Abstract
Objective
To compare outcomes of a pediatric cohort of patients compared with a matched cohort of adult patients, all diagnosed as having squamous cell carcinoma (SCC) of the oral tongue. Outcomes of oral cancer in pediatric patients have not been studied, to our knowledge.
Design
Retrospective matched-pair cohort study.
Setting
Memorial Sloan-Kettering Cancer Center, New York, New York.
Patients
A total of 10 pediatric and 40 adult patients diagnosed as having SCC of the oral tongue.
Main Outcome Measures
Overall survival (OS), disease-specific survival (DSS), and recurrence-free survival (RFS).
Results
The 5-year OS was equivalent in the 2 groups: 70% in the pediatric group and 64% in the adult group (P=.97). The 5-year DSS was also equivalent: 80% in the pediatric group and 76% in the adult group (P=.90). The 5-year RFS was 70% in the pediatric group and 78% in the adult group (P=.54).
Conclusions
When pediatric and adult patients were matched for sex, tobacco use history, TNM status, surgical procedure, and adjuvant radiotherapy, outcomes for OS, DSS, and RFS were equivalent. Pediatric patients with SCC of the oral tongue should be treated similarly to adult patients.
Squamous cell carcinoma (SCC) of the head and neck is uncommon in young adults, and rare in the pediatric age group. Approximately 1 in 1000 cases of head and neck SCC will occur in patients 20 years or younger.1–4 While there is an extensive literature examining outcomes of SCC in young adults (<40 years), to our knowledge, there is no literature on SCC in pediatric patients, other than isolated case reports.1,3,5–13 Squamous cell carcinoma in young patients is believed to be etiologically distinct from SCC in older adults owing to less significant exposure to risk factors such as tobacco and alcohol. This distinction in etiology would be especially pronounced in pediatric patients, in whom genetic syndromes such as Fanconi anemia, xeroderma pigmentosum, keratosis-ichthyosis-deafness (KID) syndrome,8,10,13 or other unidentified genetic risk factors may be contributory.
Numerous early reports of SCC in young adults concluded that the disease was more aggressive, and prognosis was poorer, than in older adults.14–16 Several recent studies, however, have provided evidence for the contrary.17–24 To our knowledge, outcomes data specific to the pediatric age group have not been reported and represent a missing piece of the literature on head and neck SCC in young patients.
In young patients with head and neck SCC, the oral tongue is the most common primary site.22,25,26 We report the outcomes of a pediatric cohort of patients with SCC of the oral tongue who were treated at a comprehensive cancer center and compare outcomes with those of an adult cohort matched for sex, smoking history, stage, and treatment modality. Although such a study would of necessity be small, these outcomes data have not yet been reported.
METHODS
Ten pediatric patients (aged ≤20 years) with SCC of the oral tongue were identified on review of medical records of patients undergoing treatment at Memorial Sloan-Kettering Cancer Center, New York, New York, for the years 1983 to 2009. Adult patients with SCC of the oral tongue were matched to the pediatric patients for sex, history of tobacco exposure, tumor status, nodal status, distant metastasis status, surgical procedure, and administration of adjuvant radiotherapy. Testing for human papillomavirus status was not routinely performed during this time period. A power analysis estimated that a 4:1 adult to pediatric patient ratio would provide a sample size sufficient to detect a clinically meaningful survival difference (hazard ratio, 1.5). Therefore, 40 adult patients were matched. Because few pediatric patients were smokers, 8 adults were not able to be matched on this 1 variable. Pertinent patient, tumor, treatment-related, and outcome details were recorded from hospital medical charts. This study was approved by the institutional review board.
The null hypothesis was that rates of overall survival (OS), disease-specific survival (DSS), and recurrence-free survival (RFS) would not differ between the pediatric and adult cohorts. The 2 groups were compared for demographic, tumor, and treatment characteristics with continuous (t test) and categorical (χ2 and Fisher exact) statistics. The OS, DSS, and RFS were calculated using the Kaplan-Meier method, and comparisons made with the 2-tailed log-rank test. The a priori level of α was .05. All analyses were performed with SPSS statistical software (version 17.0; SPSS Inc, Chicago, Illinois).
RESULTS
PEDIATRIC COHORT
From 1983 to 2009, 10 patients ages 15 to 20 years were treated for primary SCC of the oral tongue at Memorial Sloan-Kettering Cancer Center. Patient and tumor characteristics are summarized in the Table. The median duration of follow-up was 48 months (range, 6–320 months). The youngest patient was 15 years old at the time of diagnosis. One patient had a known risk factor for cancer (Fanconi anemia). One patient had a history of tobacco exposure, and 2 patients had a history of social alcohol use.
Table.
Patient and Tumor Characteristics: Pediatric and Adult Patients With Squamous Cell Carcinoma of the Oral Surface of the Tonguea
| Variable | Pediatric (n=10) | Adult (n=40) | P Value |
|---|---|---|---|
| Sex | |||
| Male | 2 (20) | 8 (20) | .99 |
| Female | 8 (80) | 32 (80) | |
| Tobacco history | |||
| Yes | 1 (11) | 18 (53) | .06 |
| Never | 7 (78) | 16 (47) | |
| Alcohol history | |||
| Yes | 2 (22) | 12 (57) | .12 |
| Never | 7 (78) | 9 (43) | |
| Clinical T stage | |||
| T1 | 3 (30) | 12 (30) | .99 |
| T2 | 4 (40) | 16 (40) | |
| T3 | 3 (30) | 12 (30) | |
| T4 | 0 | 0 | |
| Clinical N stage | |||
| N0 | 7 (70) | 28 (70) | .97 |
| N1 | 2 (20) | 6 (15) | |
| N2 | 1 (10) | 6 (15) | |
| N3 | 0 | 0 | |
| Clinical M stage | |||
| M0 | 10 (100) | 40 (100) | .99 |
| M1 | 0 (0) | 0 (0) | |
| Depth of invasion, mm | |||
| <5 | 1 (20) | 7 (21) | .57 |
| ≥5 | 4 (80) | 27 (79) | |
| Histologic grade | |||
| Well differentiated | 3 (30) | 9 (26) | .63 |
| Moderately differentiated | 7 (70) | 23 (66) | |
| Poorly differentiated | 0 (0) | 3 (9) | |
| Margin status | |||
| Positive/close | 3 (33) | 4 (10) | .23 |
| Negative | 6 (67) | 34 (89) | |
| Perineural invasion | |||
| Yes | 2 (50) | 33 (85) | .31 |
| No | 2 (50) | 6 (15) | |
| Vascular invasion | |||
| Yes | 0 | 1 (3) | .16 |
| No | 4 (100) | 38 (97) | |
| Treatment modality | |||
| Surgery | 5 (50) | 20 (50) | .99 |
| Surgery + RT | 5 (50) | 20 (50) | |
| Neck treatment | |||
| Neck dissection | 10 (100) | 40 (100) | .99 |
| No neck dissection | 0 | 0 | |
Abbreviation: RT, radiotherapy.
Data are given as number (percentage).
The median interval between the first appearance of symptoms and presentation to a physician was 15 weeks (range, 2–104 weeks), and the median time to biopsy after initial evaluation was 2 weeks (range, 0–8 weeks). Three of 10 patients experienced symptoms for longer than 3 months before presenting to a physician, and only 1 of 10 patients did not have the lesion biopsied within 1 month of presentation.
Three patients (30%) experienced recurrence after a median disease-free interval of 5.4 months (range, 2.4–7.7 months). One patient with cervical recurrence (and Fanconi anemia) died of progressive metastatic disease. Two patients developed lung metastases. Seven patients were alive at the last follow-up. Details of 2 patients who died of disease are presented herein.
A 15-year-old girl with Fanconi anemia diagnosed at age 4 years had received a bone marrow transplant at age 12 years and subsequently developed graft-vs-host disease. At age 15 years she was diagnosed as having a T1N0M0 SCC on the lateral surface of the tongue (according to the American Joint Committee on Cancer staging system), for which she underwent partial glossectomy. She developed bilateral cervical metastases 5 months postoperatively, and bilateral neck dissections were performed, confirming pathologic disease on both sides of the neck. Adjuvant concurrent chemoradiation was administered, but the patient died of progressive disease 3 months later.
A 19-year-old girl with no notable medical history and no known risk factors developed discomfort along the lateral surface of the tongue which was initially attributed to orthodontic braces. After 4 months, she was referred to an oral surgeon and, after 2 months of treatment with antibiotics, the lesion was biopsied. She was referred to our institution with a T3N0M0 SCC on the lateral surface of the tongue. A partial glossectomy with free radial forearm reconstruction was performed, and neck dissection revealed metastatic disease with extra-capsular spread in 2 lymph nodes at level 3. She completed adjuvant concurrent chemoradiation but died of lung metastases 2 months later.
ADULT COHORT
Patient and tumor characteristics are summarized in the Table. Adult cases were matched to pediatric cases in a 4:1 ratio for sex, T status, N status, M status, surgical procedure, and adjuvant radiation. Eight adults were not successfully matched on tobacco exposure history. The median follow-up in the adult cohort was 72.5 months (range, 0.8–183.7 months).
Ten patients (25%) developed recurrent disease after a median disease-free interval of 9.2 months (range, 4.2–19.8 months). Of the 10 patients with recurrence, 5 developed local recurrence, 4 developed regional recurrence, and 1 developed distant metastases. Four of the 40 adult patients developed a second primary head and neck cancer. At last follow-up, 31 adult patients were alive without evidence of disease.
OUTCOMES
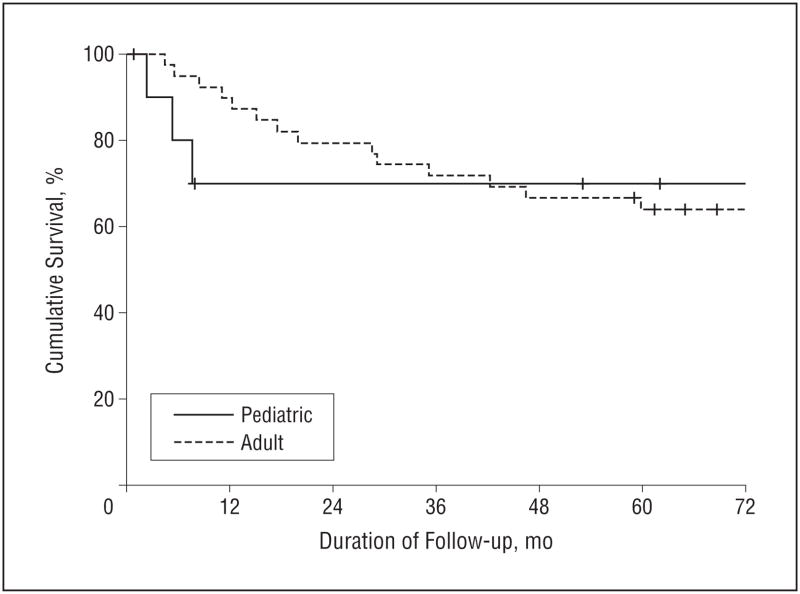
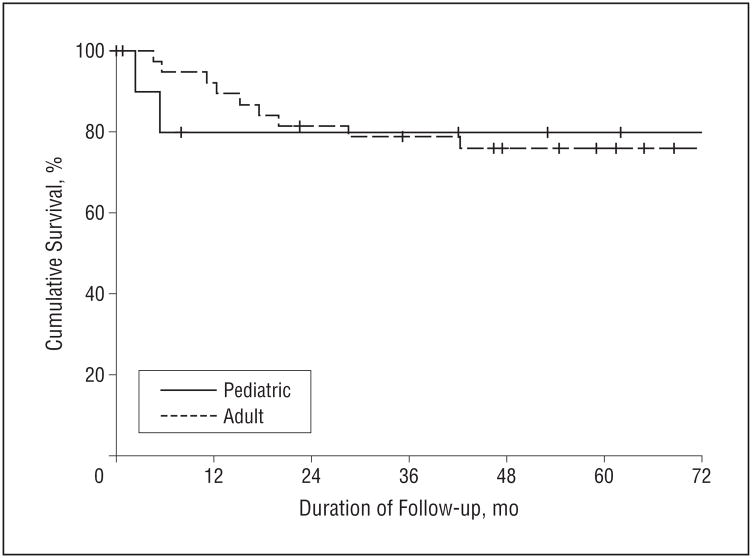
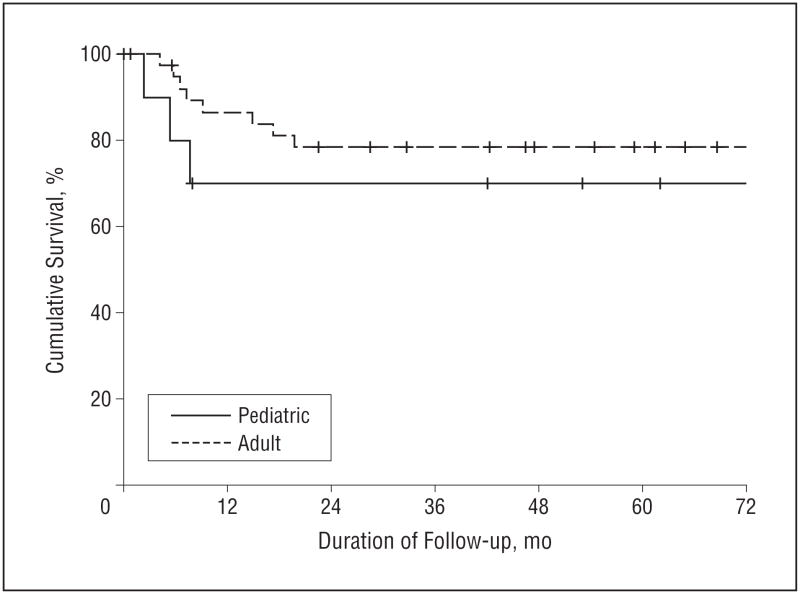
The matched adult and pediatric groups experienced equivalent survival outcomes. The 5-year OS was 70% in the pediatric group and 64% in the adult group (hazard ratio [HR] 0.97; 95% confidence interval [CI], 0.29–3.34; P=.97) (Figure 1). The 5-year DSS was also equivalent: 80% in the pediatric group and 76% in the adult group (HR, 1.11; 95% CI, 0.24–5.13; P=.90) (Figure 2). The 5-year RFS was 70% in the pediatric group and 78% in the adult group (HR, 1.50; 95% CI, 0.40–5.53; P=.54) (Figure 3).
Figure 1.
Overall survival (OS) in pediatric and adult patients with squamous cell carcinoma of the oral tongue. The 5-year OS was 70% for pediatric patients and 64% for adult patients.
Figure 2.
Disease-specific survival (DSS) in pediatric and adult patients with squamous cell carcinoma of the oral tongue. The 5-year DSS was 80% for pediatric patients and 76% for adult patients.
Figure 3.
Recurrence-free survival (RFS) in pediatric and adult patients with squamous cell carcinoma of the oral tongue. The 5-year RFS was 70% for pediatric patients and 78% for adult patients.
COMMENT
In children, adolescents, and young adults, head and neck SCC is believed to be etiologically distinct from SCC in older adults because of limited exposure to risk factors such as tobacco or alcohol. Especially in pediatric patients with no such exposure, genetic syndromes (eg, Fanconi anemia, xeroderma pigmentosum, KID syndrome,8,10,13 or other unidentified genetic risk factors) may be contributory. The subsite of primary cancer also differs considerably between younger and older patients. Funk et al,22 in a review of cases in the National Cancer Database, reported that 76% of head and neck SCC in young patients (<35 years old) originated from the oral tongue, compared with 33% in older patients.
Squamous cell carcinoma of the oral tongue in young adults has therefore been a subject of several studies. There has been inconsistency in the literature regarding outcomes in young adults with oral tongue SCC. In 1994, Sarkaria and Harari14 reported 6 cases of tongue SCC in adults younger than 40 years and reviewed 124 similar cases in the literature. They reported a 57% rate of locoregional recurrence and a 47% cancer-specific mortality rate and concluded that oral tongue SCC in young adults was an aggressive entity. Because this study and other early studies used historical controls,14–16 several recent studies have been performed, focusing on oral tongue SCC in young adults, with contemporary older adult controls. Friedlander et al17 at Memorial Sloan-Kettering Cancer Center performed a matched-pair analysis of oral tongue SCC, matching patients younger than 40 years old with patients 40 years old or older. Disease-specific survival in the 2 groups was equivalent. Since then, 7 additional studies,18–24 using institutional data, cancer registry data from the National Cancer Database, and population-based data from the Surveillance, Epidemiology, and End Results program, have demonstrated either similar or better survival outcomes for younger adult patients with oral cavity SCC.
These conflicting conclusions regarding young adults have not been readily extrapolated to pediatric patients younger than 20 years, in whom head and neck SCC is extremely rare. Only 1 in 1000 head and neck SCC cases are diagnosed in this age group.1–4 There are no data on oral cavity SCC in pediatric patients except for isolated case reports.1,3,5–13 Early reports of poor prognosis in young adults, combined with these case reports of aggressive disease in pediatric patients, have led to the conclusion that SCC of the oral tongue is aggressive in young patients. In the pediatric population, poorer prognosis has also been attributed to delay in diagnosis.5 Because malignant disease may not be suspected in children, adolescents, and young adults, symptoms may be ignored and biopsies not performed in a timely fashion.
This study reports outcomes in 10 patients aged 15 to 20 years who were treated for oral tongue SCC at our institution. In this cohort, treatment delay was uncommon. Pediatric patients were generally brought to medical attention soon after the appearance of symptoms, and a biopsy was usually performed immediately or within several weeks of presentation. In 1 patient, the biopsy was delayed by dentists and oral surgeons for 6 months, with the patient eventually presenting with a T3 tumor. Patients referred to our institution, however, may represent a selected population.
Only 1 patient had an identifiable genetic syndrome related to the development of cancer (Fanconi anemia). All patients were treated by a multidisciplinary team, including head and neck surgical oncologists, radiation oncologists, and pediatric oncologists. Treatment in each case was equivalent to appropriate therapy for an adult patient with similar disease.
Outcomes in the 10 pediatric patients were compared with adult patients treated at our institution during the same time period. Previous studies of oral tongue SCC have established that younger patients may differ from adult patients in sex distribution, tobacco exposure, or TNM stage at presentation. In addition, pediatric patients may be more likely to receive surgery and radiation. Therefore, these factors were controlled for by matching each pediatric patient to 4 adult patients on each of these variables. We report that rates of OS, DSS, and RFS were identical in the pediatric and adult cohorts.
A potential weakness of this study is the small number of cases in the pediatric cohort, which may limit statistical power to identify small differences in survival or recurrence rates. While this study was designed with a 4:1 match to obtain adequate statistical power to identify clinically meaningful differences in survival, it is possible that more subtle differences would not be identified. The absolute risk differences in this study of 6% in OS (favoring pediatric patients) (P =.97), 4% in DSS (favoring pediatric patients) (P =.90), and 8.4% in RFS (favoring adult patients) (P =.54) were not statistically significant. While outcomes are most likely equivalent between pediatric and adult patients with SCC of the oral tongue, if risk differences of this magnitude were deemed clinically important, a larger sample size would be required for evaluation. However, at the present time, these data represent the only outcomes data that have been reported for the pediatric age group. We have obtained similar results in a separate larger data set, using national cancer registry data, and are reporting these data separately owing to differences in the types of variables recorded and the necessary analysis.
Squamous cell carcinoma of the tongue in children and adolescents poses special challenges for parents and physicians owing to the emotional aspects and technical challenges of safe oncologic resection in young patients. Nevertheless, we believe that the preponderance of case reports of aggressive disease and poor outcome in pediatric patients is not an accurate reflection of true outcomes, based on this small series of patients. In fact, as a group, pediatric patients with oral cavity SCC experience better survival outcomes than adults. When pertinent differences in stage, grade, and treatment are controlled for, outcomes are identical, suggesting that pediatric patients should receive the same therapy that is the standard of care for adult patients.
Acknowledgments
Funding/Support: Dr Morris was supported by National Cancer Institute training grant T32 CA009685.
Footnotes
Financial Disclosure: None reported.
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Morris. Acquisition of data: Morris and Patel. Analysis and interpretation of data: Morris, Shah, and Ganly. Drafting of the manuscript: Morris and Ganly. Critical revision of the manuscript for important intellectual content: Morris, Patel, Shah, and Ganly. Statistical analysis: Morris. Administrative, technical, and material support: Morris. Study supervision: Morris, Patel, Shah, and Ganly.
References
- 1.de Carvalho MB, Sobrinho JdeA, Rapoport A, et al. Head and neck squamous cell carcinoma in childhood. Med Pediatr Oncol. 1998;31(2):96–99. doi: 10.1002/(sici)1096-911x(199808)31:2<96::aid-mpo9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Llewellyn CD, Johnson NW, Warnakulasuriya KAAS. Risk factors for squamous cell carcinoma of the oral cavity in young people: a comprehensive literature review. Oral Oncol. 2001;37(5):401–418. doi: 10.1016/s1368-8375(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 3.Sidell D, Nabili V, Lai C, Cheung G, Kirsch C, Abemayor E. Pediatric squamous cell carcinoma: case report and literature review. Laryngoscope. 2009;119 (8):1538–1541. doi: 10.1002/lary.20531. [DOI] [PubMed] [Google Scholar]
- 4.Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya S. An analysis of risk factors for oral cancer in young people: a case-control study. Oral Oncol. 2004;40(3):304–313. doi: 10.1016/j.oraloncology.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Stolk-Liefferink SAH, Dumans AG, van der Meij EH, Knegt PP, van der Wal KGH. Oral squamous cell carcinoma in children: review of an unusual entity. Int J Pediatr Otorhinolaryngol. 2008;72(1):127–131. doi: 10.1016/j.ijporl.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Bill TJ, Reddy VR, Ries KL, Gampper TJ, Hoard MA. Adolescent gingival squamous cell carcinoma: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(6):682–685. doi: 10.1067/moe.2001.115029. [DOI] [PubMed] [Google Scholar]
- 7.Soni S, Radel E, Smith RV, et al. Stage 4 squamous cell carcinoma of the tongue in a child: complete response to chemoradiotherapy. J Pediatr Hematol Oncol. 2001;23(9):612–615. doi: 10.1097/00043426-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Reinhard H, Peters I, Gottschling S, Ebell W, Graf N. Squamous cell carcinoma of the tongue in a 13 year old girl with Fanconi anemia. J Pediatr Hematol Oncol. 2007;29(7):488–491. doi: 10.1097/MPH.0b013e318063ef14. [DOI] [PubMed] [Google Scholar]
- 9.Earle AS, Park CH, Vlastou C. Oral squamous cell carcinoma in children. Ann Plast Surg. 1988;20(2):148–152. doi: 10.1097/00000637-198802000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Byers RM. Squamous cell carcinoma of the oral tongue in patients less than thirty years of age. Am J Surg. 1975;130(4):475–478. doi: 10.1016/0002-9610(75)90487-0. [DOI] [PubMed] [Google Scholar]
- 11.Harper JI, Copeman PW. Carcinoma of the tongue in a boy with xeroderma pigmentosum. Clin Exp Dermatol. 1981;6(6):601–604. doi: 10.1111/j.1365-2230.1981.tb02363.x. [DOI] [PubMed] [Google Scholar]
- 12.Keukens F, van Voorst Vader PC, Panders AK, Vinks S, Oosterhuis JW, Kleijer WJ. Xeroderma pigmentosum: squamous cell carcinoma of the tongue. Acta Derm Venereol. 1989;69(6):530–531. [PubMed] [Google Scholar]
- 13.Sacks HG, Holly R, Blum B, Joy ED. Case 58: maxillary alveolar mass in a 13 year old boy. J Oral Maxillofac Surg. 1985;43(12):958–963. doi: 10.1016/0278-2391(85)90011-4. [DOI] [PubMed] [Google Scholar]
- 14.Sarkaria JN, Harari PM. Oral tongue cancer in young adults less than 40 years of age: rationale for aggressive therapy. Head Neck. 1994;16(2):107–111. doi: 10.1002/hed.2880160202. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein DP, Irish JC. Head and neck squamous cell carcinoma in the young patient. Curr Opin Otolaryngol Head Neck Surg. 2005;13(4):207–211. doi: 10.1097/01.moo.0000170529.04759.4c. [DOI] [PubMed] [Google Scholar]
- 16.Wallner PE, Hanks GE, Kramer S, McLean CJ. Patterns of Care study: analysis of outcome survey data: anterior two thirds of tongue and floor of mouth. Am J Clin Oncol. 1986;9(1):50–57. doi: 10.1097/00000421-198602000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Friedlander PL, Schantz SP, Shaha AR, Yu G, Shah JP. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck. 1998;20(5):363–368. doi: 10.1002/(sici)1097-0347(199808)20:5<363::aid-hed1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Verschuur HP, Irish JC, O’Sullivan B, Goh C, Gullane PJ, Pintilie M. A matched control study of treatment outcome in young patients with squamous cell carcinoma of the head and neck. Laryngoscope. 1999;109(2 pt 1):249–258. doi: 10.1097/00005537-199902000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Pitman KT, Johnson JT, Wagner R, Myers E. Cancer of the tongue in patients less than forty. Head Neck. 2000;22(3):297–302. doi: 10.1002/(sici)1097-0347(200005)22:3<297::aid-hed14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Davidson BJ, Root WA, Trock BJ. Age and survival from squamous cell carcinoma of the oral tongue. Head Neck. 2001;23(4):273–279. doi: 10.1002/hed.1030. [DOI] [PubMed] [Google Scholar]
- 21.Annertz K, Anderson H, Biorklund A, et al. Incidence and survival of squamous cell carcinoma of the tongue in Scandinavia with special reference to young patients. Int J Cancer. 2002;101(1):95–99. doi: 10.1002/ijc.10577. [DOI] [PubMed] [Google Scholar]
- 22.Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002;24(2):165–180. doi: 10.1002/hed.10004. [DOI] [PubMed] [Google Scholar]
- 23.Manuel S, Raghavan SK, Pandey M, Sebastian P. Survival in patients under 45 years with squamous cell carcinoma of the oral tongue. Int J Oral Maxillofac Surg. 2003;32(2):167–173. doi: 10.1054/ijom.2002.0271. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg D, Brooksby C, Hollenbeak CS. Age as a determinant of outcomes for patients with oral cancer. Oral Oncol. 2009;45(8):e57–e61. doi: 10.1016/j.oraloncology.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Lacy PD, Piccirillo JF, Merritt MG, Zequeira MR. Head and neck squamous cell carcinoma: better to be young. Otolaryngol Head Neck Surg. 2000;122(2):253–258. doi: 10.1016/S0194-5998(00)70249-X. [DOI] [PubMed] [Google Scholar]
- 26.Veness MJ, Morgan GJ, Sathiyaseelan Y, Gebski V. Anterior tongue cancer: age is not a predictor of outcome and should not alter treatment. ANZ J Surg. 2003;73(11):899–904. doi: 10.1046/j.1445-2197.2003.02818.x. [DOI] [PubMed] [Google Scholar]