Abstract
The remote associates test (RAT) is a complex verbal task with associations to both creative thought and general intelligence. RAT problems require not only lateral associations and the internal production of many words but a convergent focus on a single answer. Complex problem-solving of this sort may thus require both substantial verbal processing and strong executive function capacities. Previous studies have provided evidence that verbal task performance can be enhanced by noninvasive transcranial direct current stimulation (tDCS). tDCS modulates excitability of neural tissue depending on the polarity of the current. The after-effects of this modulation may have effects on task performance if the task examined draws on the modulated region. Studies of verbal cognition have focused largely on the left dorsolateral prefrontal cortex (F3 of the 10–20 EEG system) as a region of interest. We planned to assess whether modulating excitability at F3 could affect complex verbal abilities. In Experiment 1 (anodal, cathodal, or sham stimulation over F3 with the reference electrode over the contralateral supraorbital region), we found a significant overall effect of stimulation condition on RAT performance. Post hoc tests showed an increase in performance after anodal stimulation (1 mA) compared to sham (p = .025) and to cathodal stimulation (p = .038). In Experiment 2 (either anodal stimulation at F3 or separately at its homologue F4), we replicated the anodal effect of the first study, but also showed that anodal stimulation of F4 had no effect on RAT performance. These data provide evidence that anodal stimulation of the left dorsolateral prefrontal cortex can improve performance on a complex verbal problem-solving task believed to require significant executive function capacity.
INTRODUCTION
Our study aimed to determine whether modulation of cortical activity using a noninvasive transcranial electrical stimulation technique could affect performance on a complex verbal task that included working memory and problem-solving components. Transcranial direct current stimulation (tDCS) is a recently rediscovered technology that is increasingly being applied to studies of human cognition (Wassermann & Grafman, 2005; Priori, 2003).
The remote associates test (RAT) is a complex verbal task with associations to both creative thought (Ansburg & Hill, 2003; Mednick, 1962) and general intelligence (Andrews, 1975; Mednick & Andrews, 1967). Subjects are presented with three words, for example, child–scan–wash, and must find a common linguistic associate that forms a compound noun or two-word phrase with each cue word—in this case, brain. Because many words complete one or two but not all three cue words, the problems are misleading (Bowden & Jung-Beeman, 2003), a circumstance that likely increases the need for proficient executive function capacity. In addition, RAT solution is often associated with the sudden “aha” feeling of insight (Bowden & Jung-Beeman, 2003), taken as an indicator of prior unconscious processing leading to solution and suggesting that complex insight problems may be solved in “stages” of different modes of thought (Wallas, 1926). Because RAT problems appear to involve several cognitive processes, they may be a useful task for a neurocognitive study of complex cognition that involves considerable verbal and executive function components.
tDCS, a noninvasive brain stimulation technique, has recently been shown to alter performance of several verbal abilities by modulating cortical excitability (Iyer et al., 2005). The study reported here assessed the after-effects of tDCS on a left prefrontal region that has been stimulated in previous studies of verbal working memory (Fregni et al., 2005; Marshall, Mölle, Siebner, & Born, 2005). In studies to date, the verbal tasks used have been relatively constrained. Thus, a goal of this study was to examine whether tDCS can cause measurable change in complex tasks.
tDCS creates a weak electrical current, generally 0.5 to 2.0 mA (Wagner, Valero-Cabre, & Pascual-Leone, 2007) between an active and a reference electrode. Stimulation is thought to modulate neuronal resting membrane potentials; anodal stimulation is thought to increase excitability in the region under the electrode, whereas cathodal stimulation is thought to decrease excitability of the stimulated brain region (Nitsche & Paulus, 2000, 2001).
Because tDCS is a burgeoning technology, studies on verbal phenomena are still few, but several have focused on the F3 location (left dorsolateral prefrontal cortex). Fregni et al. (2005) used the 3-back task and found that working memory was improved by anodal stimulation of F3, whereas cathodal stimulation had no effect. Motor cortex stimulation had no effect, indicating that the anodal effect on F3 was relatively focal. Marshall et al. (2005) applied bilateral concurrent stimulation to F3 and F4 with reference electrodes located over the mastoids. Stimulation was intermittent, 15 sec on and 15 sec off for a total of 15 min. On a 2-back task, both anodal and cathodal stimulation impaired performance. To explain why anodal stimulation of F3 did not replicate Fregni et al.’s result, the researchers posited that the intermittent stimulation may have debilitated the finely tuned temporal dynamics between interacting regions of the brain. A variety of other factors could have accounted for these differences, including different electrode size, bilateral stimulation, and reference electrode location. Marshall, Mölle, Hallschmid, and Born (2004) also used tDCS during deep sleep, applying low doses of intermittent anodal tDCS bilaterally to F3 and F4. Subjects were awakened during these intervals and performed two tasks, one of declarative memory (word pairs), the other nondeclarative or procedural memory (mirror tracing). Word pair performance was improved with anodal stimulation during sleep, whereas mirror tracing was unaffected. The most complex verbal task thus far investigated in a published study is verbal fluency [VF] (Iyer et al., 2005), operationalized as the number of words beginning with a specified letter that subjects can produce in 90 sec. The researchers found that anodal but not cathodal tDCS of F3 improved performance marginally at 1 mA stimulation and significantly at 2 mA.
Executive function capacity has been assessed in tDCS studies of the left dorsolateral prefrontal cortex. Kincses, Antal, Nitsche, Bártfai, and Paulus (2003) presented subjects with four shapes in various constellations that predicted one of two outcomes. Subjects were told no rules about shapes and constellations but learned to connect pattern and outcome only by trial and error. With anodal stimulation of left Fp3, just polar to F3, subjects improved implicit “probabilistic classification learning.” Fecteau, Pascual-Leone, et al. (2007) gave subjects the Stroop task and a task of ambiguous decision making. Unilateral anodal stimulation of F3 had no effect. The researchers also stimulated bilaterally, with anodal and cathodal electrodes over F3 and F4. Both montages (left anodal/right cathodal and left cathodal/right anodal) impaired absolute performance on the decision-making task while having no effect on Stroop. A further study on a risk-reward decision-making task found that right anodal–left cathodal stimulation of F3 and F4, respectively, diminished risk-taking more than when stimulation polarities were reversed (Fecteau, Knoch, et al., 2007).
There are reasons to hypothesize that either or both anodal and cathodal stimulation might aid RAT performance. In several studies, approximately half of RAT solutions were rated by subjects as occurring in a sudden insight (e.g., Jung-Beeman et al., 2004). Subjects may benefit from enhanced search strategies, yet insight problems may also benefit from decreased adherence to misleading solutions or incorrect solution heuristics (Schooler & Melcher, 1995). It thus appears possible that increasing or decreasing activity in left prefrontal regions could improve performance on this class of complex verbal task. We conducted two studies to examine tDCS effects on RAT performance. In the first, we manipulated only the polarity of stimulation over F3 (i.e., left dorsolateral prefrontal cortex) to see if this would alter performance on the RAT. In the second, we sought greater understanding of the spatial specificity of the stimulation effects found in Experiment 1 by comparing the anodal versus sham effect between an F3 and F4 stimulation site.
GENERAL METHODS
Procedure
Location of stimulation was modeled on Iyer et al.’s (2005) first experiment, which placed the active electrode over the F3 region (left dorsolateral prefrontal cortex) and the reference electrode over the right supraorbital region. In both our study and Iyer’s, stimulation duration was 20 min, and stimulation intensity was 1.0 mA. However, the size of the electrodes we used differed, thereby affecting current density. Both electrodes used by Iyer et al. were 25 cm2 leading to current densities of 40 mA/cm2. In our study, the active electrode over F3 (and F4 in Study 2) was oval shaped with an area of 16.3 cm2 (large IOGEL Iontophoresis electrodes) and the electrode over the contralateral supraorbital region was rectangular with an area of 30 cm2 (5 × 6 cm). This led to current densities of 0.061 and 0.033 mA/cm2 over the active and reference electrodes, respectively. Unlike Iyer et al.’s study, our experiment used a within-subjects design modeled on other studies in our lab (Vines, Nair, & Schlaug, 2006, 2008; Vines, Schnider, & Schlaug, 2006). Subjects underwent three stimulation conditions (anodal, cathodal, and sham) in one 3-hr session with the order of stimulation randomized across subjects. Stimulation was delivered with a battery-powered, constant current stimulator (Phoresor; Iomed Inc., Salt Lake City, UT). In the anodal and cathodal conditions, stimulation was ramped up over the first 20 sec and then remained constant. In the sham condition, stimulation ramped up similarly to the cathodal and anodal condition such that subjects experienced the initial tingling associated with onset of stimulation, and was then surreptitiously ramped down over 10 sec without the subject’s awareness. No subjects reported being aware of the sham condition. Onset of tDCS often produces a mild tingling or itching that recedes over the first minute of stimulation. Subjects are generally unable to determine whether or not they received real or sham stimulation (Gandiga, Hummel, & Cohen, 2006). Although tDCS-induced excitability changes have been shown to last up to 60 min (Nitsche et al., 2003; Nitsche & Paulus, 2001), studies have not shown performance effects after 30 min of stimulation (Vines, Nair, et al., 2006; Fregni et al., 2005; Hummel et al., 2005; Iyer et al., 2005). This was our rationale for conducting three experimental sessions within a 3-hr time window. The washout period between stimulation sessions was 30 min. After this period had fully elapsed, subjects completed a second VF pretest and then commenced the second stimulation condition. This design is illustrated in Figure 1. After subjects were introduced to and had practiced the tasks, testing began with a pre-test of VF, followed by the first stimulation condition. Order of stimulation sessions was counterbalanced across subjects.
Figure 1.
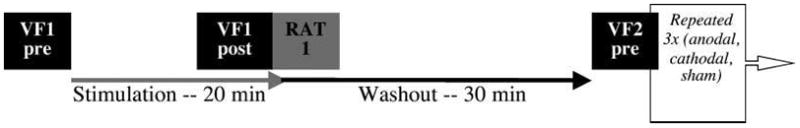
Schematic of one iteration of the experimental design of Experiment 1 showing the timing of verbal fluency (VF) and remote associates test (RAT) in relation to the stimulation sessions. Three equally difficult versions of the RAT were used. Versions of VF (e.g., A–S or C–L) and the RAT (e.g., Problem Set 1 or Problem Set 2) were counterbalanced across stimulation conditions. The order of stimulation sessions was counterbalanced across subjects. In Experiment 2, both VF and RAT were administered as posttests, and order of completion was counterbalanced.
Tasks
Two verbal tasks were used: the first, VF, and the second, the RAT. The VF protocol was modified in two ways from its typical administration (Benton, Hamsher, & Sivan, 1983; Spreen & Benton, 1977). First, because of the within-subjects design to this study, three separate tests were needed (for the anodal, cathodal, and sham sessions) and use of the most commonly used letters with no repetition therefore permitted only two per session, where usually three are used. The three combinations used were A–S, C–L, and P–R, counterbalanced across stimulation conditions and order of presentation (A–S and S–A across subjects). Subjects were given 90 sec for each of these letters. The second difference in this protocol was that subjects typed their responses instead of speaking them orally. VF is often administered to compare the verbal deficits of a clinical population to published norms. Oral administration is generally considered less taxing and faster than writing, making it appropriate for a clinical population. Our study, however, assessed normal subjects, all proficient on the keyboard. Moreover, our goal was not to compare performance to published norms, but rather to assess only pretest–posttest differences in a subject’s performance in different stimulation conditions. Thus, typed responses were a more efficient means of data collection. Visual inspection of subjects showed that typing did not impair cognitive production; toward the middle and end of each 90-sec session, all subjects had considerable pauses of several seconds, indicating that no backlog of words produced in thought but not typed quickly enough occurred.
RAT problems were taken from Bowden and Jung-Beeman (2003). Three sets of 16 problems were used, counterbalanced across conditions. Difficulty of problem sets was based on data provided by Bowden and Jung-Beeman. Average accuracy of each problem set was 48%. The test was given on a Dell laptop running DMASTR software developed at Monash University and at the University of Arizona by K. I. Forster and J. C. Forster (www.u.arizona.edu/~jforster/dmdx.htm). Problems were presented in white 22-point Verdana font on a black background. Participants had a maximum of 30 sec to answer each problem, after which time the correct answer appeared on the screen. The participant pressed the space bar when ready to move to the next problem. Subjects sat in front of a microphone and their verbal responses were recorded. Solution time was automatically recorded as the moment of voice onset, and responses were later listened to by the first author, who was blinded to condition, and determined to be correct or incorrect using Bowden and Jung-Beeman’s answers. Unclear responses were listened to and judged by a second independent rater. Total time to complete this task was between 4 and 7 min. The difference in completion time is due to the fact that subjects may take as much as 30 sec for each of 16 problems, but highly successful subjects may take a fraction of that time on a substantial number of problems.
Data Analysis
We used one-way repeated measures ANOVA to compare the three stimulation conditions for VF and RAT. VF scores were calculated as the difference between pretest and posttest in total words produced on both letters combined. The primary RAT dependent variable measured the number correct out of 16 per condition. We also used repeated measures ANOVA with mean reaction time [RT] (of correctly answered problems only) and number of false alarm errors (spoken responses that were incorrect solutions) as dependent variables. We excluded from this analysis mechanical errors, for example, when subjects coughed and triggered microphone recording. Only four subjects made a single mechanical error each. RT data on the RAT task for one subject was not recorded due to mechanical error.
EXPERIMENT 1
Methods
Stimuli and Procedure
We placed the active electrode on F3 and the reference electrode over the right supraorbital region.
In this experiment, the order in which tasks were performed was not counterbalanced. After 16 min of stimulation, subjects were given a posttest of VF (while still undergoing the last 4 min of stimulation). At 20 min, the electrodes were removed and subjects began the RAT. The order of these tasks was not counterbalanced because of the different and varying length of time the RAT takes and because the primary interest of the study was the effect of polarity of stimulation on performance of tasks that draw on brain regions underlying F3 and not a direct comparison between the tasks. The VF task was completed in 3.5 min for each subject; completion of the RAT is much more variable (explained above), thus making it more feasible to commence the task after stimulation had ceased. The intent of putting one task just before and one just after stimulation offset was to allow us to run several behavioral tests assessing the effects of stimulation within the time period that has been shown by us and others to exhibit behavioral effects of the stimulation. The total duration of the tasks together was approximately 10 min and we chose not to allow that much time to elapse after stimulation offset and while subjects were still performing experimental tasks. In addition, conducting behavioral testing during (and not after) stimulation has been shown to be effective by Kincses et al. (2003), who found an effect on task performance as early as the ninth minute during anodal stimulation.
Subjects
This study involved 18 subjects (mean age = 25.5 years, SD = 2.6, 5 men), all right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Subjects gave written informed consent of a protocol approved by the local Institutional Review Board.
Results and Discussion
All subjects completed all sections of the test and no subject reported significant discomfort. On the RAT, repeated measures ANOVA showed a significant difference in scores across the three conditions [F(2, 16) = 3.88, p = .042]. Two post-hoc paired-samples t tests compared anodal to the other conditions using a Bonferroni-adjusted p value of .025. These tests revealed that anodal stimulation of F3 significantly improved performance (M = 9.04, SD = 1.9) compared to sham stimulation [M = 7.46, SD = 2.7, t(17) = 2.46, p = .025]. Compared to cathodal stimulation (M = 7.30, SD = 2.7), the anodal enhancement was just above the stringent Bonferroni-corrected significance level [t(17) = 2.26, p = .038]. Figure 2 shows these RAT scores by condition. Mean RTs on correct RAT solutions were virtually identical and did not differ significantly [F(2, 16) = 0.004, p = .99]. Mean RTs were 10.79 sec (SD = 4.1) for anodal, 10.74 sec (SD = 2.9) for cathodal, and 10.76 sec (SD = 3.4) for sham stimulation.
Figure 2.
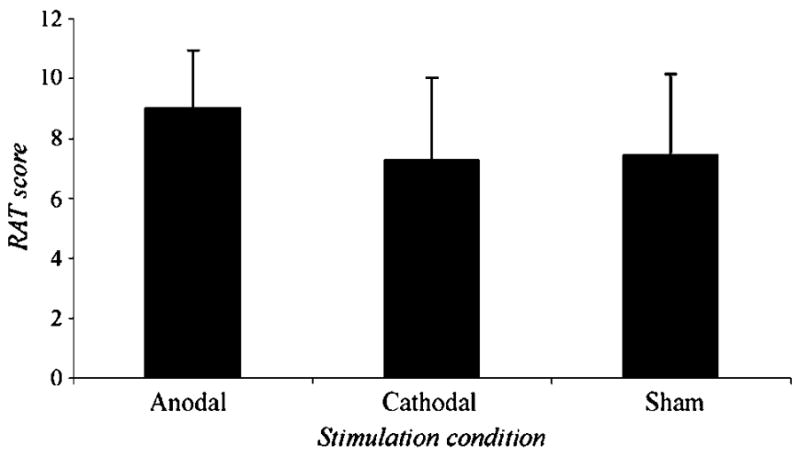
Mean number and standard deviation of remote associates problems solved correctly in each of the stimulation conditions.
Two error analyses showed no significant order effects or carryover effects from one condition to the subsequent condition. Figure 3 shows that, across the 18 subjects, scores between the first, second, and third testing sessions (regardless of stimulation condition) did not differ significantly [F(2, 16) = 0.91, p = .42]. An exploratory error analysis was conducted to examine whether one stimulation condition appeared to affect the subsequent condition. This analysis is important given that a washout interval between conditions was 30 min. Table 1 shows that no clear pattern of stimulation carryover emerged. We compared the expected difference between stimulation conditions (defined as the mean difference of overall RAT scores in the experiment) to the actual difference in mean scores when, for example, anodal was followed by cathodal stimulation. This could occur when anodal and cathodal were first and second, or second and third, respectively. When cathodal and sham followed anodal, there was virtually no deviation from the expected mean difference. At first glance, cathodal stimulation appeared to have small carryover effects; the average subject scored 1.80 higher in anodal than cathodal, but when anodal followed cathodal, the average subject’s score was 1.50 higher in anodal. The same pattern emerged when sham followed cathodal, suggesting a depressing effect of cathodal stimulation after a 30-min washout. However, the possibility that these mean differences (0.30 and 0.28) are statistical noise is presented by the sham and subsequent conditions data. No lingering effect of stimulation is possible after sham, but the mean differences between sham and subsequent anodal or cathodal were 0.35 and 0.22, respectively. This suggests that mean differences on the order of 0.30 are within normal variation.
Figure 3.

Mean number and standard deviation of RAT problems solved by order of testing session, regardless of stimulation condition.
Table 1.
Mean Differences of Consecutive Stimulation Conditions
| Consecutive Conditions | Expected Difference | Mean Difference | Expected – Actuala | |
|---|---|---|---|---|
| Anodal | Cathodal | −1.80 | −1.83 | 0.03 |
| Anodal | Sham | −1.52 | −1.50 | −0.02 |
| Cathodal | Anodal | 1.80 | 1.50 | −0.30 |
| Cathodal | Sham | 0.28 | 0.00 | −0.28 |
| Sham | Anodal | 1.52 | 1.17 | 0.35 |
| Sham | Cathodal | −0.28 | −0.50 | 0.22 |
Negative values indicate a directional effect that would confirm a carryover effect.
VF pretest–posttest differences did not differ significantly across the three stimulation conditions (p > .8). Posttest scores increased in each of the three conditions, presumably due to practice effects and memory for previously listed words. Mean posttest increases were 2.31 (SD = 4.8) for anodal, 2.45 (SD = 5.2) for cathodal, and 3.07 (SD = 4.4) for sham stimulation. These data are shown in Figure 4.
Figure 4.

Pretest and posttest verbal fluency scores and standard deviations in each of the stimulation conditions.
These results suggest that anodal but not cathodal stimulation of F3 improves RAT performance. Replicating Iyer et al.’s (2005) second experiment, we found no significant effect on VF with 1-mA stimulation over F3. Iyer et al. found significant effects with 2-mA stimulation in a third experiment. Among our subjects, stimulation produced a dissociation between tasks. Improvements in RAT performance thus appear to be due in large part to elements other than VF. This dissociation may be due to the capacities instantiated under the site of stimulation, F3, and the differential requirements of the two tasks. Because this experiment manipulated polarity of stimulation, we conducted a second experiment to explore the specificity of site of stimulation.
EXPERIMENT 2
Methods
Stimuli and Procedure
In this experiment, we compared anodal stimulation, found to have a significant effect on RAT in Experiment 1, to sham. We ran a within-subjects design separately comparing stimulation effects on F3 and its right-hemisphere homologue F4. The reference electrode was located over the contralateral supraorbital region. Two stimulation conditions (anodal and sham) were administered on each of two separate days. Half the subjects received simulation of F3 in their first session. The order of stimulation was counterbalanced across site of stimulation.
We modified our protocol by administering both tasks after stimulation offset, and the order in which tasks were given was counterbalanced. Because this 2-day protocol necessitated four separate testing sessions, we created a fourth version of both VF and RAT. The additional letter pair was D–F, and all four RAT versions were of equal difficulty.
Data Analysis
Whereas Experiment 1 had three comparisons of interest (anodal to sham, cathodal to sham, and anodal to cathodal), Experiment 2 had only two comparisons (F3 anodal vs. sham and F4 anodal vs. sham). We designed a within-subjects protocol in which each subject received real and sham stimulation on one day for one region and real and sham stimulation for the other region on a separate day. The order of F3 and F4 stimulation was counterbalanced. Because testing for each subject occurred on separate days, we thus conducted two separate paired t tests of anodal versus sham RAT score for F3 and for F4. For VF we first calculated difference scores between pretest and posttest in each condition (anodal, sham) and then ran a paired t test.
Subjects
This study involved 12 new subjects (mean age = 25.4 years, SD = 4.5, 9 men), all right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Subjects gave written informed consent of a protocol approved by the local Institutional Review Board.
Results and Discussion
Replicating Experiment 1, F3 anodal stimulation produced a significant effect on RAT score [t(11) = 2.71, p = .020]. F4 stimulation did not [t(11) = 0.13, p = .76]. Mean scores, shown in Figure 5, after F3 stimulation were 9.25 (SD = 2.99) and 7.25 (SD = 2.86) for anodal and sham, respectively. F4 means were 8.17 (SD = 2.29) and 7.92 (SD = 2.81). The F3 result is significant using a Bonferroni-adjusted threshold of 0.025.
Figure 5.

Mean number and standard deviation of RAT problems solved after anodal or sham stimulation of F3 and F4.
VF analyses showed no significant pretest–posttest differences as a result of F3 or F4 stimulation. However, the direction of the trend differed over the two sites. F3 anodal stimulation (M = 0.59, SD = 1.33) led to a non-significant increase [t(11) = 0.587, p = .57] of 0.83 comparing sham to anodal, whereas F4 anodal stimulation was associated with 2.17 points lower than sham post-test scores [t(11) = 1.85, p = .09]. This nonsignificant trend in performance after F4 stimulation might be of use in guiding future studies.
DISCUSSION
These results show that anodal stimulation of the left dorsolateral prefrontal cortex can improve complex verbal problem-solving. The RAT involves rapid recall and lateral associations, yet also concludes in a single answer and likely involves a substantial executive component. The spatial specificity of this effect appears particular to F3.
Although the tasks in this study are both verbal, RAT problems require not only the production of many words but a convergent focus on a single answer. The cue words cross–rain–tie may separately be completed by a large number of words (e.g., crosswalk or raincoat), but only a single word (“bow”) completes all three. Subjects rapidly produce associates of all three and must then undertake an elaborate sequence of testing possible solutions, many of which turn out to complete one or two but not all three cue words. The executive function and working memory components appear high. By contrast, the VF task is relatively simple, as subjects must only recall words that begin with a single letter. VF clearly necessitates search strategies, yet these appear to be less complex than in the RAT. Working memory demands in VF appear relatively low.
The left dorsolateral prefrontal cortex (F3) may instantiate verbal capacities, but our results appear to concord with other evidence to suggest that F3 houses executive functions to a greater degree. Among the tDCS literature on F3 is Fregni et al.’s (2005) study showing anodal enhancement on an n-back working memory task. The n-back task was also used in two studies using rTMS, which depresses neural activity in the affected region. In both studies, rTMS of both F3 and F4 impaired 2-back performance (Mottaghy et al., 2000). The researchers conducted their study to test the hypothesis that verbal tasks are left-dominant, but concluded that bilateral effects might have derived from a significant central executive component necessary in the 2-back task. Kincses et al. (2003) stimulated an area slightly more polar than F3 (with reference electrode at Cz) and found enhanced probabilistic classification on a nonverbal task. The cortical networks that underlie F3 thus may be responsible for executive functions primarily.
The RT from our Experiment 1 provides another window on interpretation of these results. That RT did not differ indicates that no general effect on speed of processing occurred. This appears to concur with the null finding on Stroop performance found by Fecteau, Knoch, et al. (2007) and Fecteau, Pascual-Leone, et al. (2007). The decreased errors in both anodal and cathodal, compared to sham, is intriguing. Given that anodal stimulation improved solution performance, it is possible that anodal stimulation caused a sharpening of solution recognition and made subjects more aware of wrong answers that came to mind. It is less clear why cathodal stimulation would decrease errors but not affect RT or success rate.
This study shows that tDCS can be used to affect cognitive performance on complex verbal tasks, and that the spatial focality of this effect is relatively specific. We considered this study a proof of principle testing the hypothesis that tDCS could cause measurable change in performance on a complex verbal task. We recognize that future studies will need to account for several factors that could contribute to these results. It is possible that the region underlying the “reference” electrode affected performance, but like other tDCS studies, our interpretation focuses only on our active regions of interest. As tDCS studies begin to assess more complex tasks that presumably engage multiple cognitive processes, it will be increasingly important to stimulate multiple brain regions. The RAT is believed to engage both left- and right-lateralized regions at different problem-solving stages (Jung-Beeman et al., 2004; Bowden & Jung-Beeman, 2003; Jung-Beeman & Bowden, 2000). Future studies with our problems and other complex tasks should carefully assess the multiple regions hypothesized to contribute to task performance.
In summary, our results are unique in that we were able to enhance performance on a highly complex verbal task. The results suggest that left dorsolateral prefrontal regions contribute to solving complex problems with creative and logical components.
Acknowledgments
This work was supported by grants from NIDCD (RO1 DC008796), NSF (BCS0518837), as well as the Grammy Foundation to G. S. We thank the Harvard Mind/Brain/Behavior Interfaculty Initiative for supporting C. C.’s work related to this experiment. We thank Dr. Dinesh Nair and other members of our lab for advice on the tDCS protocol.
References
- Andrews FM. Social and psychological factors which influence the creative process. In: Taylor IA, Getzels JW, editors. Perspectives in creativity. Chicago: Aldine Publishing Company; 1975. pp. 117–145. [Google Scholar]
- Ansburg PI, Hill K. Creative and analytic thinkers differ in their use of attentional resources. Personality and Individual Differences. 2003;34:1141–1152. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. 3. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- Bowden EM, Jung-Beeman M. Normative data for 144 compound remote associate problems. Behavior Research Methods, Instruments, & Computers. 2003;35:634–639. doi: 10.3758/bf03195543. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: A direct current stimulation study. Journal of Neuroscience. 2007;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Théoret H, Boggio PS, et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. Journal of Neuroscience. 2007;27:6212–6218. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Experimental Brain Research. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden MB. The right hemisphere maintains solution-related activation for yet-to-be-solved problems. Memory & Cognition. 2000;28:1231–1241. doi: 10.3758/bf03211823. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel-Liu S, Greenblatt R, et al. Neural activity when people solve verbal problems with insight [Electronic Version] PloS Biology. 2004;2 doi: 10.1371/journal.pbio.0020097. Retrieved 19 May 2004 from http://biology.plosjournals.org. [DOI] [PMC free article] [PubMed]
- Kincses TZ, Antal A, Nitsche MA, Bártfai O, Paulus W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia. 2003;42:113–117. doi: 10.1016/s0028-3932(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. Journal of Neuroscience. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neuroscience. 2005;6:1–7. doi: 10.1186/1471-2202-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SA. The associative basis of the creative process. Psychological Review. 1962;69:220–232. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Andrews FM. Creative thinking and level of intelligence. Journal of Creative Behavior. 1967;1:428–431. [Google Scholar]
- Mottaghy FM, Krause BJ, Kemna LJ, Töpper R, Tellmann L, Beu M, et al. Modulation of the neuronal circuitry subserving working memory in healthy human subjects by repetitive transcranial magnetic stimulation. Neuroscience Letters. 2000;280:167–170. doi: 10.1016/s0304-3940(00)00798-9. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. Journal of Cognitive Neuroscience. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Priori A. Brain polarization in humans: A reappraisal of an old tool for prolonged noninvasive modulation of brain excitability. Clinical Neurophysiology. 2003;114:589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- Schooler JW, Melcher J. The ineffability of insight. In: Smith SM, Ward TB, Finke RA, editors. The creative cognition approach. Cambridge, MA: MIT Press; 1995. pp. 97–133. [Google Scholar]
- Spreen O, Benton AL. Neurosensory center comprehensive examination for aphasia: Manual of directions (Revised edition) Victoria, BC, Canada: Neuropsychology Laboratory, University of Victoria; 1977. [Google Scholar]
- Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. NeuroReport: For Rapid Communication of Neuroscience Research. 2006;17:671–674. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]
- Vines BW, Nair DG, Schlaug G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. European Journal of Neuroscience. 2008;28:1667–1673. doi: 10.1111/j.1460-9568.2008.06459.x. [DOI] [PubMed] [Google Scholar]
- Vines BW, Schnider NM, Schlaug G. Testing for causality with transcranial direct current stimulation: Pitch memory and the left supramarginal gyrus. NeuroReport. 2006;17:1047–1050. doi: 10.1097/01.wnr.0000223396.05070.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annual Review of Biomedical Engineering. 2007;9:19.11–19.39. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Wallas G. The art of thought. London: J. Cape; 1926. [Google Scholar]
- Wassermann EM, Grafman J. Recharging cognition with DC brain polarization. Trends in Cognitive Neurosciences. 2005;9:503–505. doi: 10.1016/j.tics.2005.09.001. [DOI] [PubMed] [Google Scholar]


