Abstract
Long-term survival after lung transplantation is limited by acute and chronic graft rejection. Induction of immune tolerance by first establishing mixed hematopoietic chimerism (MC) is a promising strategy to improve outcomes. In a preclinical canine model, stable MC was established in recipients after reduced-intensity conditioning and hematopoietic cell transplantation from a DLA-identical donor. Delayed lung transplantation was performed from the stem cell donor without pharmacological immunosuppression. Lung graft survival without loss of function was prolonged in chimeric (n=5) vs. nonchimeric (n=7) recipients (p≤0.05, Fisher’s test). There were histological changes consistent with low grade rejection in 3/5 of the lung grafts in chimeric recipients at ≥1 year. Chimeric recipients after lung transplantation had a normal immune response to a T-dependent antigen. Compared to normal dogs, there were significant increases of CD4+INFγ+, CD4+IL-4+ and CD8+ INFγ+ T-cell subsets in the blood (p <0.0001 for each of the 3 T-cell subsets). Markers for regulatory T-cell subsets including foxP3, IL10 and TGFβ were also increased in CD3+ T cells from the blood and peripheral tissues of chimeric recipients after lung transplantation. Establishing MC is immunomodulatory and observed changes were consistent with activation of both the effector and regulatory immune response.
Introduction
In chimeric recipients, donor-specific tolerance may be established to solid organ grafts from the hematopoietic cell (HC) donor but not third-party donors (1–8). However, rejection of marrow donor-derived skin grafts has been reported in chimeric recipients (9–12). Tissue-specific minor histocompatibility antigens in organs other than skin are likely present, but skin grafts appear to be the most susceptible to rejection (11). In an outbred dog model, stable mixed hematopoietic chimerism (MC) can be achieved after nonmyeloablative conditioning and transplantation of a HC graft from a DLA-identical donor (13,14). Kidney allografts transplanted from the HC donor into these canine chimeric recipients have survived without rejection with follow-up to 5 years (3). Peripheral immune mechanisms contribute to the donor-specific tolerance in canine recipients with MC (5,15). In humans, there is some limited experience transplanting kidney allografts from HLA-identical HC donors for end stage renal disease after HCT. Only one graft rejection was observed in these patients (7,8). Because lung allografts are more immunogenic than kidney allografts, donor-specific tolerance established in chimeric recipients may not be sufficient to prevent rejection.
A major limitation to improving survival after lung transplantation is graft rejection. The overall patient survival at 1, 5 and 10 years after lung transplantation has been reported as 78%, 50% and 26%, respectively (16). Chronic graft rejection in survivors was 33% and 43% at 5 and 10 years, respectively (16). One of the strongest risk factors for chronic rejection is the number and severity of acute rejection episodes. Although pharmacological immunosuppression has reduced the incidence of acute rejection after lung transplantation and increased early survival, chronic rejection persists as a major complication (17). Also, the intensive and prolonged pharmacological immunosuppression required for lung transplantation may increase the risk for developing opportunistic infections.
Based on the substantial unmet medical need for better methods to prevent lung allograft rejection and to improve overall survival, combined hematopoietic cell and lung transplantation from DLA-identical HC donors was investigated in an outbred canine model. Combined hematopoietic cell and kidney transplantation from HLA-haploidentical donors was effective for inducing tolerance without pharmacological immunosuppression in 4 of 5 recipients (18). Although, clinically, most lung allografts are from HLA-mismatched donors, transplantation of combined HC and lung grafts from DLA-identical littermates as in this model would provide further insights into the nature of donor-specific tolerance. Also, living donor lobar transplants are now more frequently performed and could be considered for complications resulting in end-stage lung disease after allogeneic HCT from HLA-identical siblings (19–21).
Materials and Methods
Dogs
Litters of mini-mongrel cross-breeds and beagles were either purchased from commercial kennels or raised at the Fred Hutchinson Cancer Research Center (FHCRC). The dogs weighed a median of 12.1 (8.0 to 20.9) kg and were a median of 26 (11 to 50) months old at lung transplantation. They were observed for disease for at least 60 days before study. All were immunized for leptospirosis, papillomavirus, distemper, hepatitis, and parvovirus. Research was conducted according to the principles outlined in the Guide for Laboratory Animal Facilities and Care prepared by the National Academy of Sciences, National Research Council. The Institutional Animal Care and Use Committee of the FHCRC approved the research protocols and the American Association for Accreditation of Laboratory Animal Care certified the kennels. Littermate donor/recipient pairs that underwent HCT or lung transplantation were DLA-identical on the basis of matching for highly polymorphic MC class I and class II microsatellite markers (22). In addition, specific DLA DRB1 allelic identity was confirmed by direct sequencing (23).
Hematopoietic cell transplantation
Five recipients had stable MC at lung transplantation. They had first received a HC graft from the DLA-identical lung graft donor after a nonmyeloablative conditioning regimen of total body irradiation (TBI) (Table 1) (13,24). The canine model of HCT has been previously described (4,25).
Table 1.
Baseline Characteristics of Recipients with Mixed Hematopoietic Chimerism
| Hematopoietic Cell Transplantation (HCT) | Interval between HCT + OLT (months) | Orthotopic Lung Transplantation (OLT) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Recipient | Age at HCT (months) | TBI dose* (cGy) | Source of HSC | Post-HCT IS** | Donor Chimerism | ||||
| Marrow Cells/Kg (×108) | G-PBMC Cells/Kg (×108) | Age at OLT (months) | Before OLT (%) | 6 Wk Post- OLT (%) | |||||
| G190 | 9 | 200 | 4.0 | 0 | CSP/MMF | 7 | 16 | L=76.2 G=81.3 |
L=74.7 G=78.5 |
| G005 | 22 | S5/200 | 4.0 | 10.5 | CSP/MMF | 13 | 35 | L=32.0 G=57.4 |
L=53.2 G=44.7 |
| E875 | 45 | 200 | 0 | 2.8 | None | 5 | 50 | L=74.6 G=100 |
L=80.2 G=100 |
| G246 | 15 | 200 | 6.7 | 8.7 | CSP/MMF | 11 | 26 | L=60.8 G=91.1 |
L=37.4 G=68.3 |
| G183 | 15 | 200 | 4.7 | 0 | CSP/MMF | 21 | 36 | L=37.1 G=71.6 |
L=60.3 G=92.7 |
BM = bone marrow; CSP = cyclosporine; G-PBMC = granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood mononuclear cells; HCT = hematopoietic cell transplantation; HSC = hematopoietic stem cell; IS = immunosuppression; MMF = mycophenolate mofetil; OLT = orthotopic lung transplantation; S5 = CD44-specific monoclonal antibody; TBI = total body irradiation. For donor chimerism in peripheral blood: L= CD3+ lymphocytes, G= granulocytes
TBI was delivered at doses of 2 Gy from a high energy linear accelerator (Varian CLINAC 4, Palo Alto, CA) at a rate of 7 cGy/min.
For immunosuppression, oral cyclosporine (15 mg/kg b.i.d.) was administered for 35 days and mycophenolate mofetil (10 mg b.i.d., s.c.) was administered for 28 days after HCT.
Assessment of hematopoietic cell engraftment
Hematopoietic engraftment was determined by sustained recoveries of granulocyte and platelet counts after radiation nadirs and by chimerism studies from serial peripheral blood samples as previously described (26). Chimerism was assessed after both HCT and lung transplantation in recipients with MC until the end of study (see Supplement for details).
Lung transplantation
Orthotopic left lung transplantation was performed in four groups of dogs. Group 1 (n=4) underwent autologous lung transplantation. Group 2 (n=5) had stable MC and underwent lung transplantation from the DLA-identical donor of the HC graft (Table 1). Recipients in group 3 (n=7) were without donor-derived hematopoietic chimerism and received no immunosuppression before or after lung transplantation from a DLA-identical donor. Recipients in group 4 (n=3) were without donor-derived hematopoietic chimerism and received short-term immunosuppression after lung transplantation. The lung transplantation procedure has been previously described and is described in more detail in the Supplement with modifications (27).
Pulmonary function tests
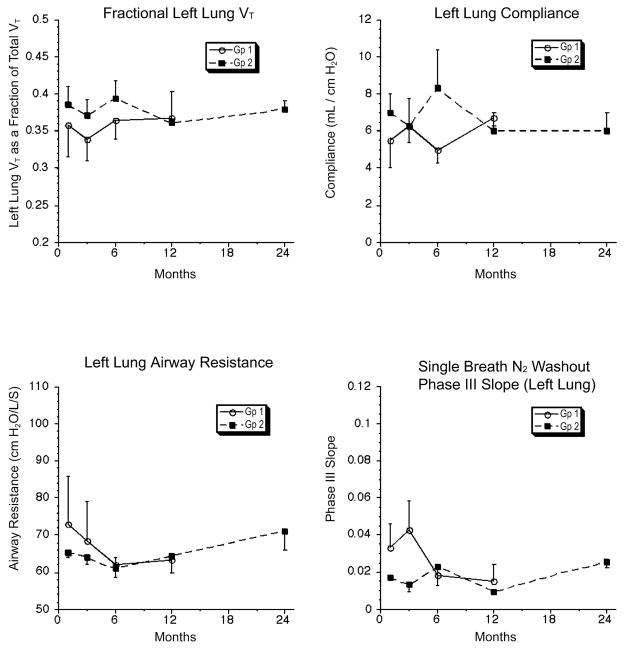
Pulmonary function studies were obtained at 1, 3, 6, and 12 months in both groups 1 and 2 and at 24 months in group 2 after lung transplantation as previously described (28–31). Pulmonary function tests could not be conducted beyond 1 month in group 3 because of graft loss. Measurements of lung volumes, carbon monoxide diffusion capacity, airway resistance, compliance and single breath nitrogen washout were performed as described in the Supplement.
Assessment of lung graft for rejection
After lung transplantation, the recipient was assessed for lung graft rejection by daily clinical evaluations, chest X-rays and pulmonary function tests. Rejection was confirmed and graded histologically on tissue obtained at necropsy (32). Sections from formalin-fixed blocks were stained with hematoxylin and eosin.
Immune response to a neoantigen
Three chimeric recipients with a lung allograft were immunized with a neoantigen (bacteriophage φx-174) according to a standard protocol (33,34). This T-cell dependent neoantigen requires the interaction of antigen-presenting cells, T cells, and B cells for the development of a normal IgG response. Bacteriophage φX174 was administered intravenously at a dose of 3 × 109 plaque forming units/kg. Serial blood draws were performed at 15 minutes (time 0) and then at 4, 7, 14 and 28 days. A secondary immunization was given at 42 days. Phage clearance and specific phage-neutralizing antibody activity, expressed as the rate of phage inactivation (K value [Kv]), was determined as previously described (35).
T-cell subset analysis by flow cytometry
T-cell subsets were identified by surface and intracellular staining for surface markers and cytokines, respectively, using a previously described procedure that had been modified (see Supplement) (36). Cytotoxic and helper T-cell subsets were identified with a combination of canine CD3-specific antibodies (clones CA17.6F9 or CA17.6B3) conjugated to FITC along with PE-conjugated (ProZyme, San Leandro, CA) antibody to CD8 (CA9.JD3) or CD4 (CA13.1E4) (gift from Peter Moore, UC Davis). Antibody to canine interferon gamma (IFNγ), tumor necrosis factor alpha (TNFα), interleukin 4 (IL4) or interleukin 10 (IL10) (R&D Systems, Minneapolis, MN) which was directly conjugated to Alexa647 (Invitrogen, Carlsbad, CA) was added. Results were reported as the percentage of cytokine positive CD3+CD8+ or CD3+CD4+ events out of the total number of CD3+CD8+ or CD3+CD4+ cells for that particular cytokine.
Quantitative reverse transcribed-PCR of intracellular cytokines and FoxP3 from CD3+ T cells
T cells were selected with canine CD3-specific monoclonal antibodies CA17.6F9 or CA17.6B3 using Miltenyi bead technology (Miltenyi Biotec, Auburn, CA). Purity of the cell sorts was determined by flow cytometry. Median CD3+ T cell purities of the sorted cells from peripheral blood, lymph node, spleen, right lung and left lung were 93 (85–99)%, 98 (92–99)%, 92 (87– 97)%, 87 (85–95)% and 90 (86–97)%, respectively. From the sorted CD3+ T cells, mRNA was extracted, isolated, and transcribed to cDNA using μMACS One Step cDNA kits (Miltenyi Biotec, Auburn, CA). FoxP3 expression was measured by quantitative reverse transcribed (RT)- PCR using primers and Taqman probe designed by Primer Express (Applied Biosystems, Foster City, CA). The sequences of the primers used for the amplification and detection of canine FoxP3 were: forward primer, 5′-AGGATTTCCTCAAGCACTGC-3′; reverse primer, 5′-TGGAAGAACTCTGGGAATGTG-3′ and probe 6FAM 5′-TGGTGCAGTCTCTGGAACAG- 3′ TAMARA. The sequences of the primers used for quantitative RT-PCR of canine interleukin- 10 (IL-10), canine transforming growth factor beta (TGFβ) and the housekeeping gene, canine glycerol-3-phosphate dehydrogenase (G3PDH) have been previously published (37). Absolute copy numbers were calculated based on FoxP3, IL-10 or TGFβ standard curves, and samples were normalized to a second standard curve for the housekeeping gene G3PDH (see Supplement for further details) (37,38).
Statistical analysis
Comparisons of effector response and expression between groups of dogs were evaluated via two-sample t-tests. All p-values are two-sided. No adjustment has been applied for multiple comparisons.
Results
Stable mixed hematopoietic chimerism after hematopoietic cell transplantation
After a nonmyeloablative conditioning regimen and HCT, all recipients were chimeric and without GVHD (Table 1). The levels of donor chimerism in the myeloid and lymphoid compartments of the peripheral blood were stable in all recipients before and after lung transplantation.
Prolonged survival of lung allografts in recipients with mixed hematopoietic chimerism
Four groups of recipients underwent orthotopic single lung transplantation (Table 2). Group 1 recipients underwent autologous lung transplantation and were followed for a median of 14 (12– 18) months. Group 2 (n=5) included chimeric recipients that received the lung graft from the DLA-identical donor of the HC graft. Stable MC was present for a median of 11 (5– 21) months before performing lung transplantation. Recipients were followed for a median of 27 (3–29) months. One recipient (G246) could only be followed for 3 months because of a complication that occurred related to open lung biopsy. Lung grafts in group 2 survived until the last scheduled follow-up. The tidal volume, compliance, airway resistance and nitrogen wash-out of the lung grafts in group 2 at 24 months were similar to group 1 at 12 months (Figure 1). There was no change in function of the lung grafts in group 2 compared to baseline evaluations. Although pulmonary function was normal, there was histological evidence of minimal-mild rejection (A1–A2) in 3 of the 5 lung grafts in group 2 (Table 2; Figure 2). There was no histological evidence for graft rejection in the right native lungs from group 2 or in the autologous lung grafts in group 1. A moderate-severe vascular rejection process (Grades A3 and A4) was observed in 5/7 of the group of DLA-identical non-chimeric recipients without posttransplant immunosuppression (group 3) compared to 0/5 in group 2 (p≤0.05, Fisher’s test). Two of the first 3 recipients in group 3 had evidence of mild vascular rejection (Grade A1) when they were euthanized at 6 and 7 days after lung transplantation for early radiographic changes in the lung grafts. Subsequent recipients were followed longer even if some changes were noted on the chest X-ray at 1 week after lung transplantation. None of the lung grafts in group 3 survived greater than 33 days after lung transplantation. Short-term immunosuppression with CSP and MMF after lung transplantation in group 4 delayed but did not prevent the loss of the lung graft in non-chimeric recipients.
Table 2.
Orthotopic Transplantation of the Left Lung from DLA-Identical Donor
| Group | UPN | Rejection (Y/N) | Histopathology of the Lung* | Outcome (mo) |
|---|---|---|---|---|
| 1. Autologous | G071 | N | A0,B0 | ET2** (18 mo) |
| G042 | N | A0,B0 | ET2 (17mo) | |
| G135 | N | A0,B0 | ET2 (14 mo) | |
| G137 | N | A0,B0 | ET2 (12 mo) | |
| 2. Allogeneic Mixed Chimerism‡ | G190 | N | A0,B0 | ET2 (27 mo) |
| G005 | N | A1,B0† | ET2 (29 mo) | |
| E875 | N | A2,B1,Ca†† | ET2 (27 mo) | |
| G246 | N | A0,B0 | ET1† (3 mo) | |
| G183 | N | A2,B0†† | ET2 (12 mo) | |
| 3. Allogeneic Nonchimeric, No CSP/MMF§ | G020 | N | A1B0 | ET2 (7 days) |
| G 260 | Y | A4B0 | ET2 (8 days) | |
| G 379 | N | A1B0 | ET2 (6 days) | |
| G 434 | Y | A3B0 | ET2 (22 days) | |
| G 392 | Y | A3B0 | ET2 (23 days) | |
| G390 | Y | A4B0 | ET2 (19 days) | |
| G533 | Y | A4,B4 | ET2 (33 days) | |
| 4. Allogeneic Nonchimeric, CSP/MMF§§ | G326 | Y | A3,B1 | ET2 (6 mo) |
| G209 | Y | A4,B4 | ET2 (3 mo) | |
| G262 | Y A4,B0 ET2 (5 mo) | |||
Lung tissue was obtained at necropsy of recipients. Acute cellular rejection can involve the vasculature and the airways of the lung graft. Using the revised criteria of the International Society for Heart and Lung Transplantation [34], acute rejection of the vasculature was graded A0-A4 based on the extent of mononuclear cell infiltration of perivascular and interstitial areas as well as whether hemorrhage and necrosis were present. An A1 grade of minimal acute rejection was characterized by rare scattered perivascular infiltrates that were not obvious at low magnification. In the A4 grade there were prominent perivascular and interstitial infiltrates associated with hemorrhage and necrosis. Acute cellular rejection involving the airways was graded B0-B4 based largely on the degree of lymphocytic, monocytic and eosinophilic infiltration in bronchi and bronchioles as well as epithelial apoptosis. A B1 grade of minimal airway rejection displayed rare mononuclear cells infiltrating the bronchial and bronchiolar submucosa.. In the B4 grade of severe airway rejection there was marked inflammatory infiltration along with epithelial necrosis. Fibrous obstruction of bronchioles was graded Ca if accompanied by infiltrating inflammatory cells or Cb if inflammation was absent.
ET1 Complication from open lung biopsy; ET2 Euthanized at end of study. Clinically stable with or without rejection of lung graft.
G005: Foci of rare perivascular infiltrates. Right native lung was normal.
G183 and E875: Had occasional foci of A2 histology. E875 had rare bronchiolar changes including bronchiolar fibrosis with mononuclear infiltrates. The right native lungs were normal.
Lung transplantation from DLA-identical HC donor into recipient with stable mixed hematopoietic chimerism (MC).
No pretransplant or posttransplant immunosuppression was administered.
Lung transplantation from DLA-identical donor into recipient without MC and no immunosuppression.
Lung transplantation from DLA-identical donor into recipient without MC and immunosuppression with oral cyclosporine (CSP) (15 mg/kg b.i.d.) for 35 days and mycophenolate mofetil (MMF) (10 mg b.i.d., s.c.) for 28 days after HCT.
Figure 1.
Pulmonary function tests on recipients after autologous lung transplantation (group1) and chimeric recipients after lung transplantation from the donor of the hematopoietic cell graft (group 2). Recipients in group1 had their last pulmonary function test at 12 months after lung transplantation. No difference was observed in tidal volume, compliance, airway resistance or nitrogen wash-out curves at 12 months between group 1 and 2. Three recipients in group 2 had pulmonary function tests at 24 months. There was no change in pulmonary function between 12 and 24 months for the 3 recipients from group 2. No pulmonary function tests were done in group 3 beyond the baseline tests since acute rejection was complete within 1 month of lung transplantation.
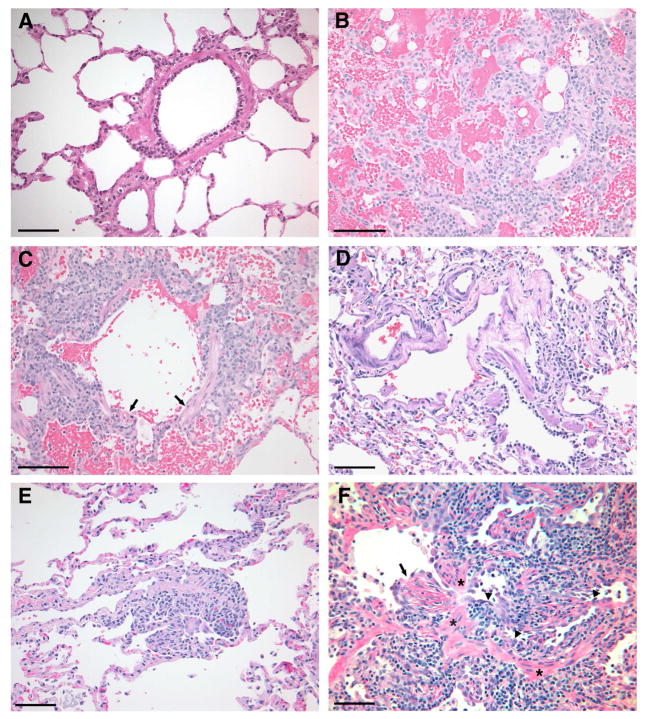
Figure 2. Necropsy histology and rejection classification of the lung grafts at the end of study (see Table 2 for a description of the grading system).
A) Autologous control (dog G135, group 1): Normal lung histology with no evidence of graft rejection (Grade A0, B0).
B) Rejection control (dog G533, group 3): The vessel in the lower right corner displays a perivascular lymphoid infiltrate which extends into the alveolar septal interstitium. There is marked alveolar hemorrhage. The alveolar septal devitalization in the upper left corner borders an area of parenchymal necrosis. (Grade A4).
C) Rejection control (dog G533, group3): In the same lung illustrated in (B), there is peribronchial lymphocytic infiltration and focal necrosis of bronchiolar epithelium (arrows). The lymphoid infiltrate extends into the alveolar interstitium. Alveolar hemorrhage is present. (Grade B4).
D) Chimeric recipient (dog G190, group 2): Normal lung histology with no evidence of graft rejection at end of study (Grade A0, B0; 27 months).
E) Chimeric recipient (dog G005, group 2): This perivascular infiltration by small numbers of lymphocytes is consistent with mild graft rejection. The airways (not shown) were uninvolved. (Grade A1, B0; 29 months).
F) Chimeric recipient (dog E875, group 2): Histological changes are observed consistent with active acute and chronic graft rejection (Grade A2, B1, Ca; 27 months). The bronchiole which extends horizontally across the photomicrograph is narrowed by lymphocytic infiltration of the mucosa (arrowheads) and proliferation of granulation tissue (arrow). Bands of smooth muscle (asterisks) in the bronchiolar wall serve as anatomic landmarks. The alterations are characteristic of early obliterative bronchiolitis, grade Ca (active) in the International Society for Heart and Lung Transplantation grading system, This is the histological expression of active chronic lung rejection as well as chronic pulmonary graft-versus-host disease. Bars represent 100 microns in all panels. Images were processed using Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Normal immune function in chimeric recipients after lung transplantation
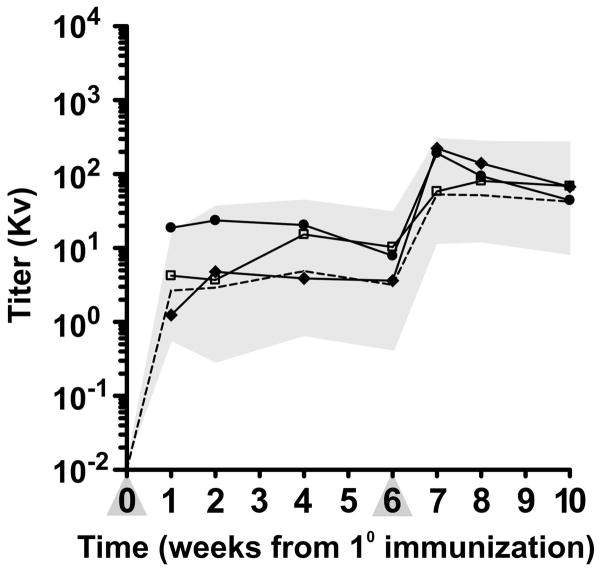
Three chimeric recipients with the longest graft survival in group 2 were immunized with bacteriophage φx174 to assess immune function at 14, 18 and 19 months after transplantation, respectively. Neutralizing antibody production after primary and secondary immunization was similar to historical normal controls (Figure 3). The normal immune response to this neoantigen in chimeric recipients after lung transplantation suggests that the prolonged graft survival resulted from tolerance and not immunosuppression.
Figure 3.
Immune response in chimeric recipients (E875, G005 and G190) after lung transplantation to a neoantigen. Antibody responses to the T cell-dependent neoantigen, bacteriophage ΦX174 (phage) were measured in three chimeric lung recipients. Phage was injected intravenously twice, 6 weeks apart (primary and secondary immunizations) at a dose of 3 × 109 PFU/kg body weight, and the production of phage-specific antibody determined. Antibody titers were measured by a neutralizing assay and expressed as rate of phage inactivation (Kv). The geometric mean antibody titers and the 95% confidence interval of normal dogs is indicated by the interrupted line and the shaded area. Percent IgG was determined for serum samples obtained 2 weeks after secondary immunization.
◆- ◆ (E875, 68% IgG), •- • (G005, 68% IgG), □- □ (G190, 100% IgG).
Increased Tc1, Th1 and Th2 effector responses in lung allograft recipients with stable mixed hematopoietic chimerism
Peripheral blood T-cell subsets based on intracellular cytokine production were determined in chimeric recipients without lung allografts (n=10) at a median of 13 (10–24) months after HCT or with lung allografts (n=4) at a median of 21 (2–29) months after lung transplantation. Compared to normal dogs (n=10), chimeric recipients without lung allografts had an increase of CD3+CD4+INFγ+ (Th1) and CD3+CD4+IL4+ (Th2) T cells (p= 0.02, p=0.006 respectively) (Table 3). After lung transplantation, there was a significant increase in levels of CD3+CD8+INFγ+ (Th1), Th1 and Th2 T cells compared to both normal controls (p<0.0001, p<0.0001, p<0.0001, respectively) and chimeric recipients without lung transplantation (p=0.03, p=0.04 and p=0.01, respectively). A significant increase was not observed in the CD3+CD4+TNFα+ or CD3+ CD4+IL10+ T-cell subsets between any of these groups. However, when chimeric recipients with other solid organ grafts (e.g. kidney and small intestine) were included with the lung graft group (n=9), CD3+CD4+TNFα+ T cells were also observed to be increased compared to normal dogs (p=0.04).
Table 3.
T-cell subsets from the peripheral blood in recipients with stable mixed hematopoietic chimerism: Effect of lung transplantation#
| Peripheral Blood | Normal (n=10) Mean % (SD) | MC (n=10) Mean % (SD) | MC/Lung (n=4) Mean % (SD) | P-value Normal vs. MC | P-value Normal vs. MC/Lung | P-value MC vs. MC/Lung |
|---|---|---|---|---|---|---|
| * CD8 INFγ | 17.0 (4.8) | 21.2 (9.4) | 33.5 (4.2) | 0.23 | <0.0001 | 0.03 |
| + CD4 INFγ | 12.6 (4.2) | 19.4 (7.4) | 29.5 (6.5) | 0.02 | <0.0001 | 0.04 |
| CD4 TNFα | 7.6 (5.0) | 9.7 (5.1) | 11.7 (3.8) | 0.38 | 0.17 | 0.50 |
| + CD4 IL-4 | 1.3 (1.1) | 4.2 (2.7) | 10.1 (4.8) | 0.006 | <0.0001 | 0.01 |
| + CD4 IL-10 | 1.7 (0.6) | 1.7 (0.8) | 2.1 (0.8) | 0.99 | 0.30 | 0.37 |
Footnotes: IL-4 = interleukin 4; IL-10 = interleukin 10; INFγ = interferon gamma; MC = mixed chimerism; MC/Lung = mixed chimerism + lung transplantation; SD = standard deviation; TNFα = tumor necrosis factor alpha.
All recipients and normal dogs had T cell subsets from the peripheral blood assessed at least twice. Transplant recipients were assessed while the grafts and clinical status were stable.
CD3+CD8+INFγ+ T cells are given as a percentage of the total number of CD3+CD8+ T cells.
CD3+CD4+ T cell subsets are given as a percentage of the total number of CD3+CD4+ T cells.
FoxP3, IL10 and TGFβ in CD3+ T cells from blood and tissue in chimeric recipients with and without lung allografts
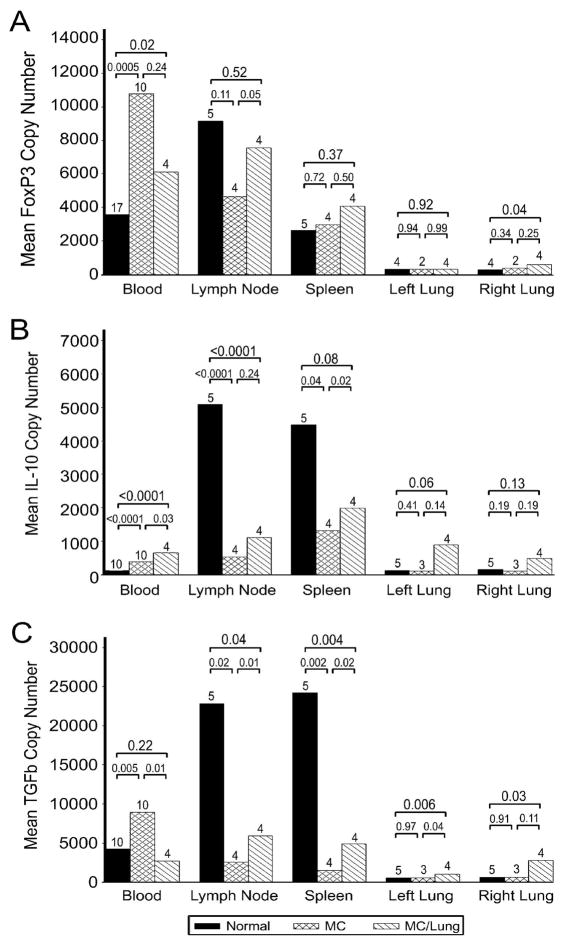
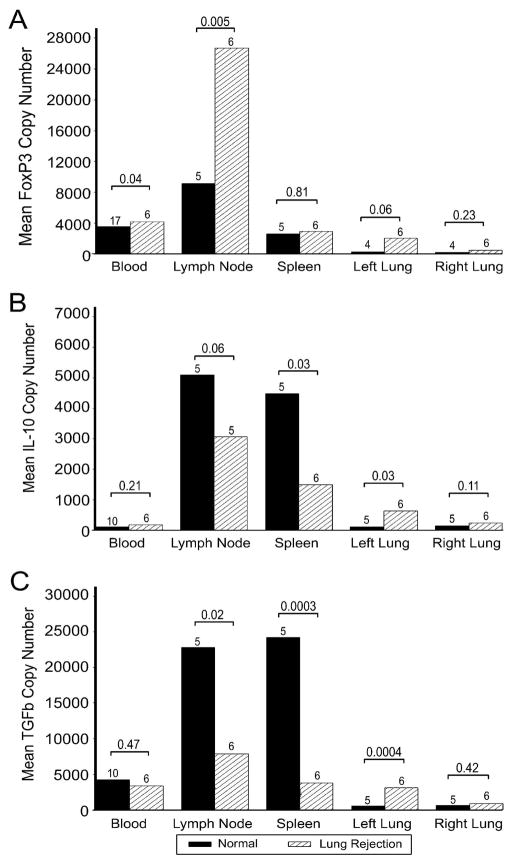
Quantitative RT-PCR for foxP3, IL10 and TGFβ was done on CD3+ T cells isolated from the peripheral blood in chimeric recipients with and without solid organ grafts. CD3+ T cells were also harvested from lymph node, spleen and the right and left lung at the end of study. Compared to normal dogs, the copy number of foxP3, IL10 and TGFβ mRNA in CD3+ T cells from the peripheral blood of chimeric recipients (n=10) was increased (n=17; p=0.0005, p<0.0001, p=0.005, respectively) (Figure 4). After lung transplantation, foxP3 and IL10 but not TGFβ copy number in the CD3+ T cells from blood from the chimeric recipients (n=4) were significantly increased compared to normal dogs (p= 0.02, p<0.0001 and p= 0.22, respectively). At necropsy, the highest levels of foxP3, IL10 and TGFβ copy number were in CD3+ T cells from the lymph nodes of normal dogs, which then decreased in chimeric recipients (p=0.11, p<0.0001, p=0.02, respectively). FoxP3 and TGFβ copy number in CD3+ T cells from lymph nodes of chimeric recipients increased significantly after lung transplantation (p=0.05, p=0.01, respectively). There was no difference in the foxP3 copy number of splenic CD3+ T cells after HCT or lung transplantation but IL10 and TGFβ copy number in splenic CD3+ T cells was decreased in chimeric recipients compared to normal dogs (p=0.04, p=0.002, respectively). In both the native and donor lungs of chimeric recipients after lung transplantation, there was in general, an increased expression of foxP3, IL10 and TGFβ in CD3+ T cells. This may reflect the indolent inflammatory process in the lung graft with a ‘sympathetic protective’ response in the native lung. The increased expression of foxP3, IL10 and TGFβ in CD3+ T cells of blood and lungs and a decrease in lymph node and spleen is consistent with a shift of regulatory activity to the peripheral tissues in chimeric recipients.
Figure 4.
Copy number of foxP3, IL10 and TGFβ in CD3+ T cells from blood, lymph nodes, spleen, lung graft (left) and native lung (right) from normal dogs, chimeric recipients and chimeric recipients after lung transplantation at end of study. Copy number of foxP3, IL10 and TGFβ was measured by quantitative RT-PCR. Absolute copy numbers were calculated based on foxP3, IL10 or TGFβ standard curves, and samples were normalized to a second standard curve for the housekeeping gene G3PDH (37). A) Copy number of foxP3 was significantly increased in CD3+ T cells from the peripheral blood in recipients with mixed hematopoietic chimerism compared to normal dogs. Copy number of foxP3 from tissue-derived CD3+ T cells were not significantly different between normal dogs and chimeric recipients except for the right (native) lung in which it was noted to be increased in chimeric recipients. B) Copy number of IL10 was significantly increased in CD3+ T cells from the peripheral blood and left (graft) lung of recipients and increased but not significantly in the right (native) lung. There were significant decreases in IL10 copy number in CD3+ T cells from lymph node and spleen in the chimeric recipients. C) Copy number of TGFβ was significantly increased in CD3+ T cells from the peripheral blood of chimeric recipients (except after lung transplantation) and in the lungs (native and graft) after lung transplantation. There were significant decreases in TGFβ copy number in CD3+ T cells from lymph node and spleen in the chimeric recipients.
Expression of FoxP3, IL10 and TGFβ in CD3+ T cells from blood and tissue during acute lung graft rejection
The expression of foxP3, IL10 and TGFβ was also evaluated in nonchimeric recipients experiencing acute lung rejection (group 3, Figure 5). FoxP3 copy number was increased in blood, lung allograft and most markedly in lymph node (p=0.04, p=0.06 and p=0.005, respectively). IL10 and TGFβ were unchanged in the blood, decreased in lymph node and spleen, and increased in the lung allograft. CD3 and foxP3 staining of lymph nodes from recipients rejecting a lung graft showed that there were an increased number of foxP3+ cells compared to a lymph node from a normal dog (Supplement, Figure 1).
Figure 5.
Copy number of foxP3, IL10 and TGFβ expression in CD3+ T cells from blood, lymph nodes, spleen, lung graft (left) and native lung (right) from normal dogs and recipients experiencing acute lung graft rejection. A) Copy number of foxP3 was increased in CD3+ T cells from peripheral blood, lymph nodes and left (graft) lung during graft rejection. The increased foxP3 copy number in the CD3+ T cells of the lymph nodes from a recipient was notable because it was substantially higher than any other blood or tissue sample studied. B) IL10 copy number in CD3+ T cells from the peripheral blood did not significantly change during graft rejection but significantly decreased in the lymph nodes and spleen and increased in the left (graft) lung. C) TGFβ copy number in CD3+ T cells from the peripheral blood, like IL10, did not significantly change during graft rejection but significantly decreased in the lymph nodes and spleen and increased in the left (graft) lung.
Discussion
In a canine model of combined hematopoietic cell and lung transplantation, it was demonstrated that lung grafts had prolonged survival without loss of function but there was histological evidence of minimal-mild rejection. Even though minimal-mild graft rejection did not significantly affect lung function during the observation period of greater than 2 years in some recipients, it did reflect persistent donor-specific alloreactivity or indolent chronic rejection. It is uncertain if there would have been progressive loss of lung function with longer follow-up of the recipients. Partial tolerance has also been observed to HC donor-derived skin grafts in this dog model of MC as well as in other transplantation models (4,10,39). Rejection of kidney, heart or heterotopic small bowel grafts has not yet been observed in chimeric large animal recipients (3,5,39,40). Rejection of skin grafts in chimeric recipients has been attributed to the presence of polymorphic minor histocompatibility antigens of the skin and also possibly, the non-vascularized nature of the skin graft (39,41). However minor histocompatibility antigens are likely expressed on most organs other than the skin and the presence of histological rejection in the lung graft may therefore relate more to other intrinsic features of the graft especially the effectiveness of donor antigen presentation for activation of the host immune system. The lung is a highly immunogenic organ based on the observation that the rates of rejection are higher after lung transplantation than kidney or liver transplantation. Even though an indolent rejection process was observed among recipients after combined hematopoietic cell and lung transplantation, this approach may improve lung transplant outcomes since the survival of the lung grafts was markedly prolonged compared to lung transplant recipients without donor chimerism.
The term ‘partial clinical tolerance’ has been proposed to describe recipients who require minimal immunosuppression for maintaining stable allograft function. Partial tolerance has been more easily achieved after liver transplantation (42). The state of ‘partial clinical tolerance,’ in most cases however, is still dependent on pharmacological immunosuppression and withdrawal of this may result in the loss of graft function. In experimental models besides the dog, partial tolerance has also been observed after small intestine and kidney transplantation in the rat (43,44). In experimental models, an indolent stable state of low grade inflammation may persist without significant impairment of graft function, possibly as the result of a balance between regulatory and effector immune mechanisms. A longer follow-up than what was performed in the current study would be required to confirm this possibility. The indolent chronic rejection of the lung allograft in mixed chimeric recipients might be prevented by converting recipients to full hematopoietic chimerism or adding low-doses of pharmacological immunosuppression.
Immune tolerance in recipients with MC is dependent upon multiple mechanisms (45). In a lymphoablative murine model, central mechanisms involving intrathymic negative selection or deletion of donor-reactive T cells were observed in the development of stable MC (46–48). Peripheral T cell depletion of donor-reactive host T cells preceded by anergy to donor-antigens has also been reported in thymectomized murine recipients (49). More recently, in a non-lymphoablative murine model, donor-specific regulatory activity and immune tolerance to skin grafts could be adoptively transferred from recipients with MC (50). In a large animal model, peripheral regulation was demonstrated in vitro when peripheral blood mononuclear cells from a mixed chimeric miniature swine suppressed alloreactivity of donor-matched responder cells to recipient-matched stimulator cells (51). In the dog, a peripheral tolerance mechanism was also evident in chimeric recipients which failed to convert from mixed to full hematopoietic chimerism after donor lymphocyte infusions (15). In the chimeric recipients, there was an increase in Th1 and Th2 cells in the peripheral blood consistent with a T helper type 1 and 2 effector response. After lung transplantation, Th1 and Th2 cells remained increased but Tc1 were then also observed to increase. In combination with the indolent rejection of the lung grafts, these observations are not consistent with a deletional mechanism of immune tolerance in this model of MC. There were also changes which involved the regulatory subsets of CD3+ T cells expressing foxP3, IL10 and TGFβ. Regulatory T cell subsets with the dominant expression of one of these molecules have been described. FoxP3 expression has been described in CD4+CD25+ regulatory T cells. IL10 and TGFβ are expressed in the Tr1 and Th3 regulatory T cells respectively. Since quantitative RT-PCR was performed on a population of CD3+ T cells, changes in the copy number of these molecules could have resulted from an increased expression within the cells without an increase in cell number. In previous studies of in vivo interleukin-2, an increase of foxP3 copy number by PCR in the blood was associated with an increase in CD4+CD25hi ± foxP3+ T cells (52,53). The decreased signal from foxP3, IL10 and TGFβ in CD3+ T cells from secondary lymphoid organs (i.e. lymph nodes and spleen) and increase in the blood and lungs was consistent with a mobilization of a regulatory response to suppress alloreactivity in peripheral tissue. Similar trafficking of foxP3+ regulatory T cells from lymph nodes to peripheral tissues during a graft-versus-host reaction has been observed in a murine model after adoptive transfer (54). The immunomodulatory changes observed in the dog model of MC and lung transplantation were consistent with the conclusion that donor-reactive helper and cytotoxic T cells persist and were only partially controlled by regulatory as well as other tolerance mechanisms.
Recipients rejecting the lung allograft (group 3) also had increased foxP3 expression in CD3+ T cells from blood similar to what had been observed in chimeric recipients. Increases of foxP3 in CD3+ T cells from the blood therefore may not be a good indicator of the presence or absence of tolerance. The largest increase of foxP3 expression in CD3+ T cells during rejection was in the lymph node compared to blood, spleen and lung graft. It had previously been shown that alloantigen-specific CD4(+)CD25(+)foxp3(+) regulatory T cells developed and are required within lymph nodes during tolerization (55). In this large animal model, the increase of foxP3 in CD3+ T cells from the lymph node likely occurred as a compensatory regulatory mechanism that was induced by factors related to rejection. There was also an increased IL10 and TGFβ copy number in CD3+ T cells in the lung graft during rejection but no change in the blood and a decrease in lymph node and spleen. These changes may represent the mobilization of regulatory T cells to the peripheral tissues including the graft for the control of alloreactivity. This demonstrates that there is a response of regulatory immune mechanisms, albeit inadequate, in parallel with an effector response of the immune system during acute rejection. In dog and human, in vitro regulatory T cell expansion (CD4+CD25+) has been observed in mixed leukocyte reactions (56,57). Alloantigen stimulation of CD4+CD25+ T cells has resulted in antigen-specific regulatory T cells (58,59). In addition, it has been demonstrated that antigen-specific CD4+CD25+ regulatory T cells can be induced from CD4+CD25− T cells by exposure to antigen- presenting cells and the cognate antigen (60). Many immunosuppressive agents commonly used in transplantation affect both effector and regulatory arms of the immune system but an approach targeting effector T cells specifically would seem to be more rational.
In summary, in the canine model of combined hematopoietic cell and lung transplantation, there was prolonged survival of lung grafts from the DLA-identical HC donor. In the lung grafts at the end of the study, there was evidence of histological changes consistent with minimal-mild chronic rejection. Further characterization of the immune response in the chimeric recipients showed that there was a T helper type 1 and 2 response and an increase in Tc1 in the blood consistent with activation of effector immune mechanisms. There was also evidence of activation of the regulatory arm of the immune system with an increase in foxP3, IL10 and TGFβ in CD3+ T cells. These observations contribute further insights into the immune profile of donor-specific tolerance in an outbred large animal model of combined hematopoietic cell and solid organ transplantation and support future investigations of interventions which would augment regulatory immune mechanisms to induce tolerance.
Acknowledgments
Funding sources: Supported in part by NIH grants AI69879, DK42716, CA78902 and CA15704 from the NIH, DHHS, Bethesda, MD. In addition, the authors have no conflicts of interest to declare.
We thank Michelle Spector, D.V.M., for providing veterinary support for the dog colony, and other investigators in Transplantation Biology and those in the Canine Facility for their contributions to the care of the dogs. Our special thanks to Eustacia Zellmer for the breeding, purchasing, and DLA typing of dogs. We thank Helen Crawford, Bonnie Larson, Karen Carbonneau, and Sue Carbonneau for their help in preparing the manuscript and for their administrative support. We thank the Experimental Histopathology Shared Resource for performing the immunohistochemistry staining and histology.
References
- 1.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307(5947):168–70. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 2.Sykes M, Szot GL, Swenson KA, Pearson DA. Induction of high levels of allogeneic hematopoietic reconstitution and donor-specific tolerance without myelosuppressive conditioning. Nat Med. 1997;3(7):783–7. doi: 10.1038/nm0797-783. [DOI] [PubMed] [Google Scholar]
- 3.Kuhr CS, Allen MD, Junghanss C, Zaucha JM, Marsh CL, Yunusov M, et al. Tolerance to vascularized kidney grafts in canine mixed hematopoietic chimeras. Transplantation. 2002;73(9):1487–93. doi: 10.1097/00007890-200205150-00020. [DOI] [PubMed] [Google Scholar]
- 4.Yunusov MY, Kuhr CS, Georges GE, Hogan WJ, Taranova AG, Lesnikova M, et al. Partial donor-specific tolerance to delayed skin grafts after rejection of hematopoietic cell graft. Transplantation. 2006;82(5):629–37. doi: 10.1097/01.tp.0000229449.09622.28. [DOI] [PubMed] [Google Scholar]
- 5.Kuhr CS, Yunusov M, Sale G, Loretz C, Storb R. Long-term tolerance to kidney allografts in a preclinical canine model. Transplantation. 2007;84(4):545–7. doi: 10.1097/01.tp.0000270325.84036.52. [DOI] [PubMed] [Google Scholar]
- 6.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DMJ, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105(2):173–81. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butcher JA, Hariharan S, Adams MB, Johnson CP, Roza AM, Cohen EP. Renal transplantation for end-stage renal disease following bone marrow transplantation: a report of six cases, with and without immunosuppression. Clin Transplant. 1999;13(4):330–5. doi: 10.1034/j.1399-0012.1999.130409.x. [DOI] [PubMed] [Google Scholar]
- 8.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6(9):2121–33. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 9.Boyse EA, Lance EM, Carswell EA, Cooper S, Old LJ. Rejection of skin allografts by radiation chimaeras: selective gene action in the specification of cell surface structure. Nature. 1970;227(261):901–3. doi: 10.1038/227901a0. [DOI] [PubMed] [Google Scholar]
- 10.Steinmuller D, Tyler JD, David CS. Cell-mediated cytotoxicity to non-MHC alloantigens on mouse epidermal cells. I. H-2 restricted reactions among strains sharing the H-2k haplotype. J Immunol. 1981;126(5):1747–53. [PubMed] [Google Scholar]
- 11.Steinmuller D. Skin allograft rejection by stable hematopoietic chimeras that accept organ allografts still is an enigma (Letter) Transplantation. 2001;72(1):8–9. doi: 10.1097/00007890-200107150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Hettiaratchy S, Melendy E, Randolph MA, Coburn RC, Neville DM, Jr, Sachs DH, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004;77(4):514–21. doi: 10.1097/01.tp.0000113806.52063.42. [DOI] [PubMed] [Google Scholar]
- 13.Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem H-P, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89(8):3048–54. [PubMed] [Google Scholar]
- 14.Storb R, Yu C, Zaucha JM, Deeg HJ, Georges G, Kiem H-P, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94(7):2523–9. [PubMed] [Google Scholar]
- 15.Georges GE, Storb R, Thompson JD, Yu C, Gooley T, Bruno B, et al. Adoptive immunotherapy in canine mixed chimeras after nonmyeloablative hematopoietic cell transplantation. Blood. 2000;95:3262–9. [PubMed] [Google Scholar]
- 16.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26(8):782–95. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Cai J, Mao Q, Ozawa M, Terasaki PI. Lung transplantation in the United States: 1990– 2005. Clinical Transplants. 2005:29–35. [PubMed] [Google Scholar]
- 18.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Date H, Yamane M, Toyooka S, Okazaki M, Aoe M, Sano Y. Current status and potential of living-donor lobar lung transplantation (Review) Frontiers in Bioscience. 2008;13:1433–9. doi: 10.2741/2772. [DOI] [PubMed] [Google Scholar]
- 20.Okumura H, Ohtake S, Ontachi Y, Ozaki J, Shimadoi S, Waseda Y, et al. Living-donor lobar lung transplantation for broncho-bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation: does bronchiolitis obliterans recur in transplanted lungs? Int J Hematol. 2007;86(4):369–73. doi: 10.1532/IJH97.07045. [DOI] [PubMed] [Google Scholar]
- 21.Sano Y, Date H, Nagahiro I, Aoe M, Shimizu N. Living-donor lobar lung transplantation for bronchiolitis obliterans after bone marrow transplantation. Ann Thorac Surg. 2005;79(3):1051–2. doi: 10.1016/j.athoracsur.2003.09.111. [DOI] [PubMed] [Google Scholar]
- 22.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–7. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397– 401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 24.Ladiges WC, Storb R, Graham T, Thomas ED. Experimental techniques used to study the immune system of dogs and other large animals. In: Gay WI, Heavener JE, editors. Methods of Animal Experimentation. New York, NY: Academic Press; 1989. pp. 103–133. [Google Scholar]
- 25.Yunusov MY, Georges GE, Storb R, Moore P, Hagglund H, Affolter V, et al. FLT3 ligand promotes engraftment of allogeneic hematopoietic stem cells without significant graft-versus-host disease. Transplantation. 2003;75:933–40. doi: 10.1097/01.TP.0000057831.93385.7D. [DOI] [PubMed] [Google Scholar]
- 26.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–6. [PubMed] [Google Scholar]
- 27.Farivar AS, Yunusov MY, Chen P, Leone RJ, Madtes DK, Kuhr CS, et al. Optimizing a canine survival model of orthotopic lung transplantation. Transplant Proc. 2006;38:1638–40. doi: 10.1016/j.transproceed.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 28.Amdur MO, Mead J. Mechanics of respiration in unanesthetized guinea pigs. Am J Physiol. 1958;192:364–8. doi: 10.1152/ajplegacy.1958.192.2.364. [DOI] [PubMed] [Google Scholar]
- 29.Borland C, Mist B, Zammit M, Vuylsteke A. Steady-state measurement of NO and CO lung diffusing capacity on moderate exercise in men. J Appl Physiol. 2001;90(2):538–44. doi: 10.1152/jappl.2001.90.2.538. [DOI] [PubMed] [Google Scholar]
- 30.Mink SN, Coalson JJ, Whitley L, Greville H, Jadue C. Pulmonary function tests in the detection of small airway obstruction in a canine model of bronchiolitis obliterans. Am Rev Respir Dis. 1984;130(6):1125–33. doi: 10.1164/arrd.1984.130.6.1125. [DOI] [PubMed] [Google Scholar]
- 31.Peters SG, Hyatt RE. A canine model of bronchial injury induced by nitric acid. Lung mechanics and morphologic features. Am Rev Respir Dis. 1986;133(6):1049–54. doi: 10.1164/arrd.1986.133.6.1049. [DOI] [PubMed] [Google Scholar]
- 32.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. Journal of Heart & Lung Transplantation. 1996;15 (Part 1 1):1–15. [PubMed] [Google Scholar]
- 33.Andrews RG, Winkler A, Potter J, Bryant E, Knitter GH, Bernstein ID, et al. Normal immunologic response to a neoantigen, bacteriophage X-174, in baboons with long-term lymphohematopoietic reconstitution from highly purified CD34+ Linallogeneic marrow cells. Blood. 1997;90(4):1701–8. [PubMed] [Google Scholar]
- 34.Felsburg PJ, Somberg RL, Hartnett BJ, Suter SF, Henthorn PS, Moore PF, et al. Full immunologic reconstitution following nonconditioned bone marrow transplantation for canine X-linked severe combined immunodeficiency. Blood. 1997;90(8):3214– 21. [PubMed] [Google Scholar]
- 35.Ochs HD, Storb R, Thomas ED, Kolb H-J, Graham TC, Mickelson E, et al. Immunologic reactivity in canine marrow graft recipients. J Immunol. 1974;113:1039–57. [Google Scholar]
- 36.Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188(1):117– 28. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 37.Peeters D, Peters IR, Clercx C, Day MJ. Quantification of mRNA encoding cytokines and chemokines in nasal biopsies from dogs with sino-nasal aspergillosis. Veterinary Microbiology. 2006;114(3–4):318–26. doi: 10.1016/j.vetmic.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 38.Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, et al. Housekeeping genes as internal standards: use and limits. Journal of Biotechnology. 1999;75(2– 3):291–5. doi: 10.1016/s0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 39.Fuchimoto Y, Gleit ZL, Huang CA, Kitamura H, Schwarze ML, Menard MT, et al. Skin-specific alloantigens in miniature swine. Transplantation. 2001;72(1):122–6. doi: 10.1097/00007890-200107150-00024. [DOI] [PubMed] [Google Scholar]
- 40.Yunusov MY, Kuhr C, Georges GE, Sale GE, Spector M, Lesnikova M, et al. Survival of small bowel transplants in canine mixed hematopoietic chimeras: preliminary results. Transplant Proc. 2002;34:3366–7. doi: 10.1016/s0041-1345(02)03614-x. [DOI] [PubMed] [Google Scholar]
- 41.Steinmuller D. The enigma of skin allograft rejection. Transplantation Reviews. 1998;12(1):42–57. [Google Scholar]
- 42.Cortesini R, Renna-Molajoni E, Cinti P, Pretagostini R, Ho E, Rossi P, et al. Tailoring of immunosuppression in renal and liver allograft recipients displaying donor specific T-suppressor cells. Hum Immunol. 2002;63(11):1010–8. doi: 10.1016/s0198-8859(02)00442-1. [DOI] [PubMed] [Google Scholar]
- 43.Hewitt CW, Black KS, Harman JC, Beko KR, Lee HS, Patel AP, et al. Partial tolerance in rat renal allograft recipients following multiple blood transfusions and concomitant cyclosporine. Transplantation. 1990;49(1):194–8. doi: 10.1097/00007890-199001000-00043. [DOI] [PubMed] [Google Scholar]
- 44.Nakao A, Nalesnik MA, Ishikawa T, Azhipa O, Demetris AJ, Murase N. Chimerism and tolerance in rat recipients of intestinal allografts from ALS-treated donors with and without adjunct naive-donor-strain bone-marrow cells. Transplantation. 2003;75(9):1575–81. doi: 10.1097/01.TP.0000061225.81051.06. [DOI] [PubMed] [Google Scholar]
- 45.Sykes M. Immune tolerance: mechanisms and application in clinical transplantation (Review) J Intern Med. 2007;262(3):288–310. doi: 10.1111/j.1365-2796.2007.01855.x. [DOI] [PubMed] [Google Scholar]
- 46.Manilay JO, Pearson DA, Sergio JJ, Swenson KG, Sykes M. Intrathymic deletion of alloreactive T cells in mixed bone marrow chimeras prepared with a nonmyeloablative conditioning regimen. Transplantation. 1998;66(1):96–102. doi: 10.1097/00007890-199807150-00015. [DOI] [PubMed] [Google Scholar]
- 47.Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996;62(3):380–7. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 48.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153(3):1087–98. [PubMed] [Google Scholar]
- 49.Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood. 2004;103(11):4336–43. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 50.Bigenzahn S, Blaha P, Koporc Z, Pree I, Selzer E, Bergmeister H, et al. The role of nondeletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am J Transplant. 2005;5(6):1237–47. doi: 10.1111/j.1600-6143.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 51.Kunisaki SM, Haller GW, Fuchimoto Y, Huang CA, Sachs DH. Peripheral regulation of graft-versus-host alloreactivity in mixed chimeric miniature swine. Transplantation. 2001;72(3):523–6. doi: 10.1097/00007890-200108150-00027. [DOI] [PubMed] [Google Scholar]
- 52.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107(6):2409–14. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11(11):1238–43. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109(6):2649–56. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 55.Ochando JC, Yopp AC, Yang Y, Garin A, Li Y, Boros P, et al. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174(11):6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 56.Lesnikova M, Nikitine A, Pogosov L, Nash RA, Georges GE. Peripheral CD4+CD25+ regulatory T cells (Treg) block alloreactive host anti-donor T cells in canine mixed hematopoietic chimeras [abstract] Blood. 2005;106(Part 1 11):867a–3101. [Google Scholar]
- 57.Martin PJ, Pei J, Gooley T, Anasetti C, Appelbaum FR, Deeg J, et al. Evaluation of a CD25-specific immunotoxin for prevention of graft-versus-host disease after unrelated marrow transplantation. Biol Blood Marrow Transplant. 2004;10:552–60. doi: 10.1016/j.bbmt.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Joffre O, Gorsse N, Romagnoli P, Hudrisier D, van Meerwijk JP. Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood. 2004;103(11):4216–21. doi: 10.1182/blood-2004-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang S, Camara N, Lombardi G, Lechler RI. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood. 2003;102(6):2180–6. doi: 10.1182/blood-2003-04-1164. [DOI] [PubMed] [Google Scholar]
- 60.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25- cells. Proc Natl Acad Sci USA. 2005;102(11):4103–8. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]