Abstract
Many indirect developing animals create specialized multipotent cells in early development to construct the adult body and perhaps to hold the fate of the primordial germ cells. In sea urchin embryos, small micromeres formed at the fifth division appear to be such multipotent cells: they are relatively quiescent in embryos, but contribute significantly to the coelomic sacs of the larvae, from which the major tissues of the adult rudiment are derived. These cells appear to be regulated by a conserved gene set that includes the classic germline lineage genes vasa, nanos and piwi. In vivo lineage mapping of the cells awaits genetic manipulation of the lineage, but previous research has demonstrated that the germline is not specified at the fourth division because animals are fertile even when micromeres, the parent blastomeres of small micromeres, are deleted. Here, we have deleted small micromeres at the fifth division and have raised the resultant larvae to maturity. These embryos developed normally and did not overexpress Vasa, as did embryos from a micromere deletion, implying the compensatory gene regulatory network was not activated in small micromere-deleted embryos. Adults from control and micromere-deleted embryos developed gonads and visible gametes, whereas small micromere-deleted animals formed small gonads that lacked gametes. Quantitative PCR results indicate that small micromere-deleted animals produce background levels of germ cell products, but not specifically eggs or sperm. These results suggest that germline specification depends on the small micromeres, either directly as lineage products, or indirectly by signaling mechanisms emanating from the small micromeres or their descendants.
Keywords: Sea urchin, Vasa, Nanos, Piwi, Small micromeres, GFP reporter, Germ cells
INTRODUCTION
Primordial germ cells (PGCs) are the precursor stem cells to the germline. In well-characterized animals such as Drosophila (Huettner, 1923; Illmensee and Mahowald, 1974), Caenorhabditis elegans (Sulston et al., 1983) and the mouse (Seydoux and Braun, 2006), PGCs form early in development through either autonomous mechanisms of germ plasm localization (e.g. fly and worm) or by cell interactions (e.g. mouse) (Extavour and Akam, 2003). Formation early in development means that these cells are often segregated from normal developmental activities and are relatively quiescent for much of embryogenesis. Animals in other taxa, however, appear to have a distinct developmental program for establishing their germline (e.g. Juliano et al., 2010a). In this group of animals, which includes lophotrochozoans and echinoderms, multipotent cells are established in embryogenesis that form the major tissues of the adult, probably including cells that give rise to the germline. The multipotent cells segregate early in development and may remain metabolically quiescent during the major changes of embryogenesis. At some point in larval development, this lineage begins rapid cell cycle progression, acquires diversity of fate and differentiates into many adult tissues, including the germline. Thus, the mechanism of germline segregation in these animals may be distinct from animals in which PGCs are formed early in embryogenesis (Juliano and Wessel, 2010; Juliano et al., 2010a).
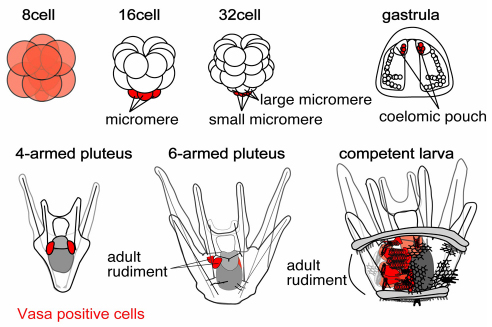
Sea urchins are members of the phylum Echinodermata, a sister group to the chordates. They use a developmental strategy whereby some cells are set aside in embryogenesis as multipotent cells that will eventually contribute to major structures of the adult. These multipotent cells include the descendants of small micromeres, formed at the fifth cleavage, and contribute only to the coelomic sacs (Pehrson and Cohen, 1986; Tanaka and Dan, 1990), a precursor of the adult rudiment in postembryonic development. The small micromeres do not contribute to any functional structures of the embryo (Cameron et al., 1987; Cameron et al., 1991). Ransick et al. (Ransick et al., 1996) tested whether the small micromeres constitute a definitive primordial germ cell lineage during embryonic development by depleting micromeres, the parent cells of small micromeres that are formed at the fourth cleavage (Fig. 1), and raising those embryos to adults. The animals developed and metamorphosed normally, and most of the adults made gametes. They concluded that the germ cell lineage is not formed obligatorily by the fourth cell division, and instead, concluded it must segregate in postembryonic development of the rudiment or even following metamorphosis (Ransick et al., 1996).
Fig. 1.
A schematic drawing of sea urchin embryonic and larval development. Vasa-positive cells and tissues are labeled in red.
The micromeres are a major signaling center of the sea urchin embryo and their removal either at the 16-cell stage, or in blastulae, results in significant compensatory development and fate transitions within the embryo (Ransick and Davidson, 1993). The micromeres are the precursor to both the large and small micromeres, and the large micromeres have a singular fate of making the larval skeletal system. Removal of the large micromeres just prior to gastrulation results in trans-fating, whereby other mesodermal cells change fate to become skeletogenic cells, a fate they would not normally display (Ettensohn and McClay, 1988). In the early embryo, micromeres have a capacity to induce new axial development when transplanted to ectopic positions, and their removal results in a delay of development in the mesendodermal tissues of the embryo (Horstadius, 1950; Ransick and Davidson, 1995). Removal of the micromeres also results in a significant upregulation of Vasa, the RNA helicase, in all remaining cells of the embryo from Strongylocentrotus purpuratus (Voronina et al., 2008). This is significant in that Vasa is long believed to be involved in germline specification and maintenance (Lasko and Ashburner, 1988; Lasko and Ashburner, 1990; Styhler et al., 1998; Tomancak et al., 1998; Tanaka et al., 2000; Salinas et al., 2007). Indeed, the micromeres and then the small micromeres are the sole cells that retain Vasa and its upregulation throughout the embryo following micromere removal may have a compensatory function in re-specifying the multipotent cell line or another germline lineage.
The small micromeres are now thought to be a multipotent cell that uses a core gene set ancestral to the derivation of primordial germ cells early in embryogenesis (Juliano et al., 2010a). In addition to vasa, the small micromeres specifically accumulate other determinants long thought to be exclusive to the germline (Duboc et al., 2005; Juliano et al., 2006). This repertoire includes nanos, piwi and soxE (Huang et al., 1999; Kobayashi et al., 1996; Cox et al., 1998; Forbes and Lehmann, 1998; Tsuda et al., 2003; Wang and Lin, 2004; Suzuki et al., 2007). nanos and soxE appear to be specifically transcribed in the small micromeres, whereas piwi and vasa mRNAs are distributed uniformly in the early embryo but their cognate proteins accumulate selectively in the small micromere lineage by selective protein turnover. A significant fate transition appears to occur in the micromere lineage from the fourth to the fifth cell division: the large micromere fate becomes restricted to a skeletogenic cell fate (Ettensohn and McClay, 1988), whereas the small micromeres, the cells that retain Vasa, retain broad developmental potency but lose the signaling capacity held by the micromeres (Kurihara and Amemiya, 2005). To test the hypothesis that the small micromeres contribute to the germline in adult sea urchins, we depleted small micromeres of Lytechinus variegatus, raised these embryos to adulthood, and tested them for their ability to make gametes.
MATERIALS AND METHODS
Micromere depletion
Micromeres or small micromeres were depleted as described previously (Ransick et al., 1996; Yajima, 2007a) with slight modifications. Cross-linking of the fertilization envelope was inhibited by 1 mM amino-triazole and was removed by gentle pipetting. Micromeres or small micromeres were then micro-surgically depleted in Ca2+-free seawater (Fig. 2A). Resulting embryos were fed on Chaetoceros gracilis (UTEX, Texas) (Yajima, 2007b) during larval development, and on seaweed and an artificial diet (George et al., 2004) for 1.5 years at 20°C.
Fig. 2.
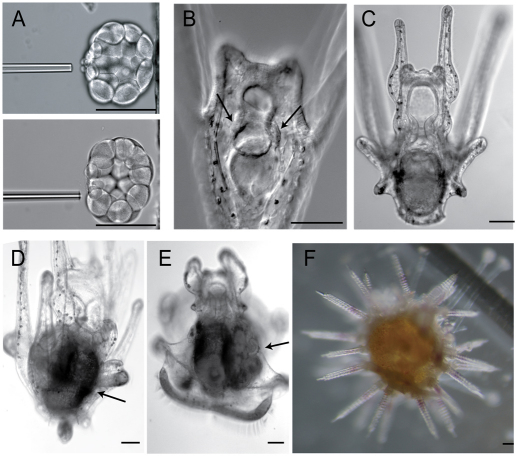
Embryos depleted of their small micromeres develop and metamorphose normally. (A) Before (top) and after (bottom) small micromeres (smms) are removed by a needle. (B,C) Smm-removed pluteus larva formed coelomic pouches (arrows), as did normal larvae. (D-F) Smm-removed larvae formed adult rudiments (arrows) after 4-6 weeks and metamorphosed normally after 6 weeks of fertilization. Scale bars: 100 μm.
Immunolabeling
SpVasa immunolabeling was performed as previously described (Juliano et al., 2010b) and the SpVasa-GFP fusion construct (Gustafson et al., 2010) was injected into fertilized eggs and micromeres were removed at 16-cell or 28-cell stage. Photomicrographs were taken by confocal microscopy (Zeiss, 510) for fluorescent images, by epifluorescent microscopy (Zeiss Axioplan) for gonad samples and fluorescent intensity measurements, or with a dissecting microscope (Olympus SZX16) for juvenile and adult morphology.
Quantitative PCR
Quantitative PCR (qPCR) was performed as described previously (Juliano et al., 2006). Gonadal tissues of each animal were collected and subjected to total RNA extraction with Trizol (Invitrogen). The RNA was made into cDNA with TaqMan RT-PCR kit (Roche), and 1 μl of each cDNA was used for qPCR reactions. PCR primers for each gene are as follows: SFE1, 5′-GGTTTCCCATAAAACAGCACA-3′ and 5′-TGGTACTTGGAAGGACCAATC-3′; Ovoperoxidase, 5′-CTCGAACCATGGAGGAGTAG-3′ and 5′-TCTTGACCGTCAAGAGTACC-3′; Bindin, 5′-GAATGTCTTGAACAACCTG-3′ and 5′-CCCTGATTGTAACCTTGT-3′; Ubiquitin, 5′-CACAGGCAAGACCATCAC-3′ and 5′-GAGAGAGTGCGACCATCC-3′. The level of expression of each gene was normalized to that of Ubiquitin.
RESULTS
Small micromere depletion has no effect on normal development
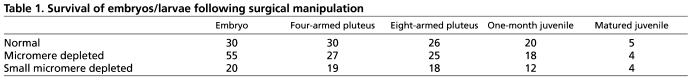
Micromeres (mms) or small micromeres (smms) were depleted at the 16-cell stage or at the 32-cell stage, respectively. When mms were depleted, as previously reported (Tanaka and Dan, 1990; Ransick and Davidson, 1995), we noted a significant delay in development and nearly half of them failed in gastrulation and died before reaching the larval stage (Table 1). This outcome might be because, in response to the major signaling center of the micromeres, a dynamic reorganization of development occurs: embryos reset the gene regulatory network (GRN) and macromere descendants compensate for the loss of micromere lineages (Ransick and Davidson, 1995). By contrast, smm-depleted embryos developed on schedule, underwent gastrulation successfully and developed normally in terms of timing and size for post-embryonic development (Fig. 2A,B-F; Table 1). These smm-depleted embryos formed coelomic pouches in larvae (Fig. 2B,C, arrows), adult rudiments within 3-4 weeks of late larval development (Fig. 2D,E, arrows), and became juveniles in 4-6 weeks (Fig. 2F) as did control embryos that were unmanipulated (Table 1). Although lethality was higher in mm-depleted samples during early embryogenesis, the survival ratio after the larval stage was similar among the three different embryo cohorts. From these results, we conclude that smms either do not contribute to larval development as was previously speculated (Cameron et al., 1987; Cameron et al., 1991), or their loss results in seamless compensatory fate transitions. Unfortunately, the long-term lineage analysis of smms is complicated by a lack of sensitive long-lasting genetic labeling mechanisms used to trace lineages for years.
Table 1.
Survival of embryos/larvae following surgical manipulation
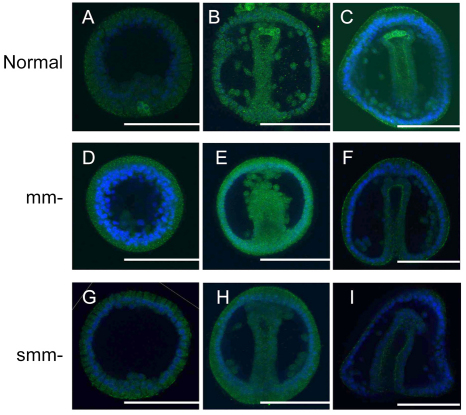
To determine the effect of mm and smm loss on the embryo with respect to Vasa accumulation, we immunostained embryos with SpVasa antibody at multiple developmental stages. The results were analyzed by confocal microscopy, using the same settings for each developmental stage and for each embryo cohort, to determine relative Vasa protein accumulation. In normal L. variegatus and S. purpuratus embryos, we find Vasa-positive cells as previously described (Fig. 3A-C) (Voronina et al., 2008; Juliano and Wessel, 2009). In mm-depleted embryos (Fig. 3D-F), as reported previously in S. purpuratus (Voronina et al., 2008), Vasa protein upregulates from a maternal load of mRNA and does so uniformly (Fig. 3D,E). However, the level of upregulation in response to mm loss is significantly lower in L. variegatus than in S. purpuratus. This may be because the amount of maternal L. variegatus vasa mRNA is substantially lower than that of S. purpuratus (Juliano et al., 2006) so that the up-regulation of L. variegatus vasa translation is limited. However, the developmental program was still reset, resulting in compensatory development of skeletons and a small overexpression of Vasa in blastulae. In smm-depleted embryos (Fig. 3G-I), however, no significant upregulation of Vasa was detected (Fig. 3G,H) so that the entire Vasa signal in the embryos was lost. These results suggest that the L. variegatus embryos do not compensate for the loss of smms, at least according to the criteria of gross morphogenesis and Vasa compensation.
Fig. 3.
Vasa expression in micromere- or small micromere-removed embryos. Normal or treated embryos were immunolabeled with Vasa (green) and DAPI (blue) at various developmental stages. (A-C) Normal embryos. Vasa expression is restricted to small micromere (smm) descendants. (D-F) Micromere (mm)-removed embryos. Specific Vasa expression in the smm lineage is lost and Vasa is overexpressed ubiquitously in blastula (D) but goes back to normal levels by prism stage (F). (G-I) Smm-removed embryos. Vasa expression is lost with no detectable overexpression throughout the early embryo. (A,D,G) Mesenchyme blastula; (B,E,H) gastrula; (C,F,I) prism larva. Scale bars: 50 μm.
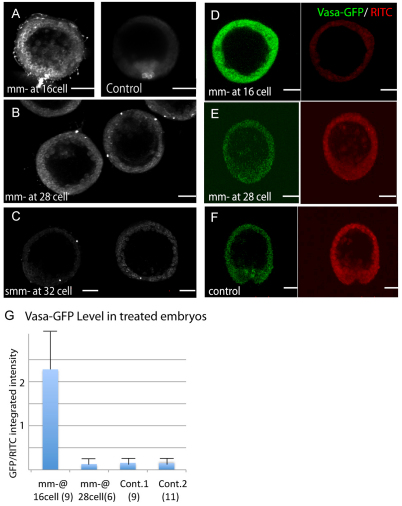
Removal of the mm at the 16-cell stage of S. purpuratus showed significant Vasa upregulation and the resultant animals developed gametes (Ransick et al., 1996; Voronina et al., 2008). We hypothesize that in S. purpuratus, removal of mms at the 16-cell stage induces a reset of the mesendodermal developmental program that results in the large upregulation of Vasa and the compensatory mechanisms that lead to gametogenesis in S. purpuratus adults. Thus, we further examined the Vasa response to mm removal in S. purpuratus, the major signaling center of the early embryo. Micromeres or smms were removed at the 16-cell stage or the 32-cell stage, respectively, from S. purpuratus. As the four smms resulting from the mm division are delayed in cytokinesis compared with other blastomeres, a transient 28-cell stage is formed prior to the 32-cell stage (Endo, 1966; Pehrson and Cohen, 1986), so we also tested mm removal at the 28-cell stage. The results (Fig. 4A-C) are as follows: mm removal at the 16-cell stage caused an overexpression of Vasa, whereas mm removal at 28-cell or smm removal at 32-cell stage showed a control level of Vasa expression compared with unmanipulated embryos. We also examined the regulation of a Vasa-GFP fusion reporter injected into fertilized eggs (Gustafson et al., 2010) (Fig. 4D-G). This vasa-GFP reporter mimicked the endogeneous Vasa expression pattern: it showed an overexpression of GFP in mm-removed embryos at the 16-cell stage but not in mm-removed embryos at the 28-cell stage. The level of overexpression in both the endogenous and the reporter assays are variable in mm-removed embryos at 16-cell stage, but not in normal embryos nor in mm-deleted embryos at the 28-cell stage (Fig. 4G). These results suggest that a narrow window is present at the 16-cell stage to respond to a loss of mms by upregulating Vasa. A huge error bar in mm-removed embryos at 16-cell stage also supports this model; the extent of interaction between mms and macromeres prior to mm removal results in differential levels of Vasa overexpression during subsequent development.
Fig. 4.
No Vasa overexpression is detected in micromere-removed embryos at the 28-cell stage or in small micromere-removed embryos of S. purpuratus. (A-C) Micromeres (mms) or small mms were removed at the 16-cell stage (A, left panel), 28-cell stage (B) or 32-cell stage (C), and each of the embryos was raised to mesenchyme blastula stage, and then fixed and immunolabeled with Vasa antibody. The smm lineage-specific Vasa localization seen in normal embryos (A, right panel) is lost in the treated embryos. Significant Vasa overexpression was observed in mm-removed embryos at the 16-cell stage (A, left panel), but not in mm-removed embryos at the 28-cell stage (B) or in smm-removed embryos at the 32-cell stage (C). (D-F) A Vasa-GFP fusion reporter was introduced into fertilized eggs, and mms were removed at the 16-cell stage (D) or the 28-cell stage (E). Embryos were raised to the mesenchyme blastula stage and Vasa-GFP (green) levels were quantified by normalizing to the co-injected RITC (red) intensity, as measured using Metamorph software. (G) Embryos injected with Vasa-GFP and RITC but without the surgery were used as controls (Cont. 1 and Cont. 2). Cont. 2 was performed independently from the Cont.1 experiment to test the technical consistency of this method. The numbers in parenthesis indicates the number of specimens measured. Results are relative values ± s.e.m. Scale bars: 20 μm.
Small micromeres contribute to the germline in adult L. variegatus
Culture conditions for post-metamorphic L. variegates development are well established and it is known that the timing of sexual maturation of young adults of L. variegates is dependent on size rather than on age, and that adults produce gametes by the time they reach 10 millimeters in diameter (George et al., 2004) (Sophie George, personal communication). At 14-17 months post-fertilization, four unmanipulated (normal), four mm-depleted and four smm-depleted juveniles successfully exceeded 9 or 10 millimeters in diameter. A second batch of normal juveniles was also produced to re-test the premise. In the normal cohort, 11 out of 11 adults from both batches that exceeded 9 millimeters in diameter produced significant gametes (Fig. 5A,D, arrows) apparent within the gonads (Fig. 5G,J,J′).
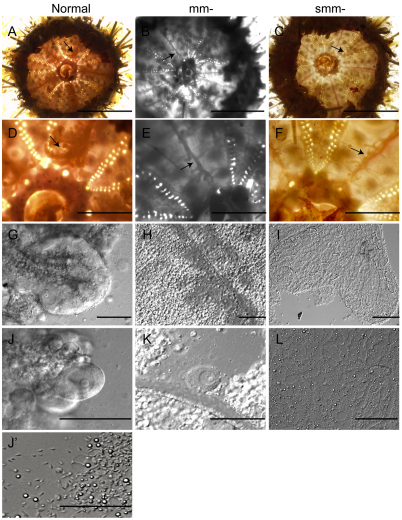
Fig. 5.
Gametogenesis in micromere- or small micromere-removed adult sea urchins. (A,D,G,J,J') Significant gonads are formed (A,D, arrows) in normal sea urchins, and oocytes (G,J) or sperm (J′) were found in these gonadal tubes. (B,E,H,K) Gonads were less apparent in micromere (mm)-removed animals (B,E, arrows) but oocytes (H,K) were sometimes apparent. (C,F,I,L) Poor gonadal tubes (C,F, arrows) developed in small mm-removed animals and no oocytes or sperm were found (I,L). Scale bars: 5 mm in A-C; 1.3 mm in D-F; 100 μm in G-L.
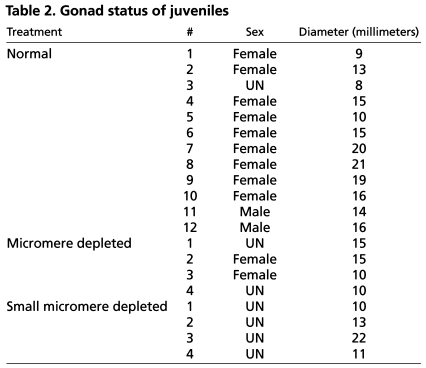
Adults derived from mm-depleted embryos showed intriguing results. Although two out of four adults produced gonads (Fig. 5B,E, arrows) containing gametes (Fig. 5H,K), both adults produced relatively small gonads compared with the normal cohort and two of the adults failed to produce gametes (Table 2). Thus, in L. variegatus, although mm depletion has some negative effect on gonad development and sometimes may cause infertility, some animals are capable of making gametes, consistent with the previous report by Ransick et al. (Ransick et al., 1996) in S. purpuratus. The less effective production of gametes in L. variegates might be explained by the species-dependent variability of the smm potential, the timing of adult rudiment specification, and/or the level of compensatory Vasa expression.
Table 2.
Gonad status of juveniles
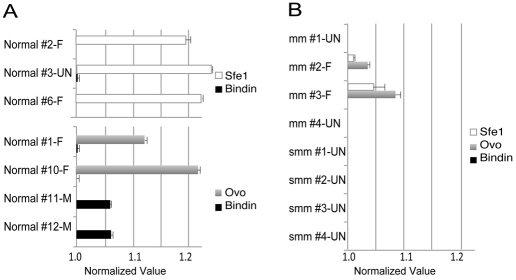
In smm-depleted adults of L. variegatus, each of the four animals showed poorly developed gonads (Fig. 5C,F, arrows) with no gametes visually detectable (Fig. 5I,L). Although we detect yolk platelets (Fig. 5L), which normally function in the transfer of nutrients from somatic cells to oocytes/spermatocytes in the gonadal tissues, we could not find any mature or developing gametes. To survey whether any immature gametes are present that are not detectable under the microscope, we performed a quantitative RT-PCR analysis against oocyte or spermatocyte-specific genes. Probes for sfe1 and ovoperoxidase (ovo) were used as oocyte indicators, and bindin was used as a spermatocyte indicator (Wong and Wessel, 2004). These genes are specific indicators of sea urchin gametes. In normal females (Normal#-F in Fig. 6A), both sfe1 and ovo expression always exceeded the level of bindin, whereas bindin expression was higher than ovo or sfe1 in normal males (Normal #-M in Fig. 6A). The one normal animal examined at 8 millimeters in diameter (Normal #3-UN in Fig. 6A) contained gonads and, although no mature gametes were apparent, the sfe1/bindin index showed that it was a female. Two of the mm-depleted animals (mm #2 and #3 in Fig. 6B) that produced gametes showed a reduced but otherwise similar gene expression profile as normal animals, one that is consistent with their morphology. However, two adults resulting from mm-depletion (mm #4 and #1 in Fig. 6B) and all of the adults from smm-depleted embryos (smm #1-4 in Fig. 6B) showed only background levels of sfe1, ovo and bindin expressions, implying that there is no definitive gamete differentiation in those animals. Taken together, we conclude that smms contribute to gamete formation either directly by cell lineage, or indirectly through signaling to other cells that result in proper gamete development.
Fig. 6.
Gene expression profile of gonads in micromere- or small micromere-removed animals. Gene expression levels of the ovary specific gene Sfe1or Ovoperoxidase (Ovo) and the spermatocyte specific gene Bindin were measured by RT-qPCR in normal animals (A) and in treated animals (B). The value was normalized to an internal control, ubiquitin, and then standardized to the average background level of Bindin in normal females (valued as 1). Each specimen number (#) corresponds to the numbers in Table 2. Results are mean ± s.e.m. –M, male; –F, female; –UN, unknown.
DISCUSSION
Germline specification and Vasa expression in sea urchins
Embryos and larvae segregate a population of cells from which germ cells are ascribed later in development (Wylie and Heasman, 1993; Ransick et al., 1996; Seydoux and Braun, 2006). These cells may have a restricted developmental potential in vivo normally to form only germ cells (e.g. the primordial germ cells of many ecdysosoans), or they may be segregated as multipotent cells to form diverse tissues, eventually including germ cells (e.g. many lophotrochozoans). The potency of the cell being segregated, either strictly for the germline or for diverse tissues (including germ cells) appears to form a continuum over diverse taxa (Juliano and Wessel, 2010). It is clear from the present study that the smms of the sea urchin are required for germ cell development, although the exact potency and lineage fates of the smms are yet unknown. One possible conclusion from these results is that the smms are the PGCs of this organism. It is, however, premature to draw this conclusion as a thorough lineage analysis has yet to be conducted.
A partial explanation to the phenomenon of developmental compensation of the germline in sea urchins appears to be the narrow temporal window in 16-cell stage embryos to respond to mm removal with upregulation of Vasa. The ability to initiate Vasa upregulation begins at the 16-cell stage and was essentially lost within one cell division, by the 28/32 cell stage. The lack of response prior to the 16-cell stage was shown by separating blastomeres from the two-cell to the eight-cell stage; no change was seen in the overall level of Vasa accumulation in the embryo (Voronina et al., 2008). The compensatory response begins with the asymmetric cell division forming the macromeres and mms. Removal of the nascent mms leads to a compensatory Vasa accumulation and the level of compensation appears to decrease with time during the 16-cell stage. Although this Vasa response appears essential for recovery of reproductive capacity in the juvenile, responding to mm removal involves other pathways and targets. For example, the timing of endo 16 expression responds to mm-removal at the fourth, fifth and sixth divisions (Ransick and Davidson, 1995); endo 16 expression levels were delayed and decreased when mms were removed at the fourth division, whereas endo 16 was higher and more consistent between embryos when they were removed at the fifth or sixth divisions. Thus, the duration of contact between the mms and macromeres at the 16-cell stage is relevant to the initial specification of the vegetal plate in the blastula. Prolonged mm/macromere contact leads to increased endo16 in the vegetal plate, but repression of Vasa in non-mms. Additionally, as the coelomic pouches otherwise appear to form normally in smm-depleted embryos, distinct compensatory development for coelomic pouch cells must have occurred from other lineages.
The levels of compensatory Vasa overexpression differ not only in the timing of mm removal but also between species. In L. variegatus, compared with S. purpuratus, only a slight recovery of Vasa expression was observed in mm-depleted embryos, resulting in an incomplete compensatory alteration of the reproductive program. This difference in Vasa response correlates well with the levels of maternal vasa mRNA in each species: L. variegatus has a lower reproductive compensation, a lower level of vasa mRNA and a lower Vasa compensation when compared with S. purpuratus. Thus, Vasa upregulation appears relevant to the compensatory reproductive process, if not causally, then it is at least indicative of the potential for germline specification. This species variability in characters of mm lineage has also been observed in other cases. For example, in Hemicentrotus pulcherrimus, which is phylogenetically close to S. purpuratus, smms have a weak potential to induce endo-mesodermal structures and to differentiate into skeletogenic cells, whereas, in the sand dollar Scaphechinus mirabilis, smms have neither potential (Kurihara and Amemiya, 2005). Furthermore, the left coelomic sac can be regenerated in H. pulcherrimus, but not in S. mirabilis (M. Aihara, PhD thesis, University of Tokyo, 2000). This tissue is the main precursor of the adult rudiment in the larva and contains derivatives of the smm lineage. Perhaps the more rapidly developing species such as S. mirabilis and L. variegatus have less flexibility in terms of smm lineage compensation compared with S. purpuratus and H. pulcherrimus.
A shared mechanism for germline segregation
Vasa is consistently seen as a germline and multipotent cell marker throughout the animal kingdom. Yet, the manner in which Vasa accumulates selectively in these cells is widely different (Raz, 2000); transcriptional specificity, mRNA translation and localization, and post-translational protein stability each plays a major role in Vasa-selective localizations, depending on the organism (e.g. Raz, 2000; Shirae-Kurabayashi et al., 2006; Voronina et al., 2008). The mechanism of Vasa compensatory accumulation in relation to the germline specification seen here, and the narrow temporal window in which the embryo may respond to this induction, may also be conserved. One example that supports this contention is Vasa accumulation and the pattern of germ cell specifications in Ciona intestinalis. C. intestinalis, an urochordate phylogenically close to echinoderms, does not have autonomously specified germ cells, but germline segregation does occur during embryonic development (Shirae-Kurabayashi et al., 2006). The B7.6 cells of C. intestinalis embryos express the Ciona Vasa homolog, and they subsequently undergo an asymmetric cell division to produce two daughter cells, B8.11 and B8.12. The B8.11 cells do not contribute to germ cells and lose Vasa localization, whereas Vasa production in B8.12 cells is further upregulated, resulting in the formation of the germ granules and incorporation into the gonad in juveniles. This process is similar to what we have seen here: the significant Vasa protein expression starts first in mms, but large mms lose Vasa expression and do not contribute to the adult structures (Okazaki, 1975; Yajima, 2007a; Yajima, 2007b), whereas smms increase Vasa accumulation and contribute to the germline. Therefore, we hypothesize that the Vasa function in formation and maintenance of the smms is similar to that of the B8.12 cells of C. intestinalis. Furthermore, the pattern of Vasa distribution and the lineages of germlines seem to be closely linked in these animals.
The regulation of Vasa accumulation leading to reproductive success may be related to the mechanism of selective Vasa turnover. Some reports indicate that E3-ubiquitin ligase complexes contribute to establishing a finely tuned steady state of Vasa ubiquitylation (Kugler et al., 2010; Gustafson et al., 2010). Thus, in response to the mm removal, a repression of the degradation mediated E3-ubiquitin ligase may occur that leads selectively to the overexpression of Vasa in all blastomeres to restart the developmental program related to the germline specifications. Understanding this inducible process will be essential in the future for understanding reproductive success.
In summary, we conclude that the smms contribute to the germline in adult sea urchins and that developmental compensation for removal of the mms, a major signaling center of the embryo, is related to levels of Vasa expression in the remaining embryo. The compensatory activity of Vasa expression upon mm, or smm removal appears to explain in part the species differences seen here between S. purpuratus and L. variegatus, and their ability to make, or not to make, respectively, gametes following mm removal. The regulation and timing of Vasa expression appears relevant to the process, if not causally, then it is at least indicative of the potential for normal and compensatory germline specification.
Acknowledgments
This work was supported by NIH and NSF grants to G.M.W., and by a HFSP long-term fellowship to M.Y. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Cameron R. A., Hough-Evans B. R., Britten R. J., Davidson E. H. (1987). Lineage and fate of each blastomere of the eight-cell sea urchin embryo. Genes Dev. 1, 75-85 [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Fraser S. E., Britten R. J., Davidson E. H. (1991). Macromere cell fates during sea urchin development. Development 113, 1085-1092 [DOI] [PubMed] [Google Scholar]
- Cox D. N., Chao A., Baker J., Chang L., Qiao D., Lin H. (1998). A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 12, 3715-3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboc V., Röttinger E., Lapraz F., Besnardeau L., Lepage T. (2005). Left-right asymmetry in the sea urchin embryo is regulated by nodal signaling on the right side. Dev. Cell 9, 147-158 [DOI] [PubMed] [Google Scholar]
- Endo Y. (1966). Development and differentiation. In Biology of Today (in Japanese), pp. 1-61 Tokyo: Iwanami Shoten; [Google Scholar]
- Ettensohn C. A., McClay D. R. (1988). Cell lineage conversion in the sea urchin embryo. Dev. Biol. 125, 396-409 [DOI] [PubMed] [Google Scholar]
- Extavour C. G., Akam M. (2003). Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130, 5869-5884 [DOI] [PubMed] [Google Scholar]
- Forbes A., Lehmann R. (1998). Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 125, 679-690 [DOI] [PubMed] [Google Scholar]
- George S. B., Lawrence J. M., Lawrence A. L. (2004). Complete larval development of the sea urchin Lytechinus variegatus fed an artificial feed. Aquaculture 242, 213-224 [Google Scholar]
- Gustafson E. A., Yajima M., Juliano C. E., Wessel G. M. (2010). Posttranslational regulation by gustavus contributes to selective Vasa protein accumulation in multipotent cells during embryogenesis. Dev. Biol. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstadius S. (1950). The mechanics of sea urchin development. Annee Biol. 26, 381-398 [PubMed] [Google Scholar]
- Huang B., Wang S., Ning Y., Lamb A. N., Bartley J. (1999). Autosomal XX sex reversal caused by duplication of SOX9. Am. J. Med. Genet. 87, 349-353 [DOI] [PubMed] [Google Scholar]
- Huettner A. F. (1923). The origin of the germ cells in Drosophila melanogaster. J. Morphol. 2, 385-422 [Google Scholar]
- Illmensee K., Mahowald A. P. (1974). Transplantation of posterior polar plasm in Drosophila. Induction of germ cells at the anterior pole of the egg. Proc. Natl. Acad. Sci. USA 71, 1016-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C. E., Wessel G. M. (2009). An evolutionary transition of vasa regulation in echinoderms. Evol. Dev. 11, 560-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C., Wessel G. M. (2010). Versatile germline genes. Science 329, 640-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C. E., Voronina E., Stack C., Aldrich M., Cameron A. R., Wessel G. M. (2006). Germ line determinants are not localized early in sea urchin development, but do accumulate in the small micromere lineage. Dev. Biol. 300, 406-415 [DOI] [PubMed] [Google Scholar]
- Juliano C. E., Swartz S. Z., Wessel G. M. (2010a). A conserved germline multipotency program. Development 137, 4113-4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano C. E., Yajima M., Wessel G. M. (2010b). Nanos functions to maintain the fate of the small micromere lineage in the sea urchin embryo. Dev. Biol. 337, 220-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Yamada M., Asaoka M., Kitamura T. (1996). Essential role of the posterior morphogen nanos for germline development in Drosophila. Nature 380, 708-711 [DOI] [PubMed] [Google Scholar]
- Kugler J. M., Woo J. S., Oh B. H., Lasko P. (2010). Regulation of Drosophila vasa in vivo through paralogous cullin-RING E3 ligase specificity receptors. Mol. Cell. Biol. 30, 1769-1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H., Amemiya S. (2005). Developmental potential of small micromeres in sea urchin embryos. Zool. Sci. 22, 845-852 [DOI] [PubMed] [Google Scholar]
- Lasko P. F., Ashburner M. (1988). The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature 335, 611-617 [DOI] [PubMed] [Google Scholar]
- Lasko P. F., Ashburner M. (1990). Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 4, 905-921 [DOI] [PubMed] [Google Scholar]
- Okazaki K. (1975). Normal development to metamorphosis. In The Sea Urchin Embryo: Biochemistry and Morphogenesis (ed. Czihak G.), pp. 177-232 New York: Springer-Verlag; [Google Scholar]
- Oliveri P., Tu Q., Davidson E. H. (2008)., Global regulatory logic for specification of an embryonic cell lineage. Proc. Natl. Acad. Sci. USA 105, 5955-5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson J. R., Cohen L. H. (1986). The fate of the small micromeres in sea urchin development. Dev. Biol. 113, 522-526 [DOI] [PubMed] [Google Scholar]
- Ransick A., Davidson E. H. (1993). A complete second gut induced by transplanted micromeres in the sea urchin embryo. Science 259, 1134-1138 [DOI] [PubMed] [Google Scholar]
- Ransick A., Davidson E. H. (1995). Micromeres are required for normal vegetal plate specification in sea urchin embryos. Development 121, 3215-3222 [DOI] [PubMed] [Google Scholar]
- Ransick A., Cameron R. A., Davidson E. H. (1996). Postembryonic segregation of the germ line in sea urchins in relation to indirect development. Proc. Natl. Acad. Sci. USA 93, 6759-6763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz E. (2000). The function and regulation of vasa-like genes in germ-cell development. Genome Biol. 1, 1017.1-1017.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas L. S., Maldonado E., Macías-Silva M., Blackwell T. K., Navarro R. E. (2007). The DEAD box RNA helicase VBH-1 is required for germ cell function in C. elegans. Genesis 45, 533-546 [DOI] [PubMed] [Google Scholar]
- Seydoux G., Braun R. E. (2006). Pathway to totipotency: lessons from germ cells. Cell 127, 891-904 [DOI] [PubMed] [Google Scholar]
- Shirae-Kurabayashi M., Nishikata T., Takamura K., Tanaka K. J., Nakamoto C., Nakamura C., Nakamura A. (2006). Dynamic redistribution of vasa homolog and exclusion of somatic cell determinants during germ cell specification in Ciona intestinalis. Development 133, 2683-2693 [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119 [DOI] [PubMed] [Google Scholar]
- Styhler S., Nakamura A., Swan A., Suter B., Lasko P. (1998). Vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development 125, 1569-1578 [DOI] [PubMed] [Google Scholar]
- Tanaka S., Dan K. (1990). Study of the lineage and cell cycle of small micromeres in embryos of the sea urchin, Hemicentrotus pulcherrimus. Dev. Growth Differ. 32, 145-156 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Tsuda M., Saga Y. (2007). Functional redundancy among Nanos proteins and a distinct role of Nanos2 during male germ cell development. Development 134, 77-83 [DOI] [PubMed] [Google Scholar]
- Tanaka S. S., Toyooka Y., Akasu R., Katoh-Fukui Y., Nakahara Y., Suzuki R., Yokoyama M., Noce T. (2000). The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 14, 841-853 [PMC free article] [PubMed] [Google Scholar]
- Tomancak P., Guichet A., Zavorszky P., Ephrussi A. (1998). Oocyte polarity depends on regulation of gurken by Vasa. Development 125, 1723-1732 [DOI] [PubMed] [Google Scholar]
- Tsuda M., Sasaoka Y., Kiso M., Abe K., Haraguchi S., Kobayashi S., Saga Y. (2003). Conserved role of nanos proteins in germ cell development. Science 301, 1239-1241 [DOI] [PubMed] [Google Scholar]
- Voronina E., Lopez M., Juliano C. E., Gustafson E., Song J. L., Extavour C., George S., Oliveri P., McClay D., Wessel G. M. (2008). Vasa protein expression is restricted to the small micromeres of the sea urchin, but is inducible in other lineages early in development. Dev. Biol. 314, 276-286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Lin H. (2004). Nanos maintains germline stem cell self renewal by preventing differentiation. Science 303, 2016-2019 [DOI] [PubMed] [Google Scholar]
- Wong J. L., Wessel G. M. (2004). Major components of a sea urchin block to polyspermy are structurally and functionally conserved. Evol. Dev. 6, 134-153 [DOI] [PubMed] [Google Scholar]
- Wylie C. C., Heasman J. (1993). The biology of primordial germ cells. Semin. Dev. Biol. 4, 161-170 [DOI] [PubMed] [Google Scholar]
- Yajima M. (2007a). Evolutionary modification of mesenchyme cells in sand dollars in the transition from indirect to direct development. Evol. Dev. 9, 257-266 [DOI] [PubMed] [Google Scholar]
- Yajima M. (2007b). A switch in the cellular basis of skeletogenesis in late-stage sea urchin larvae. Dev. Biol. 307, 272-281 [DOI] [PubMed] [Google Scholar]