regulation of tau through the MAP kinase pathway remains unclear as this has only been shown in vitro. More recently, a separate role for Akt in regulating trafficking of APP has also been reported, and may hold implications for the pathogenesis of AD (Shineman et al., 2009). Activation of Akt can result in feedback inhibition of the PI3K/Akt/mTOR pathway through insulin receptor substrate 1, inhibiting PI3K. Although the mechanisms are unknown, inhibition of PI3K has been shown to decrease intracellular accumulation of Aβ. Therefore, PTEN and Akt have a role in the pathogenesis of AD. It remains unclear whether the primary pathway involves regulation of tau protein or APP, and more work will need to be done to clarify this. Nevertheless, these findings show that the regulatory roles of the PI3K/Akt/mTOR pathway include not only cell growth and survival, but also critical aspects of neuronal development and degeneration.
mTOR and TSC: regulators of autophagy
The mTOR protein, a key component of the PI3K/Akt/mTOR signaling pathway, may also have a role in neurodegenerative disease, as a regulator of autophagy. MTOR provides an illustrative example of the potential importance of autophagy as a contributor to both cancer and neuronal degeneration.
Autophagy is the process of sequestration of cytoplasm and organelles, which are delivered to the lysosome in vesicles and degraded. This process permits the maintenance of essential cellular functions in conditions of starvation, but also serves the role of eliminating damaged organelles or proteins, thereby protecting cells from death signals (Klionsky and Ohsumi, 1999; Levine and Yuan, 2005). Inhibition of autophagy is associated with neurodegeneration in otherwise normal neurons (Wang et al., 2009). With respect to cancer, autophagy can be both oncogenic, promoting tumor cell survival, as well as tumor suppressive, reducing DNA damage and inflammation (Gozuacik and Kimchi, 2004, 2007; Tsuchihara et al., 2009). The inherited neurodegenerative disorder Huntington’s disease is believed to be in partly because of a deficit in autophagy-mediated degradation of the mutant Huntingtin protein (Ravikumar et al., 2008). Rapamycin, an mTOR inhibitor, reduces Huntingtin accumulation and cell death in models of Huntington’s disease (Berger et al., 2006; Ravikumar and Rubinsztein, 2006). This has been attributed to inhibition of autophagy by mTOR, and induction of autophagy with rapamycin, both of which have been shown in Drosophila (Wang et al., 2009). PTEN and TSC, both tumor suppressors, have been shown to induce autophagy (Arico et al., 2001; Feng et al., 2005). Therefore, there is preliminary evidence to support a role for mTOR in neurodegeneration through inhibition of autophagy, but further investigation is required.
Protein tyrosine phosphatase delta: axonal development
Protein tyrosine phosphatase delta (PTPRD) was recently identified by our group and others as a tumor suppressor, which is frequently mutated in glioblastoma, lung, colon, melanoma, and head and neck cancers (Weir et al., 2007; Solomon et al., 2008; Veeriah et al., 2009a, b). Epigenetic silencing and reduced expression of PTPRD has also been identified in colon, breast, and glioblastoma tumors (Chan et al., 2008; Veeriah et al., 2009a). PTPRD is a protein tyrosine phosphatase that suppresses tumor growth by dephosphorylating the oncoprotein STAT3 (Chan et al., 2008; Chan and Heguy, 2009; Veeriah et al., 2009a). Interestingly, the PTPRD phosphatase was also recently identified in a genome-wide association study as a locus significantly associated with the restless legs syndrome. Restless legs syndrome is a neurological disorder with motor and sensory components, characterized by unpleasant sensations in the legs and an irresistible urge to move the legs. The mechanism of restless legs syndrome is unknown, although a familial neurodegenerative process is a possibility: 60% of cases are familial, and there is a pathologic evidence of dopamine dysfunction related to the substantia nigra (Connor et al., 2003). Receptor tyrosine phosphatases are believed to have critical roles in neural development, underpinning functions such as neuronal differentiation and migration (den Hertog et al., 1999). PTPRD in particular has been implicated in axonal growth and guidance in mammals, specifically in motor neurons (Bixby, 2000; Johnson and Van Vactor, 2003; Stepanek et al., 2005). Furthermore, investigators have observed that germline PTPRD mutations in mice affect motor neuron development and learning (Uetani et al., 2000, 2006).
Concluding remarks
Certain genetic alterations are able to contribute to either cancer or neurodegeneration. The genes PARK2, PTEN, mTOR, ATM, APP, and PTPRD provide instructive examples of a number of mechanisms by which these dichotomous outcomes may occur. Strongest evidence supports dual roles for PARK2 and ATM, in which the same mutation results in neuronal degeneration or cancer through similar molecular mechanisms, with outcome highly dependent on cellular context (that is germline vs somatic; neuron vs cycling cell). In the case of PTEN, mTOR, and related proteins, the risk of neurodegenerative disease shows that derangement of the PI3K/Akt/mTOR pathway has implications beyond carcinogenesis. In addition to its tumor suppressive role, PTEN appears to have central roles in neuronal survival through several pathways. The importance of autophagy as a potential common link between cancer and neurologic disease is suggested by mTOR, although further investigation is needed. In the cases of APP and PTPRD, alterations in the same gene appear to lead to cancer or neuronal dsyfunction through independent mechanisms. Indeed, the genes included in this review show the potential value of identifying shared risk factors. The early observation of increased cancer risk and Alzheimer’s-type neuropathology in patients with trisomy 21 prompted investigation into the role of APP in both diseases. The identification of PTPRD as a tumor suppressor and STAT3 inhibitor will encourage further investigation into the mechanism of predisposition to restless legs syndrome. These findings provide significant insight and may pay clinical dividends by stimulating development of biomarkers or therapeutic agents for affected patients.
Acknowledgments
We thank the Head and Neck Service, Department of Surgery at Memorial Sloan-Kettering Cancer Center for support (LGTM). LGTM is supported by National Cancer Institute T32 Grant CA009685. TAC is supported by the Doris Duke Charitable Foundation, the Society of Memorial Sloan-Kettering Foundation, the Flight Attendant Medical Research Institute, the Louis Gerstner Foundation, the Memorial Sloan-Kettering Brain Tumor Center, and the STARR Cancer Consortium.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Abbas N, Lucking CB, Ricard S, Durr A, Bonifati V, De Michele G, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson’s disease genetics study group and the European consortium on genetic susceptibility in parkinson’s disease. Hum Mol Genet. 1999;8:567–574. doi: 10.1093/hmg/8.4.567. [DOI] [PubMed] [Google Scholar]
- Ackman JB, Ramos RL, Sarkisian MR, Loturco JJ. Citron kinase is required for postnatal neurogenesis in the hippocampus. Dev Neurosci. 2007;29:113–123. doi: 10.1159/000096216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirre X, Roman-Gomez J, Vazquez I, Jimenez-Velasco A, Garate L, Montiel-Duarte C, et al. Abnormal methylation of the common PARK2 and PACRG promoter is associated with downregulation of gene expression in acute lymphoblastic leukemia and chronic myeloid leukemia. Int J Cancer. 2006;118:1945–1953. doi: 10.1002/ijc.21584. [DOI] [PubMed] [Google Scholar]
- Alderton GK, Joenje H, Varon R, Borglum AD, Jeggo PA, O’Driscoll M. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Hum Mol Genet. 2004;13:3127–3138. doi: 10.1093/hmg/ddh335. [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Devi L. Amyloid precursor protein and mitochondrial dysfunction in Alzheimer’s disease. Neuroscientist. 2007;13:626–638. doi: 10.1177/1073858407303536. [DOI] [PubMed] [Google Scholar]
- Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, et al. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–35246. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- Arvidsson Y, Andersson E, Bergstrom A, Andersson MK, Altiparmak G, Illerskog AC, et al. Amyloid precursor-like protein 1 is differentially upregulated in neuroendocrine tumours of the gastrointestinal tract. Endocr Relat Cancer. 2008;15:569–581. doi: 10.1677/ERC-07-0145. [DOI] [PubMed] [Google Scholar]
- Auclair Y, Rouget R, Affar el B, Drobetsky EA. ATR kinase is required for global genomic nucleotide excision repair exclusively during S phase in human cells. Proc Natl Acad Sci USA. 2008;105:17896–17901. doi: 10.1073/pnas.0801585105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus CD, Liyanarachchi S, Mrozek K, Auer H, Tanner SM, Guimond M, et al. Acute myeloid leukemia with complex karyotypes and abnormal chromosome 21: Amplification discloses overexpression of APP, ETS2, and ERG genes. Proc Natl Acad Sci USA. 2004;101:3915–3920. doi: 10.1073/pnas.0400272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LG, Xiao W. Molecular basis of ataxia telangiectasia and related diseases. Acta Pharmacol Sin. 2005;26:897–907. doi: 10.1111/j.1745-7254.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- Bao S, Tibbetts RS, Brumbaugh KM, Fang Y, Richardson DA, Ali A, et al. ATR/ATM-mediated phosphorylation of human Rad17 is required for genotoxic stress responses. Nature. 2001;411:969–974. doi: 10.1038/35082110. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- Bixby JL. Receptor tyrosine phosphatases in axon growth and guidance. Neuroreport. 2000;11:R5–10. doi: 10.1097/00001756-200007140-00001. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J. Pattern of brain destruction in Parkinson’s and Alzheimer’s diseases. J Neural Transm. 1996;103:455–490. doi: 10.1007/BF01276421. [DOI] [PubMed] [Google Scholar]
- Brion JP, Couck AM. Cortical and brainstem-type Lewy bodies are immunoreactive for the cyclin-dependent kinase 5. Am J Pathol. 1995;147:1465–1476. [PMC free article] [PubMed] [Google Scholar]
- Burtelow MA, Roos-Mattjus PM, Rauen M, Babendure JR, Karnitz LM. Reconstitution and molecular analysis of the hRad9-hHus1-hRad1 (9–1–1) DNA damage responsive checkpoint complex. J Biol Chem. 2001;276:25903–25909. doi: 10.1074/jbc.M102946200. [DOI] [PubMed] [Google Scholar]
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H, et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc Natl Acad Sci USA. 2003;100:5956–5961. doi: 10.1073/pnas.0931262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TA, Glockner S, Yi JM, Chen W, Van Neste L, Cope L, et al. Convergence of mutation and epigenetic alterations identifies common genes in cancer that predict for poor prognosis. PLoS Med. 2008;5:e114. doi: 10.1371/journal.pmed.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TA, Heguy A. The protein tyrosine phosphatase receptor D, a broadly inactivated tumor suppressor regulating STAT function. Cell Cycle. 2009;8:3063–3064. doi: 10.4161/cc.8.19.9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HK, Fernandez-Funez P, Acevedo SF, Lam YC, Kaytor MD, Fernandez MH, et al. Interaction of Akt-phosphorylated ataxin-1 with 14–3–3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003;113:457–468. doi: 10.1016/s0092-8674(03)00349-0. [DOI] [PubMed] [Google Scholar]
- Cheng G, Yu Z, Zhou D, Mattson MP. Phosphatidylinositol-3-kinase-Akt kinase and p42/p44 mitogen-activated protein kinases mediate neurotrophic and excitoprotective actions of a secreted form of amyloid precursor protein. Exp Neurol. 2002;175:407–414. doi: 10.1006/exnr.2002.7920. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Chun HH, Gatti RA. Ataxia-telangiectasia, an evolving phenotype. DNA Repair (Amst) 2004;3:1187–1196. doi: 10.1016/j.dnarep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, et al. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Ondo WG, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–309. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- Corti O, Hampe C, Darios F, Ibanez P, Ruberg M, Brice A. Parkinson’s disease: from causes to mechanisms. C R Biol. 2005;328:131–142. doi: 10.1016/j.crvi.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Darios F, Corti O, Lucking CB, Hampe C, Muriel MP, Abbas N, et al. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum Mol Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- Delgado-Esteban M, Martin-Zanca D, Andres-Martin L, Almeida A, Bolanos JP. Inhibition of PTEN by peroxynitrite activates the phosphoinositide-3-kinase/Akt neuroprotective signaling pathway. J Neurochem. 2007;102:194–205. doi: 10.1111/j.1471-4159.2007.04450.x. [DOI] [PubMed] [Google Scholar]
- den Hertog J, Blanchetot C, Buist A, Overvoorde J, van der Sar A, Tertoolen LG. Receptor protein-tyrosine phosphatase signalling in development. Int J Dev Biol. 1999;43:723–733. [PubMed] [Google Scholar]
- Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–2915. doi: 10.1038/sj.onc.1208618. [DOI] [PubMed] [Google Scholar]
- Donnellan R, Chetty R. Cyclin E in human cancers. FASEB J. 1999;13:773–780. doi: 10.1096/fasebj.13.8.773. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000;37:828–830. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radio-resistant DNA synthesis. Nature. 2001;410:842–847. doi: 10.1038/35071124. [DOI] [PubMed] [Google Scholar]
- Farina L, Uggetti C, Ottolini A, Martelli A, Bergamaschi R, Sibilla L, et al. Ataxia-telangiectasia: MR and CT findings. J Comput Assist Tomogr. 1994;18:724–727. [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fegan C, Robinson H, Thompson P, Whittaker JA, White D. Karyotypic evolution in CLL: identification of a new sub-group of patients with deletions of 11q and advanced or progressive disease. Leukemia. 1995;9:2003–2008. [PubMed] [Google Scholar]
- Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine B, Hodakoski C, Koujak S, Su T, Saal LH, Maurer M, et al. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science. 2009;325:1261–1265. doi: 10.1126/science.1173569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakhar-Koppole N, Hundeshagen P, Mandl C, Weyer SW, Allinquant B, Muller U, et al. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur J Neurosci. 2008;28:871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- Gatti RA, Tward A, Concannon P. Cancer risk in ATM heterozygotes: a model of phenotypic and mechanistic differences between missense and truncating mutations. Mol Genet Metab. 1999;68:419–423. doi: 10.1006/mgme.1999.2942. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Schapira AH, Taanman JW. Silencing of PINK1 expression affects mitochondrial DNA and oxidative phosphorylation in dopaminergic cells. PLoS One. 2009;4:e4756. doi: 10.1371/journal.pone.0004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina SF, Beart PM. Kainate receptor-mediated apoptosis in primary cultures of cerebellar granule cells is attenuated by mitogen-activated protein and cyclin-dependent kinase inhibitors. Br J Pharmacol. 2002;135:1733–1742. doi: 10.1038/sj.bjp.0704636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken HH, Becker D, et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy and cell death. Curr Top Dev Biol. 2007;78:217–245. doi: 10.1016/S0070-2153(06)78006-1. [DOI] [PubMed] [Google Scholar]
- Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- Gronbaek K, Zeuthen J, Guldberg P, Ralfkiaer E, Hou-Jensen K. Alterations of the MMAC1/PTEN gene in lymphoid malignancies. Blood. 1998;91:4388–4390. [PubMed] [Google Scholar]
- Gumy-Pause F, Wacker P, Maillet P, Betts DR, Sappino AP. ATM variants and predisposition to childhood T-lineage acute lymphoblastic leukaemia. Leukemia. 2006;20:526–527. doi: 10.1038/sj.leu.2404091. author reply 527. [DOI] [PubMed] [Google Scholar]
- Hampe C, Ardila-Osorio H, Fournier M, Brice A, Corti O. Biochemical analysis of Parkinson’s disease-causing variants of Parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum Mol Genet. 2006;15:2059–2075. doi: 10.1093/hmg/ddl131. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Rahman A, Wehner S, Herzog V, Yeo CJ, Maitra A. Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res. 2003;63:7032–7037. [PubMed] [Google Scholar]
- Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- Husseman JW, Nochlin D, Vincent I. Mitotic activation: a convergent mechanism for a cohort of neurodegenerative diseases. Neurobiol Aging. 2000;21:815–828. doi: 10.1016/s0197-4580(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Huynh DP, Nguyen DT, Pulst-Korenberg JB, Brice A, Pulst SM. Parkin is an E3 ubiquitin-ligase for normal and mutant ataxin-2 and prevents ataxin-2-induced cell death. Exp Neurol. 2007;203:531–541. doi: 10.1016/j.expneurol.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275:35661–35664. doi: 10.1074/jbc.C000447200. [DOI] [PubMed] [Google Scholar]
- Inglis KJ, Chereau D, Brigham EF, Chiou SS, Schobel S, Frigon NL, et al. Polo-like kinase 2 (PLK2) phosphorylates alpha-synuclein at serine 129 in central nervous system. J Biol Chem. 2009;284:2598–2602. doi: 10.1074/jbc.C800206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskip HM, Kinlen LJ, Taylor AM, Woods CG, Arlett CF. Risk of breast cancer and other cancers in heterozygotes for ataxia-telangiectasia. Br J Cancer. 1999;79:1304–1307. doi: 10.1038/sj.bjc.6690209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzelberg R, Jankovic J. Are Parkinson disease patients protected from some but not all cancers? Neurology. 2007;69:1542–1550. doi: 10.1212/01.wnl.0000277638.63767.b8. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Ohsawa I, Takamura C, Morimoto T, Kohsaka S. Secreted form of beta-amyloid precursor protein activates protein kinase C and phospholipase Cgamma1 in cultured embryonic rat neocortical cells. Brain Res Mol Brain Res. 1998;53:24–32. doi: 10.1016/s0169-328x(97)00280-5. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Van Vactor D. Receptor protein tyrosine phosphatases in nervous system development. Physiol Rev. 2003;83:1–24. doi: 10.1152/physrev.00016.2002. [DOI] [PubMed] [Google Scholar]
- Kerr F, Rickle A, Nayeem N, Brandner S, Cowburn RF, Lovestone S. PTEN, a negative regulator of PI3 kinase signalling, alters tau phosphorylation in cells by mechanisms independent of GSK-3. FEBS Lett. 2006;580:3121–3128. doi: 10.1016/j.febslet.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Kim RH, Mak TW. Tumours and tremors: how PTEN regulation underlies both. Br J Cancer. 2006;94:620–624. doi: 10.1038/sj.bjc.6602994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Su LK, Oh Y, Kemp BL, Hong WK, Mao L. Alterations of PTEN/MMAC1, a candidate tumor suppressor gene, and its homologue, PTH2, in small cell lung cancer cell lines. Oncogene. 1998;16:89–93. doi: 10.1038/sj.onc.1201512. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Ohsumi Y. Vacuolar import of proteins and organelles from the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:1–32. doi: 10.1146/annurev.cellbio.15.1.1. [DOI] [PubMed] [Google Scholar]
- Ko SY, Lin SC, Chang KW, Wong YK, Liu CJ, Chi CW, et al. Increased expression of amyloid precursor protein in oral squamous cell carcinoma. Int J Cancer. 2004;111:727–732. doi: 10.1002/ijc.20328. [DOI] [PubMed] [Google Scholar]
- Kohno T, Takahashi M, Manda R, Yokota J. Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosomes Cancer. 1998;22:152–156. doi: 10.1002/(sici)1098-2264(199806)22:2<152::aid-gcc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Krause K, Karger S, Sheu SY, Aigner T, Kursawe R, Gimm O, et al. Evidence for a role of the amyloid precursor protein in thyroid carcinogenesis. J Endocrinol. 2008;198:291–299. doi: 10.1677/JOE-08-0005. [DOI] [PubMed] [Google Scholar]
- Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, et al. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41:549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–942. doi: 10.4161/cc.6.8.4180. [DOI] [PubMed] [Google Scholar]
- Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901–1915. doi: 10.2741/1933. [DOI] [PubMed] [Google Scholar]
- Lee Y, McKinnon PJ. Responding to DNA double strand breaks in the nervous system. Neuroscience. 2007;145:1365–1374. doi: 10.1016/j.neuroscience.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KA, Mullany S, Thomas B, Chien J, Loewen R, Shridhar V, et al. Heterozygous ATR mutations in mismatch repair-deficient cancer cells have functional significance. Cancer Res. 2005;65:7091–7095. doi: 10.1158/0008-5472.CAN-05-1019. [DOI] [PubMed] [Google Scholar]
- Liang Y, Lin SY, Brunicardi FC, Goss J, Li K. DNA damage response pathways in tumor suppression and cancer treatment. World J Surg. 2009;33:661–666. doi: 10.1007/s00268-008-9840-1. [DOI] [PubMed] [Google Scholar]
- Lindsey-Boltz LA, Bermudez VP, Hurwitz J, Sancar A. Purification and characterization of human DNA damage checkpoint Rad complexes. Proc Natl Acad Sci USA. 2001;98:11236–11241. doi: 10.1073/pnas.201373498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovestone S, Reynolds CH. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience. 1997;78:309–324. doi: 10.1016/s0306-4522(96)00577-5. [DOI] [PubMed] [Google Scholar]
- Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, Gasser T, et al. Association between early-onset Parkinson’s disease and mutations in the parkin gene. N Engl J Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- Mao JH, Perez-Losada J, Wu D, Delrosario R, Tsunematsu R, Nakayama KI, et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature. 2004;432:775–779. doi: 10.1038/nature03155. [DOI] [PubMed] [Google Scholar]
- Marino S, Krimpenfort P, Leung C, van der Korput HA, Trapman J, Camenisch I, et al. PTEN is essential for cell migration but not for fate determination and tumourigenesis in the cerebellum. Development. 2002;129:3513–3522. doi: 10.1242/dev.129.14.3513. [DOI] [PubMed] [Google Scholar]
- Matrone C, Di Luzio A, Meli G, D’Aguanno S, Severini C, Ciotti MT, et al. Activation of the amyloidogenic route by NGF deprivation induces apoptotic death in PC12 cells. J Alzheimers Dis. 2008;13:81–96. doi: 10.3233/jad-2008-13109. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Wang PY, Ma W, Sung HJ, Matoba S, Hwang PM. Polo-like kinases mediate cell survival in mitochondrial dysfunction. Proc Natl Acad Sci USA. 2009;106:14542–14546. doi: 10.1073/pnas.0904229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrou A, Tsangaris GT, Roma E, Kolialexi A. The ATM gene and ataxia telangiectasia. Anticancer Res. 2008;28:401–405. [PubMed] [Google Scholar]
- Mazieres J, He B, You L, Xu Z, Jablons DM. Wnt signaling in lung cancer. Cancer Lett. 2005;222:1–10. doi: 10.1016/j.canlet.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Meulmeester E, Pereg Y, Shiloh Y, Jochemsen AG. ATM-mediated phosphorylations inhibit Mdmx/Mdm2 stabilization by HAUSP in favor of p53 activation. Cell Cycle. 2005;4:1166–1170. doi: 10.4161/cc.4.9.1981. [DOI] [PubMed] [Google Scholar]
- Milne RL. Variants in the ATM gene and breast cancer susceptibility. Genome Med. 2009;1:12. doi: 10.1186/gm12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller H, Mellemkjaer L, McLaughlin JK, Olsen JH. Occurrence of different cancers in patients with Parkinson’s disease. BMJ. 1995;310:1500–1501. doi: 10.1136/bmj.310.6993.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dikeman DA, Dawson VL, Dawson TM. Parkin mediates the degradation-independent ubiquitination of Hsp70. J Neurochem. 2008;105:1806–1819. doi: 10.1111/j.1471-4159.2008.05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell D, Cromartie E, Swift M. Mortality and cancer incidence in 263 patients with ataxia-telangiectasia. J Natl Cancer Inst. 1986;77:89–92. [PubMed] [Google Scholar]
- Nguyen MD, Mushynski WE, Julien JP. Cycling at the interface between neurodevelopment and neurodegeneration. Cell Death Differ. 2002;9:1294–1306. doi: 10.1038/sj.cdd.4401108. [DOI] [PubMed] [Google Scholar]
- Nishimura I, Takazaki R, Kuwako K, Enokido Y, Yoshikawa K. Upregulation and antiapoptotic role of endogenous Alzheimer amyloid precursor protein in dorsal root ganglion neurons. Exp Cell Res. 2003;286:241–251. doi: 10.1016/s0014-4827(03)00066-1. [DOI] [PubMed] [Google Scholar]
- Nouspikel T, Hanawalt PC. When parsimony backfires: neglecting DNA repair may doom neurons in Alzheimer’s disease. Bioessays. 2003;25:168–173. doi: 10.1002/bies.10227. [DOI] [PubMed] [Google Scholar]
- Nowak-Wegrzyn A, Crawford TO, Winkelstein JA, Carson KA, Lederman HM. Immunodeficiency and infections in ataxia-telangiectasia. J Pediatr. 2004;144:505–511. doi: 10.1016/j.jpeds.2003.12.046. [DOI] [PubMed] [Google Scholar]
- Nunan J, Small DH. Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 2000;483:6–10. doi: 10.1016/s0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- O’Driscoll M, Jackson AP, Jeggo PA. Microcephalin: a causal link between impaired damage response signalling and microcephaly. Cell Cycle. 2006;5:2339–2344. doi: 10.4161/cc.5.20.3358. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Olsen JH, Friis S, Frederiksen K, McLaughlin JK, Mellemkjaer L, Moller H. Atypical cancer pattern in patients with Parkinson’s disease. Br J Cancer. 2005;92:201–205. doi: 10.1038/sj.bjc.6602279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orii KE, Lee Y, Kondo N, McKinnon PJ. Selective utilization of nonhomologous end-joining and homologous recombination DNA repair pathways during nervous system development. Proc Natl Acad Sci USA. 2006;103:10017–10022. doi: 10.1073/pnas.0602436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Levine B, Ferrari G, Greene LA. Cyclin dependent kinase inhibitors and dominant negative cyclin dependent kinase 4 and 6 promote survival of NGF-deprived sympathetic neurons. J Neurosci. 1997;17:8975–8983. doi: 10.1523/JNEUROSCI.17-23-08975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Morris EJ, Stefanis L, Troy CM, Shelanski ML, Geller HM, et al. Multiple pathways of neuronal death induced by DNA-damaging agents, NGF deprivation, and oxidative stress. J Neurosci. 1998;18:830–840. doi: 10.1523/JNEUROSCI.18-03-00830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WD., Jr Cytochrome oxidase deficiency in Alzheimer’s disease. Ann N Y Acad Sci. 1991;640:59–64. doi: 10.1111/j.1749-6632.1991.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JJ, Khatoon S, An WL, Nordlinder M, Tanaka T, Braak H, et al. Role of protein kinase B in Alzheimer’s neurofibrillary pathology. Acta Neuropathol. 2003;105:381–392. doi: 10.1007/s00401-002-0657-y. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, O’Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopcova J, Kleibl Z, Banwell CM, Pohlreich P. The role of ATM in breast cancer development. Breast Cancer Res Treat. 2007;104:121–128. doi: 10.1007/s10549-006-9406-6. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Rauen M, Burtelow MA, Dufault VM, Karnitz LM. The human checkpoint protein hRad17 interacts with the PCNA-like proteins hRad1, hHus1, and hRad9. J Biol Chem. 2000;275:29767–29771. doi: 10.1074/jbc.M005782200. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Rubinsztein DC. Role of autophagy in the clearance of mutant huntingtin: a step towards therapy? Mol Aspects Med. 2006;27:520–527. doi: 10.1016/j.mam.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Rubinsztein DC. Clearance of mutant aggregate-prone proteins by autophagy. Methods Mol Biol. 2008;445:195–211. doi: 10.1007/978-1-59745-157-4_13. [DOI] [PubMed] [Google Scholar]
- Rawal N, Corti O, Sacchetti P, Ardilla-Osorio H, Sehat B, Brice A, et al. Parkin protects dopaminergic neurons from excessive Wnt/beta-catenin signaling. Biochem Biophys Res Commun. 2009;388:473–478. doi: 10.1016/j.bbrc.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38:873–875. doi: 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- Rickle A, Bogdanovic N, Volkman I, Winblad B, Ravid R, Cowburn RF. Akt activity in Alzheimer’s disease and other neurodegenerative disorders. Neuroreport. 2004;15:955–959. doi: 10.1097/00001756-200404290-00005. [DOI] [PubMed] [Google Scholar]
- Rickle A, Bogdanovic N, Volkmann I, Zhou X, Pei JJ, Winblad B, et al. PTEN levels in Alzheimer’s disease medial temporal cortex. Neurochem Int. 2006;48:114–123. doi: 10.1016/j.neuint.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Rideout HJ, Wang Q, Park DS, Stefanis L. Cyclin-dependent kinase activity is required for apoptotic death but not inclusion formation in cortical neurons after proteasomal inhibition. J Neurosci. 2003;23:1237–1245. doi: 10.1523/JNEUROSCI.23-04-01237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos-Mattjus P, Vroman BT, Burtelow MA, Rauen M, Eapen AK, Karnitz LM. Genotoxin-induced Rad9-Hus1-Rad1 (9–1–1) chromatin association is an early checkpoint signaling event. J Biol Chem. 2002;277:43809–43812. doi: 10.1074/jbc.M207272200. [DOI] [PubMed] [Google Scholar]
- Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Tikkanen R, Kirfel G, Herzog V. The biological role of the Alzheimer amyloid precursor protein in epithelial cells. Histochem Cell Biol. 2002;117:171–180. doi: 10.1007/s00418-001-0351-5. [DOI] [PubMed] [Google Scholar]
- Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R, et al. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson’s disease. Science. 2001;293:263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- Shineman DW, Dain AS, Kim ML, Lee VM. Constitutively active Akt inhibits trafficking of amyloid precursor protein and amyloid precursor protein metabolites through feedback inhibition of phosphoinositide 3-kinase. Biochemistry. 2009;48:3787–3794. doi: 10.1021/bi802070j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DA, Kim JS, Cronin JC, Sibenaller Z, Ryken T, Rosenberg SA, et al. Mutational inactivation of PTPRD in glioblastoma multiforme and malignant melanoma. Cancer Res. 2008;68:10300–10306. doi: 10.1158/0008-5472.CAN-08-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram SR, Li X, Ko HS, Chung KK, Wong E, Lim KL, et al. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet. 2005;14:2571–2586. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- Staropoli JF. Tumorigenesis and neurodegeneration: two sides of the same coin? Bioessays. 2008;30:719–727. doi: 10.1002/bies.20784. [DOI] [PubMed] [Google Scholar]
- Staropoli JF, McDermott C, Martinat C, Schulman B, Demireva E, Abeliovich A. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron. 2003;37:735–749. doi: 10.1016/s0896-6273(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Stein TD, Johnson JA. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein is associated with increased levels of transthyretin and the activation of cell survival pathways. J Neurosci. 2002;22:7380–7388. doi: 10.1523/JNEUROSCI.22-17-07380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanek L, Stoker AW, Stoeckli E, Bixby JL. Receptor tyrosine phosphatases guide vertebrate motor axons during development. J Neurosci. 2005;25:3813–3823. doi: 10.1523/JNEUROSCI.4531-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba Rao K. Mechanisms of disease: DNA repair defects and neurological disease. Nat Clin Pract Neurol. 2007;3:162–172. doi: 10.1038/ncpneuro0448. [DOI] [PubMed] [Google Scholar]
- Swift M, Morrell D, Cromartie E, Chamberlin AR, Skolnick MH, Bishop DT. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet. 1986;39:573–583. [PMC free article] [PubMed] [Google Scholar]
- Tabira T, Chui DH, Kuroda S. Significance of intracellular Abeta42 accumulation in Alzheimer’s disease. Front Biosci. 2002;7:a44–a49. doi: 10.2741/tabira. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Suzuki T, Chiba T, Shimura H, Hattori N, Mizuno Y. Parkin is linked to the ubiquitin pathway. J Mol Med. 2001;79:482–494. doi: 10.1007/s001090100242. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Garcia-Higuera I, Xu B, Andreassen PR, Gregory RC, Kim ST, et al. Convergence of the fanconi anemia and ataxia telangiectasia signaling pathways. Cell. 2002;109:459–472. doi: 10.1016/s0092-8674(02)00747-x. [DOI] [PubMed] [Google Scholar]
- TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways (Cancer Genome Atlas Network) Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Duedal S, Kirner J, McGuffog L, Last J, Reiman A, et al. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst. 2005;97:813–822. doi: 10.1093/jnci/dji141. [DOI] [PubMed] [Google Scholar]
- Toma MI, Grosser M, Herr A, Aust DE, Meye A, Hoefling C, et al. Loss of heterozygosity and copy number abnormality in clear cell renal cell carcinoma discovered by high-density affymetrix 10K single nucleotide polymorphism mapping array. Neoplasia. 2008;10:634–642. doi: 10.1593/neo.08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihara K, Fujii S, Esumi H. Autophagy and cancer: dynamism of the metabolism of tumor cells and tissues. Cancer Lett. 2009;278:130–138. doi: 10.1016/j.canlet.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci. 2006;26:5872–5880. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetani N, Kato K, Ogura H, Mizuno K, Kawano K, Mikoshiba K, et al. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 2000;19:2775–2785. doi: 10.1093/emboj/19.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Veeriah S, Brennan C, Meng S, Singh B, Fagin JA, Solit DB, et al. The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc Natl Acad Sci USA. 2009a;106:9435–9440. doi: 10.1073/pnas.0900571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeriah S, Taylor BS, Meng S, Fang F, Yilmaz E, Vivanco I, et al. Somatic mutations of the Parkinson’s disease-associated gene PARK2 in glioblastoma and other human malignancies. Nat Genet. 2009b;42:77–82. doi: 10.1038/ng.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdaguer E, Garcia-Jorda E, Canudas AM, Dominguez E, Jimenez A, Pubill D, et al. Kainic acid-induced apoptosis in cerebellar granule neurons: an attempt at cell cycle re-entry. Neuroreport. 2002;13:413–416. doi: 10.1097/00001756-200203250-00010. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA. 2009;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Lao U, Edgar BA. TOR-mediated autophagy regulates cell death in Drosophila neurodegenerative disease. J Cell Biol. 2009;186:703–711. doi: 10.1083/jcb.200904090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Wang X, Kusiak JW. Signaling events in amyloid beta-peptide-induced neuronal death and insulin-like growth factor I protection. J Biol Chem. 2002;277:17649–17656. doi: 10.1074/jbc.M111704200. [DOI] [PubMed] [Google Scholar]
- Weir BA, Woo MS, Getz G, Perner S, Ding L, Beroukhim R, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2009 doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier AC, Ge Y, Taub JW. Down syndrome and malignancies: a unique clinical relationship: a paper from the 2008 william beaumont hospital symposium on molecular pathology. J Mol Diagn. 2009;11:371–380. doi: 10.2353/jmoldx.2009.080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Wang D, Chen L, Choo YS, Ma H, Tang C, et al. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest. 2009;119:650–660. doi: 10.1172/JCI37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhou HY, Li B, Niu GZ, Chen SD. Downregulation of parkin damages antioxidant defenses and enhances proteasome inhibition-induced toxicity in PC12 cells. J Neuroimmune Pharmacol. 2007;2:276–283. doi: 10.1007/s11481-007-9082-2. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao J, Chung KK, Huang H, Dawson VL, Dawson TM. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hoell P, Ahlemeyer B, Krieglstein J. PTEN: a crucial mediator of mitochondria-dependent apoptosis. Apoptosis. 2006;11:197–207. doi: 10.1007/s10495-006-3714-5. [DOI] [PubMed] [Google Scholar]
- Zighelboim I, Schmidt AP, Gao F, Thaker PH, Powell MA, Rader JS, et al. ATR mutation in endometrioid endometrial cancer is associated with poor clinical outcomes. J Clin Oncol. 2009;27:3091–3096. doi: 10.1200/JCO.2008.19.9802. [DOI] [PMC free article] [PubMed] [Google Scholar]