Abstract
Objective
This study aimed (1) to investigate the relationship between the presence of lymph node central necrosis, viewed on pre-operative computed tomography imaging, and the occurrence of histopathologically determined metastatic lymph node extracapsular spread and (2) to determine whether a larger scale study would be valuable.
Materials and methods
Pre-operative computed tomography scans, surgical records and post-operative histopathological analysis results were reviewed for 19 consecutive neck dissections performed in 17 patients with head and neck squamous cell carcinoma.
Results
A total of 20/26 (77 per cent) lymph nodes with central necrosis had extracapsular spread on histopathological analysis. Twenty of 21 (95 per cent) lymph nodes with extracapsular spread had central necrosis on pre-operative computed tomography. Thirty-four of 40 (85 per cent) lymph nodes without extracapsular spread had no evidence of central necrosis on computed tomography. Only three of 12 (25 per cent) patients with lymph node central necrosis identified on pre-operative computed tomography were found to have actual necrosis on final histopathological analysis.
Conclusions
Lymph node central necrosis viewed on pre-operative computed tomography scans is a useful indicator of metastatic lymph node extracapsular spread, with a sensitivity of 95 per cent, a specificity of 85 per cent, a positive predictive value of 69 per cent and a negative predictive value of 98 per cent. Lymph node diameter is not a sensitive indicator of extracapsular spread.
Keywords: Lymph Node, Cervical, Central, Necrosis, Extension, Squamous Cell Carcinoma, Metastasis, Computerized Tomography Scan, Computerized Axial Tomography Scan
Introduction
In head and neck squamous cell carcinoma cases, the histopathological identification of lymph nodes containing tumour metastasis with extracapsular spread is a poor prognostic indicator; it has been associated with a 50 per cent decrease in survival and an approximately 1.5- to 3.5-fold increase in regional recurrence.1–3 In addition, large, multicentre studies have established histologically identified extracapsular spread as a major determinant of whether a patient would benefit from adjuvant chemotherapy.4 Therefore, the ability to predict lymph node extracapsular spread prior to treatment may be helpful in guiding subsequent therapy, both in non-surgical cases (which would benefit from concurrent chemo-radiotherapy) and surgical cases (which may require adjuvant chemotherapy).
Contrast-enhanced computed tomography (CT) is the imaging modality most commonly used to evaluate cervical lymph node status. The CT criteria for lymph node analysis include: size, the presence of central necrosis, and the appearance of a cluster of lymph nodes in the expected drainage path of a tumour. However, such analysis may not be accurate in the setting of recent surgery, radiation or infection.5
The best radiological predictor of lymph node metastasis is a finding of central lymph node necrosis, which has been reported to carry nearly 100 per cent accuracy in predicting the presence of metastatic disease.6,7 Radiologically, lymph node central necrosis is defined as a central area of low attenuation surrounded by an irregular rim of enhancing tissue.5
Two entities may mimic malignant lymph node central necrosis: lipid metaplasia and abscess. Lipid metaplasia is fatty degeneration secondary to inflammation or irradiation, and is usually found at the periphery of the node, while an abscess can typically be differentiated clinically.
Radiological findings which suggest lymph node extracapsular spread comprise nodal capsular enhancement, infiltration of adjacent fat or muscle planes, and capsular contour irregularity.8,9 The use of CT for the identification of extracapsular spread has a sensitivity of 81 per cent and a specificity of 72 per cent, compared with 57–77 per cent and 57–72 per cent, respectively, for magnetic resonance imaging (MRI).10 For MRI scanning, pre-contrast T1- and T2-weighted images are more sensitive than gadolinium-enhanced T1-weighted images.9 Ultrasonography has been shown to be acceptably sensitive, but less specific, for the detection of extracapsular spread.11
While there is some existing radiographic evidence of the prognostic usefulness of lymph node extracapsular spread, it remains unclear whether a finding of lymph node central necrosis on pre-operative CT is predictive of histopathologically determined extra-capsular spread.
Lymph node central necrosis is clearly related to lymph node size, and is observed as a relatively late event in tumour progression, occurring particularly after massive tumour infiltration. Don et al. found that metastatic lymph nodes 20.0 mm in diameter or greater tended to have central necrosis.12 In addition, the ability to palpate tumours beneath the strap muscles or sternocleidomastoid muscle is a fairly specific indicator of central necrosis.12
Increased lymph node size on radiographic imaging is significantly related to histologically identified extracapsular spread. Many studies have shown that nodal (N) staging, which is based on the diameter of the largest lymph node, is related to capsular rupture.13–16 Snyderman et al. noted that lymph nodes 3 cm or more in diameter had a risk of rupture 14.3 times greater than that of nodes less than 3 cm in diameter.2 However, small nodes less than 1 cm in diameter may also have extracapsular spread, with an incidence as high as 25 per cent.2,17
It is not known whether an association exists between radiographic lymph node central necrosis and histopathological extracapsular spread.
This study of patients undergoing neck dissection for metastatic head and neck squamous cell carcinoma investigated the relationship between the presence of cervical lymph nodes with central necrosis, as seen on pre-operative CT imaging, and the post-operative histopathological identification of lymph node extracapsular spread. A limited number of patients were studied, in order to determine whether a larger study would be valuable.
Materials and methods
We undertook a clinicopathological study of 17 consecutive patients diagnosed with head and neck squamous cell carcinoma, who underwent 19 consecutive neck dissections. This was a retrospective chart review.
The study was approved by the New York University School of Medicine IRB.
Pre-operative CT appearances, operative findings and post-operative histopathological analysis results were correlated for these 17 patients’ 19 neck dissections.
Pre-operative CT scans were assessed by a head and neck radiologist and the senior surgeon (DM), with attention to lymph node size, level, and the presence or absence of central necrosis. Figure 1 shows an example of a representative lymph node with central necrosis, visible on an axial, contrast-enhanced CT scan of the neck.
Fig. 1.
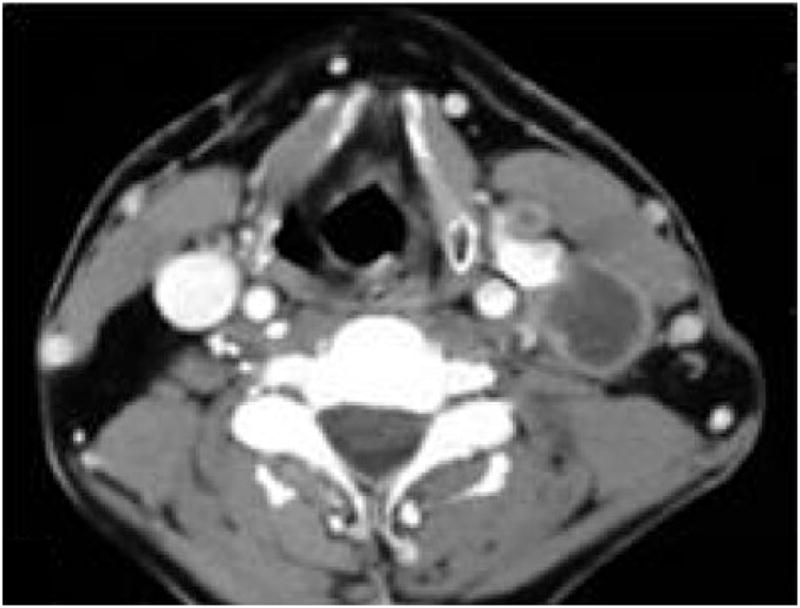
Axial computed tomography scan of patient with left cervical metastatic lymphadenopathy, showing a low attenuation focus of central necrosis.
All specimen were oriented by having lymph node levels I through V labeled individually with staples and/or sutures.
The resected surgical specimens were then examined, during the present study, by an experienced surgical pathologist (HY), specifically noting lymph node involvement, size, calcification, fibrosis, necrosis, and microscopic and/or macroscopic extracapsular spread. Signs of microscopic and macroscopic extra-capsular spread were grouped together as showing ‘extracapsular spread’. Peri-operative records of lymph node size and location were used to match the lymph nodes observed radiographically with the resected lymph nodes analysed histopathologically. In no surgical specimen was there more than one lymph node with radiologically identified central necrosis within a single nodal level.
These data were used to correlate radiological identification of lymph node central necrosis with his-topathological findings of lymph node diameter, calcification, fibrosis, necrosis and extracapsular spread.
Statistical analysis was undertaken using Fisher’s exact test, calculated utilising the Winstat version 2006.2 software package (Wistat, Lehigh Valley, Pennsylvania, USA).
Results and analysis
All 17 patients were male. The mean patient age was 57 years; patients’ ages ranged from 47 to 76 years.
These patients’ primary tumour sites were: the larynx (four patients), floor of the mouth (three), tongue (three), hypopharynx (three), tonsil (three) and unknown (one). Patients’ tumour (T) stages were: T1 (one patient), T2 (eight), T3 (seven) and T4 (one). Their N stages were: N1 (four patients), N2 (10) and N3 (three).
Twelve patients underwent modified radical neck dissection, and four underwent selective neck dissection. The mean time interval between pre-operative CT and surgery was 86 days. Nine of the 17 patients (53 per cent) received pre-operative radiation therapy, and seven of those nine underwent this radiation between CT scanning and surgery. All but two patients received chemotherapy.
Of the 19 neck dissections, 13 (68 per cent) involved patients with radiographic evidence of lymph node central necrosis. On final histopathological analysis, five of the 19 neck dissections (26 per cent) involved nodes with extracapsular spread. In no case did we observe more than one lymph node with radiographic central necrosis or histopathological extracapsular spread in a single nodal station, which helped ensure reliable clinicopathological matching.
Mean nodal diameter did not differ between nodes with and without histopathological extracapsular spread (p = 0.83). However, lymph nodes showing central necrosis on pre-operative CT scanning were significantly larger than those without central necrosis. (1.32 versus 0.79 cm, respectively; p = 0.03).
Twenty of the 26 (77 per cent) lymph nodes with radiological central necrosis had extracapsular spread on histopathological analysis. Six of the 26 (23 per cent) lymph nodes with central necrosis had central fibrosis of 20 per cent or greater on histopathological analysis. Twenty of the 21 (95 per cent) lymph nodes with extracapsular spread had central necrosis on pre-operative CT, and 34 of the 40 (85 per cent) nodes without extracapsular spread had no evidence of necrosis on pre-operative CT (Table I). Only three of the 12 (25 per cent) patients with lymph node central necrosis on pre-operative CT were found to have actual central necrosis on histopathological analysis.
TABLE I.
FINDINGS FOR EXTRACAPSULAR SPREAD AND CENTRAL NECROSIS
| Central necrosis? | Extracapsular spread? |
Total | |
|---|---|---|---|
| Yes | No | ||
| Yes | 20 | 6 | 26 |
| No | 1 | 34 | 35 |
| Total | 21 | 40 | 61 |
Data represent lymph node numbers.
For the determination of histopathological extra-capsular spread, a finding of lymph node central necrosis on pre-operative CT had a sensitivity of 95 per cent and a specificity of 85 per cent. The two-tailed p value, calculated by Fisher’s exact test, was statistically significant, with p < 0.0001.
Positive predictive value and negative predictive value can be calculated based on Bayes’s theorem. The results depend on the overall estimate of the incidence of extracapsular spread in all cases of metastatic head and neck squamous cell carcinoma. Previous studies have placed this incidence at anywhere between 22 and 75 per cent, but favouring the lower end of this range.1,2 Therefore, we used a prevalence of 25 per cent. Incorporating this figure, we calculated that lymph node central necrosis on pre-operative CT had a positive predictive value of 69 per cent and a negative predictive value of 98 per cent as an indicator of histopathological lymph node extracapsular spread.
Discussion
Our results indicate that a finding of lymph node central necrosis on pre-operative CT is associated with the presence of histopathologically verified metastatic lymph node extracapsular spread, and that a lack of radiographic lymph node central necrosis carries a 98 per cent negative predictive value for extracapsular spread. Given its low number of patients, the current study can only generate hypotheses, but it does demonstrate that a larger scale study of this type would be worthwhile. As lymph node extracapsular spread is a critical prognostic factor in head and neck squamous cell carcinoma, information from a larger study may be helpful in informing therapeutic decision-making.
In a study of laryngeal and hypopharyngeal carcinoma cases, Carvalho demonstrated that macroscopic lymph node extracapsular spread was the most important independent prognostic indicator of survival; its presence increased the risk of recurrence 3.5-fold. Carvalho inferred that lymph node extracapsular spread should also be confirmed histologically, as such identification could also assist treatment planning.3 Jose et al. reported that actuarial and disease-free survival were both negatively affected by the finding of lymph node extracapsular spread, whether macroscopic or microscopic, suggesting that cases with extracapsular spread of any kind warrant more aggressive therapy.18
In the current study, pre-operative CT scanning indicated that lymph nodes with central necrosis were on average larger than those without necrosis; however, final histopathological analysis showed that the mean diameter of nodes with and without extracapsular spread did not differ. This seems contrary to many previous studies which showed a direct correlation between lymph node size and extracapsular spread. This apparent contradiction may have occurred due to a wider range of lymph node sizes encountered in our study, compared with previous studies.2,17
In order to demonstrate that extracapsular spread was common in lymph nodes smaller than 3 cm in size, Johnson and colleagues conducted two studies; both showed that extracapsular spread was found in approximately 60 per cent of patents with cervical metastases consisting of nodes less than 3 cm in diameter.1,13 Furthermore, patients with metastatic lymph node extracapsular extension had decreased survival rates and shorter disease-free intervals. This suggests that lymph node extracapsular spread can be prognostic even in small lymph nodes. One of Johnson and colleagues’ studies, a retrospective analysis of 349 patients, demonstrated that histological evidence of extracapsular spread was a statistically significant predictor of decreased patient survival. In addition, the disease-free interval and the time to development of recurrent disease were shorter in patients with extracapsular spread, including those with nodal metastases smaller than 3 cm in size.1,13
Metastatic lymph node extracapsular spread may be most common in patients who develop distant metastases. In a retrospective cohort of 130 patients, Alvi and Johnson found that 88 per cent of patients with distant metastases had lymph node extracapsular spread, compared with only 60 per cent without distant metastases (p < 0.001).19 Lefebvre et al. obtained similar results in a retrospective study of patients with hypopharyngeal and laryngeal cancer.19 Patients with more than three metastatic lymph nodes and lymph node extracapsular spread had approximately three times the rate of distant metastases, compared with patients without nodal metastasis or extracapsular spread.
All the aforementioned studies support the well accepted theory that lymph node extracapsular spread is associated with advanced or high risk disease.
In the present study, final analysis indicated that the sensitivity and specificity of radiographic lymph node central necrosis in detecting extracapsular spread were 95 and 85 per cent, respectively, and that radiographic lymph node central necrosis had a positive predictive value of 68.8 per cent for extracapsular spread. Furthermore, radiographic lymph node central necrosis had a high negative predictive value, 98 per cent, thus indicating that a patient without radiographic central necrosis will probably not have extracapsular spread.
The finding of lymph node central necrosis on pre-operative computed tomography (CT) is associated with the presence of histopathologically identified metastatic lymph node extracapsular spread
Such central necrosis on pre-operative CT is a sensitive indicator of metastatic lymph node extracapsular spread; lymph node diameter is not
Lack of such central necrosis has a high negative predictive value for metastatic lymph node extracapsular spread
Interestingly, only 25 per cent of lymph nodes identified as having central necrosis on CT were found to have actual necrosis on final histopathological analysis. This may be attributed to the prolonged time interval between initial CT scanning and surgery (on average approximately three months), the effects of intervening radiation therapy in some cases, and the specific technique used by the surgical pathologist. Additionally, almost 25 per cent of lymph nodes identified as having central necrosis on CT were found to have central fibrosis when the specimens were subsequently re-examined for the present study. This probably resulted from gradual post-operative transformation of a necrotic core.
We do acknowledge several weaknesses of this study. (However, it should be borne in mind that this was a pilot study, and that a larger study along the same lines is needed to provide stronger evidence.) One major weakness was the small sample size. The possibility of selection bias exists; however, cases were included consecutively. Negative findings may be due to low sample size and low statistical power. There was no way to be absolutely certain that the histopathologically examined lymph nodes were identical to the nodes identified on CT. However, we are confident that nodes were reliably matched, in that nodal stations were meticulously delineated intra-operatively, and there were no cases in which a nodal station harboured more than one lymph node with either central necrosis or extracapsular spread. Radiographically and histopathologically identified nodes were matched in collaboration with the attending surgeon, pathologist and radiologist. In general, however, this kind of error would tend to add heterogeneity to our sample, and to understate any correlations. A second caveat with our series was that several patients received intervening radiation therapy between their CT scan and neck dissection. We cannot be sure that this did not alter our results, although we do not believe that it would have introduced any systematic bias – there is no evidence that radiation would cause extracapsular spread in lymph nodes with central necrosis, but not in other lymph nodes. If anything, such radiation would tend to cause lymph node regression, and such an effect would dilute (rather than increase) the strength of our associations. On subgroup analysis (data not shown), there were no differences in rates of central necrosis or extracapsular spread, comparing irradiated and non-irradiated cases.
Conclusion
This study represents a multidisciplinary investigation of the relationship between cervical lymph nodes with central necrosis, as viewed on pre-operative CT, and the finding of metastatic lymph node extracapsular spread upon post-operative histopathological analysis.
While the results support the hypothesis that lymph node central necrosis on pre-treatment CT is a highly sensitive marker of histopathologically identified extracapsular spread, a larger study of similar design would be valuable. This would consist of a prospective trial with larger patient numbers, in which the specific nodes seen on CT would be matched to the nodes undergoing histopathological analysis. Such a study would generate important prognostic information, the early availability of which would be of value to patients, surgeons and the entire healthcare team in coordinating appropriate risk stratification and informing therapeutic decisions.
Footnotes
Dr R A Zoumalan takes responsibility for the integrity of the content of the paper.
Competing interests: None declared
Presented as a poster at the American Head and Neck Society 7th International Conference on Head and Neck Cancer, 20 September 2008, San Francisco, California, USA.
References
- 1.Johnson JT, Myers EN, Bedetti CD, Barnes EL, Schramm VL, Thearle PB. Cervical lymph node metastases: incidence and implications of extracapsular carcinoma. Arch Otolaryngol. 1985;111:534–7. doi: 10.1001/archotol.1985.00800100082012. [DOI] [PubMed] [Google Scholar]
- 2.Snyderman NL, Johnson JT, Schramm VL, Myers EN, Bedetti CD, Thearle P. Extracapsular spread of carcinoma in cervical lymph nodes: impact upon survival in patients with carcinoma of the supraglottic larynx. Cancer. 1985;56:1597–9. doi: 10.1002/1097-0142(19851001)56:7<1597::aid-cncr2820560722>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho BM. Quantitative analysis of the extent of extra-capsular invasion and its prognostic significance: a prospective study of 170 cases of carcinoma of the larynx and hypopharynx. Head Neck. 1998;20:16–21. doi: 10.1002/(sici)1097-0347(199801)20:1<16::aid-hed3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl Med J. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 5.Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992;158:961–9. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- 6.Van den Brekel MW, Stel HV, Castelijins JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastases: assessment of radiological criteria. Radiology. 1990;177:379–84. doi: 10.1148/radiology.177.2.2217772. [DOI] [PubMed] [Google Scholar]
- 7.Steinkamp HJ, van der Hoeck E, Böck JC, Felix R. The extracapsular spread of cervical lymph node metastases: the diagnostic value of computed tomography [in German] Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 1999;170:457–62. doi: 10.1055/s-2007-1011073. [DOI] [PubMed] [Google Scholar]
- 8.Van den Brekel MW, van der Waal I, Meijer CJ, Freeman JL, Castelijns JA, Snow B. The incidence of micrometastases in neck dissection specimens obtained from elective neck dissections. Laryngoscope. 1996;106:987–91. doi: 10.1097/00005537-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Yousem DM, Som PM, Hackney DB, Schwaibold F, Hendrix RA. Central nodal necrosis and extracapsular neoplastic spread in cervical lymph nodes: MR imaging versus CT. Radiology. 1992;182:753–9. doi: 10.1148/radiology.182.3.1535890. [DOI] [PubMed] [Google Scholar]
- 10.Steinkamp HJ, Hosten N, Richter C, Schedel H, Felix R. Enlarged cervical lymph nodes at helical CT. Radiology. 1994;191:795–8. doi: 10.1148/radiology.191.3.8184067. [DOI] [PubMed] [Google Scholar]
- 11.Tartaglione T, Summaria V, Medoro A, Brunetti D, Di Lella GM, Zacchei P. Metastatic lymphadenopathy from ENT carcinoma: role of diagnostic imaging. Rays. 2000;25:429–46. [PubMed] [Google Scholar]
- 12.Don DM, Anzai Y, Lufkin RB, Fu YS, Calcaterra TC. Evaluation of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Laryngoscope. 1995;105:669–74. doi: 10.1288/00005537-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JT, Barnes EL, Myers EN, Schramm VL, Jr, Borochovitz D, Sigler BA. The extracapsular spread of tumors in cervical node metastasis. Arch Otolaryngol. 1981;107:725–9. doi: 10.1001/archotol.1981.00790480001001. [DOI] [PubMed] [Google Scholar]
- 14.Hirabayashi H, Koshii K, Uno K, Ohgaki H, Nakasone Y, Fujisawa T, et al. Extracapsular spread of squamous cell carcinoma in neck lymph nodes: prognostic factor of laryngeal cancer. Laryngoscope. 1991;101:502–6. doi: 10.1288/00005537-199105000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Grandi C, Alloisio M, Moglia D, Podrecca S, Sala L, Salvatori P, et al. Prognostic significance of lymphatic spread in head and neck carcinomas: therapeutic implications. Head Neck Surg. 1985;8:67–73. doi: 10.1002/hed.2890080202. [DOI] [PubMed] [Google Scholar]
- 16.Carter RL, Bliss JM, Soo K, O’Brien CJ. Radical neck dissection for squamous carcinomas: pathological findings and their clinical implications with particular reference to trans-capsular spread. Int J Radiat Oncol Biol Phys. 1987;13:825–32. doi: 10.1016/0360-3016(87)90094-0. [DOI] [PubMed] [Google Scholar]
- 17.Giancarlo T, Palmieri A, Giacomarra V, Russolo M. Pre-operative evaluation of cervical adenopathies in tumours of the upper aerodigestive tract. Anticancer Res. 1998;18:2805–9. [PubMed] [Google Scholar]
- 18.Jose J, Coatesworth AP, Johnston C, MacLennan K. Cervical node metastases in squamous cell carcinoma of the upper aerodigestive tract: the significance of extracapsular spread and soft tissue deposits. Head Neck. 2003;25:451–6. doi: 10.1002/hed.10214. [DOI] [PubMed] [Google Scholar]
- 19.Alvi A, Johnson JT. Extracapsular spread in the clinically negative neck (N0): implications and outcome. Otolaryngol Head Neck Surg. 1996;114:65–70. doi: 10.1016/S0194-59989670285-1. [DOI] [PubMed] [Google Scholar]


