Abstract
Cellular recruitment during inflammatory/immune responses is tightly regulated. The ability to dampen inflammation is imperative for prevention of chronic immune responses, as in asthma. Here we investigated the ability of lipoxin A4 (LXA4) stable analogs to regulate airway responses in two allergen-driven models of inflammation. A 15-epi-LXA4 analog (ATLa) and a 3-oxa-15-epi-LXA4 analog (ZK-994) prevented excessive eosinophil and T lymphocyte accumulation and activation after mice were sensitized and aerosol-challenged with ovalbumin. At <0.5 mg/kg, these LXA4 analogs reduced leukocyte trafficking into the lung by >50% and to a greater extent than equivalent doses of the CysLT1 receptor antagonist montelukast. Distinct from montelukast, ATLa treatment led to marked reductions in cysteinyl leukotrienes, interleukin-4 (IL-4), and IL-10, and both ATLa and ZK-994 inhibited levels of IL-13. In cockroach allergen-induced airway responses, both intraperitoneal and oral administration of ZK-994 significantly reduced parameters of airway inflammation and hyper-responsiveness in a dose-dependent manner. ZK-994 also significantly changed the balance of Th1/Th2-specific cytokine levels. Thus, the ATLa/LXA4 analog actions are distinct from CysLT1 antagonism and potently block both allergic airway inflammation and hyper-reactivity. Moreover, these results demonstrate these analogs’ therapeutic potential as new agonists for the resolution of inflammation.
Keywords: resolution, lipid mediators, leukocytes
Airway inflammation in asthma is characterized by mucosal infiltration of eosinophils (Eos) and T lymphocytes (Lymphs) and by elaboration of lipid and peptide mediators that regulate the host inflammatory response to inhaled allergens (1). Chronic anti-inflammatory treatment is a principal tenet of current asthma management, yet corticosteroids, the main anti-inflammatory modality in clinical use, can have deleterious effects on normal physiological mechanisms of host defense and homeostasis (1). In addition, ~5% of asthmatic patients are poorly controlled with corticosteroids and develop severe asthma that is refractory to treatment (2). Moreover, no therapy is currently available to modify asthma pathobiology and resolve the aberrant immune activation. New approaches are needed.
A rapidly expanding number of counter-regulatory mediators were recently uncovered that are endogenously generated to limit acute inflammatory responses and promote resolution (3). Lipoxins are the first described endogenous lipid mediators of anti-inflammation and resolution. Lipoxin A4 (LXA4) is a short-acting, naturally occurring eicosanoid with potent anti-inflammatory actions in vitro and in vivo (for a recent review, see ref. 3). In humans, the lion’s share of LXA4 is produced locally at sites of inflammation by transcellular biosynthesis, and endogenous LXA4 synthesis can be primed by cytokines. Aspirin, which is known to inhibit prostaglandin and thromboxane biosynthesis, has a unique ability to trigger formation of the 15-epimer of LXA4, both in vitro and in vivo, via a mechanism involving cyclooxygenase-2 inhibition (3). Aspirin-triggered lipoxins (ATLs) retain the anti-inflammatory properties of LXA4 and may mediate, in part, aspirin’s therapeutic effects. LXA4 and the ATL or 15-epi-LXA4 exert anti-inflammatory effects through signals generated by binding to a high-affinity, G-protein-coupled LXA4 receptor, denoted ALX. ALX receptors are conserved in mammalian species and constitutively expressed on neutrophils, Eos, monocytes, and epithelium, thus being ideally localized to play key roles in modulating cell-cell interactions and cell-mediated immune responses in the airway. High-level ALX expression on neutrophils and Eos correlates with the ability of LXA4 to potently stop chemotaxis and transcellular migration of these inflammatory cells. Also of note, both LXA4 and 15-epi-LXA4 can compete with leukotriene D4 (LTD4) for specific binding at cysteinyl leukotriene 1 (CysLT1) receptors to serve in vivo as an antagonist for CysLT signaling (4), similar to existing asthma therapeutic agents, such as montelukast (1).
Rapid metabolic inactivation of LXA4 and 15-epi-LXA4 occurs via oxidation at C-15 and reduction at C13–C14 (5). These LXA4 metabolites have a reduced affinity for ALX and lower potency as anti-inflammatory agents in vitro. Chemical modifications to the C15–C20 region of LXA4 and ATLs prevent metabolic inactivation, providing metabolically stable analogs with superior pharmaceutical characteristics (6). These analogs have been used to establish that both LXA4 and ATLs stop neutrophil diapedesis, reduce epithelial cell cytokine release, and decrease vascular permeability of murine skin exposed to inflammatory stimuli (3). Eos-driven allergic reactions are also blocked by LXA4 and its stable analogs (7, 8). In the airway, LXA4 and specific LX analogs administered intravenously dampen allergic airway inflammation and hyper-reactivity (8), and promote resolution of mild acute lung injury from aspiration of hydrochloric acid (9). Hence, LXA4 serves as a selective agonist at ALX receptors for cell type-specific actions and as an antagonist at CysLT1 receptors to block LTD4-mediated airway responses. Of particular interest in asthma, glucocorticoids, the most common anti-inflammatory agent used in its treatment, can induce LX signaling circuits by increasing expression of leukocyte ALX receptors (10) and the counter-regulatory peptide mediator annexin 1 that signals via ALX (11). Taken together, these data indicate involvement of the endogenous LXA4/ALX pathway in promoting attenuation and/or resolution of responses to diverse proinflammatory mediators in airway inflammation.
Here we provide new findings on the actions and mechanisms for LXA4 analogs in altering allergic airway inflammation and hyper-reactivity in two distinct murine models of allergen-driven asthmatic responses and determine a structure activity relationship for LXA4-specific actions in the airway that are distinct from CysLT1 receptor antagonism.
MATERIALS AND METHODS
LXA4 analogs
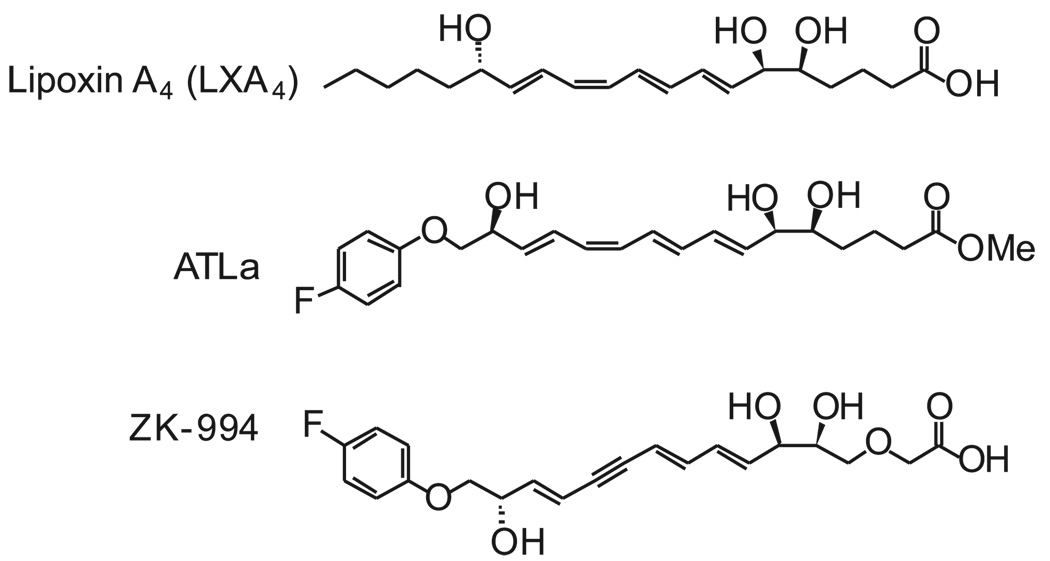
The metabolically stable analog of the aspirin-triggered 15-epi-LXA4 methyl [5S,6R,7E,9E,11Z,13E,15S)-16-(4-fluorophenoxy)-5,6,15-trihydroxy-7,9,11,13-hexadecatetraenoate (ATLa; see Fig. 1)] was prepared at Berlex using published procedures (12). Material of >95% purity was qualified using a synthetic LXA4 standard by 1H-NMR, HPLC coinjection with photodiode array UV-visible detection (two methods), and LC-MS-MS. HPLC analyses were carried out on a LUNA 5 µ C18(2) column (250×4.60 mm) using a gradient of methanol in water containing 0.1% acetic acid on a Varian ProStar HPLC equipped with a diode array detector. The plasma half-life of ATLa after i.v. injection was determined to be ~ 15 min (13). The second-generation analog, 5S,6R,7E,9E,13E,15S)-16-(4-fluorophenoxy)-3-oxa-5,6,15-trihydroxy-7,9,13-hexadecatrien-11-ynoic acid (ZK-994; see Fig. 1), was designed and prepared at Berlex to block metabolism by β-oxidation through insertion of a 3-oxa group and improve chemical stability through substitution of the tetraene group for a trienyne group (13). The changes resulted in significant improvements in acid and light stability, and an improved plasma half-life, but displayed similar biological activity with a better pharmacokinetic profile over ATLa (14, 15). The montelukast sodium was isolated from Singulair tablets by extraction and chromatography at Berlex Biosciences. Chemical and NMR analytical data were consistent with the desired structure.
Figure 1.
Structure of LXA4 stable analogs. Structural analogs of LXA4 were prepared to resist rapid conversion by PGDH and ω-oxidation as well as β-oxidation (ATLa) (see text for further details). In addition, the tetraene was converted to a trienyne to enhance chemical stability in light and acid (3-oxa-LXA4).
Ovalbumin sensitization and challenge
Five- to 7-wk-old male FVB (Charles River Laboratories, Wilmington, MA, USA) mice were housed under pathogen-free conditions. After Harvard Medical Area IRB approval (protocol #03618), mice were sensitized with i.p. injections of ovalbumin (OVA; Grade III, Sigma-Aldrich Co., St. Louis, MO, USA) (200 µg) plus 1 mg aluminum hydroxide (Sigma-Aldrich) as adjuvant in 0.2 ml sterile saline (0.9%) on days 0 and 7. On days 14–17, mice received test compound (~500 µg/kg) by gavage, including 15-epi-16-parafluorophenoxy LXA4-me (ATLa; 10 µg=22.8 nmol), 3-oxa-trienyne-16-parafluorophenoxy LXA4 (3-oxa-LXA4; 10 µg=23.5 nmol), montelukast (10 µg=16.4 nmol), or vehicle (0.5% ethanol) in 0.2 ml sterile 0.9% saline 60 min before nebulization with 6% OVA (25 min). On day 18, mice were anesthetized and underwent bronchoalveolar lavage (BAL) with two instillations of 1 ml PBS plus 0.6 mM EDTA. After inflation to 25 cm H2O, lung tissues were excised into 10% neutral buffered formalin (Fisher Scientific, Pittsburgh, PA, USA) for histological examination.
Cockroach allergen sensitization and induction of airway responses
Five to seven week old Balb/c mice were immunized by i.p. injection with 10 µg of cockroach allergen (CRA, Bayer Pharmaceuticals, West Haven, CT, USA) emulsified in incomplete Freund’s adjuvant on day 0. After 14 days the mice were lightly anesthetized and given an intranasal challenge of 10 µg of CRA in 10 µl diluent to localize the response to the airway. Seven days later animals were given an intratracheal injection of 4 µg of CRA in 40 µl of sterile PBS or with PBS alone (vehicle). A second intratracheal administration of allergen was performed 48 h later. This procedure has demonstrated a strong Th2-mediated, eosinophil-rich response. Animals were treated with an LXA4 analog or vehicle at the time of the final two intratracheal allergen challenges or provided in the drinking water (0, 1, or 10 µg/ml) for 3 days beginning on day 14, 2 h before intranasal allergen challenge. The amount of water the animals consumed in the 3 day period of treatment was measured and averaged on a per mouse basis. To conserve material, animals were analyzed 24 h after the final allergen challenge.
Allergen-initiated respiratory inflammation
BAL fluid was centrifuged (800 g, 10 min, 4°C), and the supernatant was aliquotted and stored at −80°C for later determination of mediator levels, including IL-4, IL-5, IL-10, RANTES (R&D systems, Minneapolis, MN, USA), CysLTs (Cayman Chemical, Ann Arbor, MI, USA), and LXA4 (Neogen, Lexington, KY, USA). The cell pellet was gently resuspended in PBS for total cell counts. To determine differentials, cells were concentrated onto microscope slides by cytocentrifuge (STATspin, Norwood, MA, USA) (265 g) and stained with a Wright-Giemsa stain (Sigma-Aldrich). At least 200 cells were counted per sample.
Lung homogenates
The right upper lobe from each mouse was flash-frozen in liquid nitrogen and kept at −80°C. Just prior to running the ELISA assays, the lungs were homogenized in 1 ml of homogenization buffer containing protease inhibitors (Complete, Roche, Indianapolis, IN, USA) and 0.1% Triton-X 100 in PBS.
Lung cytokine and chemokine ELISAs
Cytokines were quantitated from homogenized lung aqueous extracts using a double ligand ELISA system as in ref. 16. Briefly, flat-bottomed 96-well microtiter plates were coated with capture antibody (3.2 µg/ml, overnight, 4°C). Nonspecific binding sites were blocked with 2% BSA in PBS (1 h, 37°C). Plates were washed and specimens were added in triplicate, followed by incubation at 37°C and washing. Biotinylated antibody was added for 1 h at 37°C. After washing, bound antibody was conjugated with streptavidin-peroxidase and detected with chromogen substrate. The individual polypeptides were standardized to total protein (ng/µg total protein). The lower limit of detection for these assays was ~50 pg/ml. These ELISAs are specific and do not cross-react to other chemokine or cytokines.
Morphometric analysis of peribronchial Eos accumulation
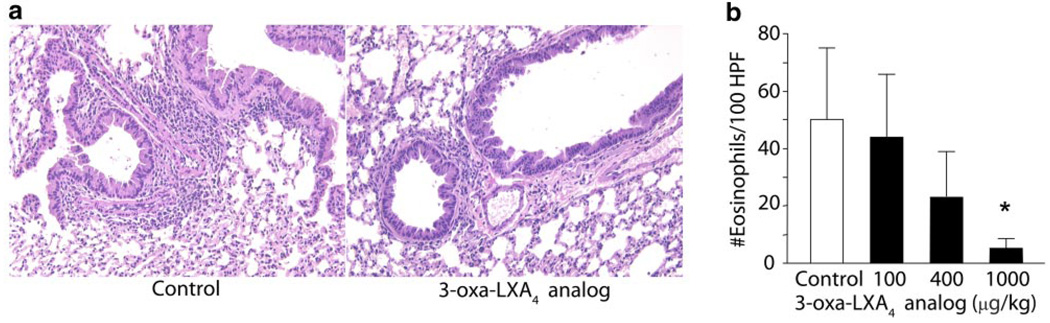
Lungs from mice immunized and challenged with CRA or vehicle were preserved with 4% paraformaldehyde (1 ml) on day 18 postchallenge. The fixed lungs were embedded in paraffin and multiple 50 µm sections were stained with Wright-Giemsa to identify Eos. Individual Eos were counted from 100 high-power fields (HPF, 1000×) per lung using multiple-step sections of lung. The Eos counted were only in the peribronchial region to ensure the enumeration of only those Eos within or immediately adjacent to an airway. The inflammation observed in this model was completely associated with the airway with little or no alveolitis.
Measurement of airway hyper-reactivity
Airway hyper-reactivity was measured using a Buxco mouse plethysmograph specifically designed for low tidal volumes (Buxco, Troy, NY, USA) as described (16). Briefly, the mice were anesthetized, intubated, and ventilated with a Harvard pump ventilator (tidal volume=0.4 ml, frequency=120 breaths/min, positive end-expiratory pressure 2.5–3.0 cm H2O). Initial readings were acquired after 5 min of ventilation. Once baseline levels were stabilized and initial readings taken, a methacholine challenge was given via the cannulated tail vein. After determining a dose response curve (0.001 to 0.5 mg), an optimal dose of methacholine (0.1 mg) was chosen and used throughout the rest of the experiments in this study. After methacholine challenge, peak airway resistance was recorded as a measure of airway hyper-reactivity.
Statistical analyses
Numerical results were expressed as mean ± se. Analysis of variance was used to determine the level of difference between groups. Pairs of groups were compared by unpaired 2-tailed Student’s t test. Significance was determined with P values of <0.05.
RESULTS
LXA4 analogs differ from montelukast in regulating key parameters of allergic airway responses to OVA
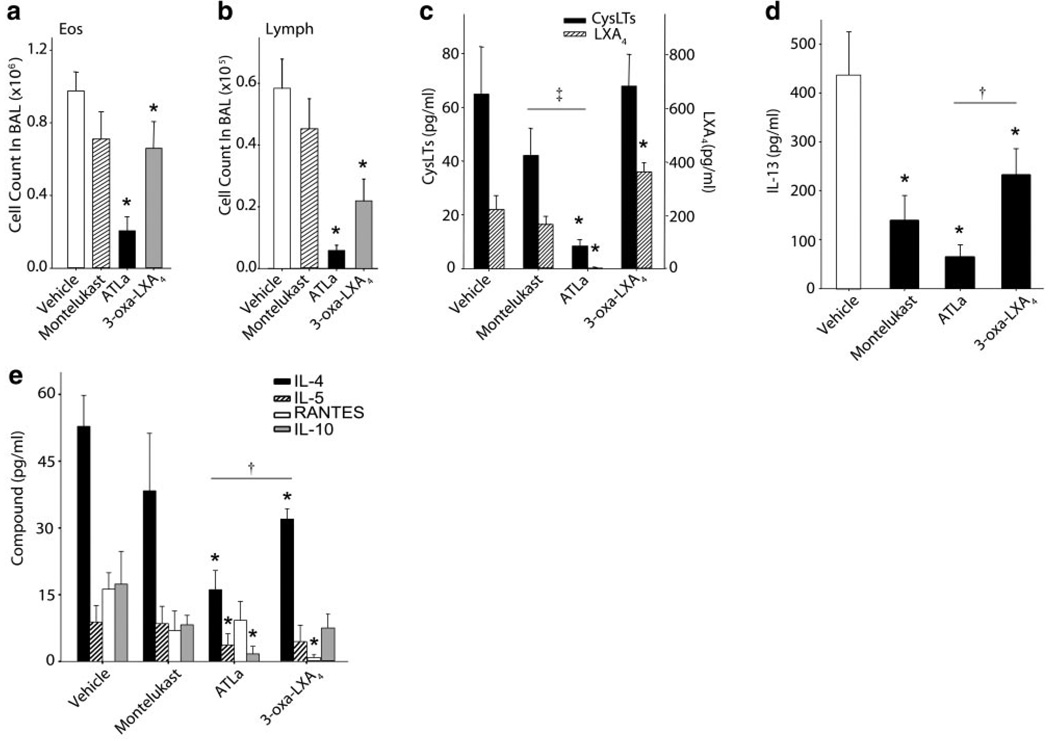
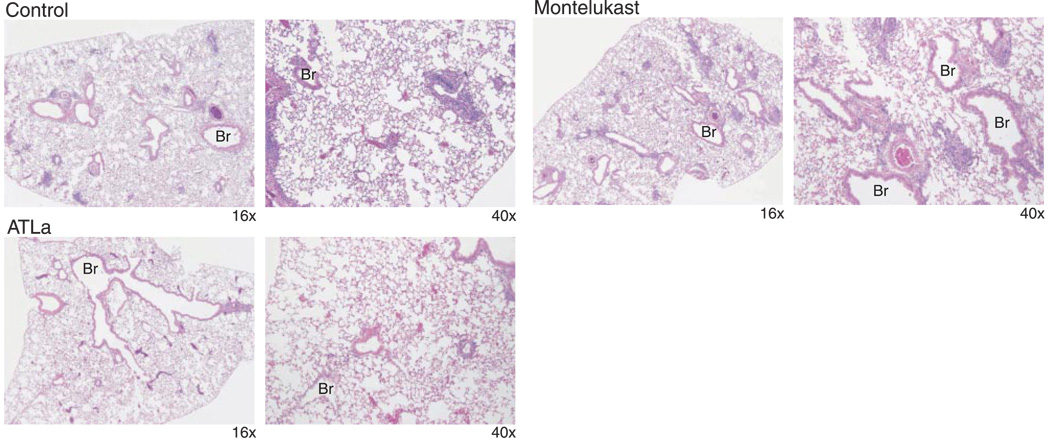
In a model of systemic allergen sensitization and airway challenge, mice were sensitized to OVA and aerosol-challenged on 4 successive days with 6% OVA in the presence of ~500 µg/kg of either ATLa, ZK-994, or montelukast, which were administered by gavage 60 min before aerosol challenge. Twenty-four hours after the last of four daily OVA aerosol challenges, BAL was performed or lung tissues were collected for microscopic analyses (see Materials and Methods). Similar to results with intravenous administration (8), ATLa was bioavailable after enteric administration, leading to significant reductions in BALF Eos and Lymphs (Fig. 2a, b). ZK-994 also significantly decreased both Eos and Lymphs in BALF, but these decrements were substantially less marked than with ATLa. Since only a single dose was used, the differences in the regulation pattern of mediators could be due to differences in the dose response curves for the two analogs. Administration of an equivalent amount of montelukast did not significantly change the allergic airway inflammation in antigen-sensitized and aerosol-challenged animals. ATLa regulated lipid mediator levels, as both CysLTs and endogenous LXA4 were decreased in these mice (Fig. 2c). ATLa also led to decreased Th2 cytokines IL-13, IL-4, and IL-5 as well as IL-10 in BAL fluids from OVA-sensitized and challenged animals (Fig. 2d, e). Inhibition appeared selective, since levels of RANTES were not similarly reduced when determined in the same samples of BAL fluid (Fig. 2d). ZK-994 gave a significantly different pattern for regulation of mediators (Fig. 2c, d). Decrements in IL-13 and IL-4 were observed with ZK-994, but the reductions were significantly less than with ATLa (Fig. 2c, d). Of note, ZK-994, unlike ATLa, led to higher levels of immunoreactive LXA4 (Fig. 2c) and a marked inhibition of RANTES (Fig. 2e). There was no significant change in CysLTs, IL-5, or IL-10. Apart from inhibition of IL-13, these findings with the LX analogs were in sharp contrast to montelukast-treated mice, which displayed no significant change in any of the other peptide or lipid mediators, including CysLTs (Fig. 2c–e). These results indicate that administration of LXA4 mimetics can significantly inhibit allergic pulmonary inflammation, including leukocyte infiltration and formation of specific mediators of interest in airway pathophysiology such as key eicosanoids and cytokines. Differences in actions between LX analogs and montelukast in BALF leukocytes were also reflected in lung histopathology (Fig. 3). Administration of ATLa, but not montelukast, significantly reduced leukocyte infiltration, particularly tissue Eos and Lymphs. Together, these findings indicate that LXA4 regulates leukocyte trafficking in allergic airway inflammation principally via ALX signaling and not antagonism of CysLT1 receptors.
Figure 2.
LX stable analogs differ from montelukast in the regulation of allergen-induced airway inflammation. BAL fluids were obtained from OVA-sensitized and challenged mice that had been treated with ~500 mg/kg ATLa, ZK994, or montelukast by gavage. a) Eos and b) Lymphs were enumerated and identified after Wright-Giemsa stain. Results are expressed as means ± se (n≥6). c–e) Lipid mediators, cytokine and chemokine amounts in BAL fluids from animals treated with vehicle, montelukast, or LX analogs were determined by sensitive and specific EIAs. Results are expressed as the mean ± se (n≥6, duplicate determinations). *P < 0.05 by Student’s t test vs. vehicle control, ‡P < 0.01 vs. montelukast, and †P < 0.03 vs. ATLa-treated animals.
Figure 3.
Lung histopathology from montelukast and ATLa-treated mice. Male FVB mice were sensitized and aerosol-challenged with OVA in the absence (top row) or presence of ~500 µg/kg of either montelukast (middle row) or ATLa (bottom row). Representative (n=3) lung tissue sections were obtained from formalin-fixed, paraffin-embedded lung tissue prepared and stained with H&E. Magnifications, ×16 (left) and ×40 (right). Br, bronchus.
Oral ATLa treatment blocks airway responses to CRA
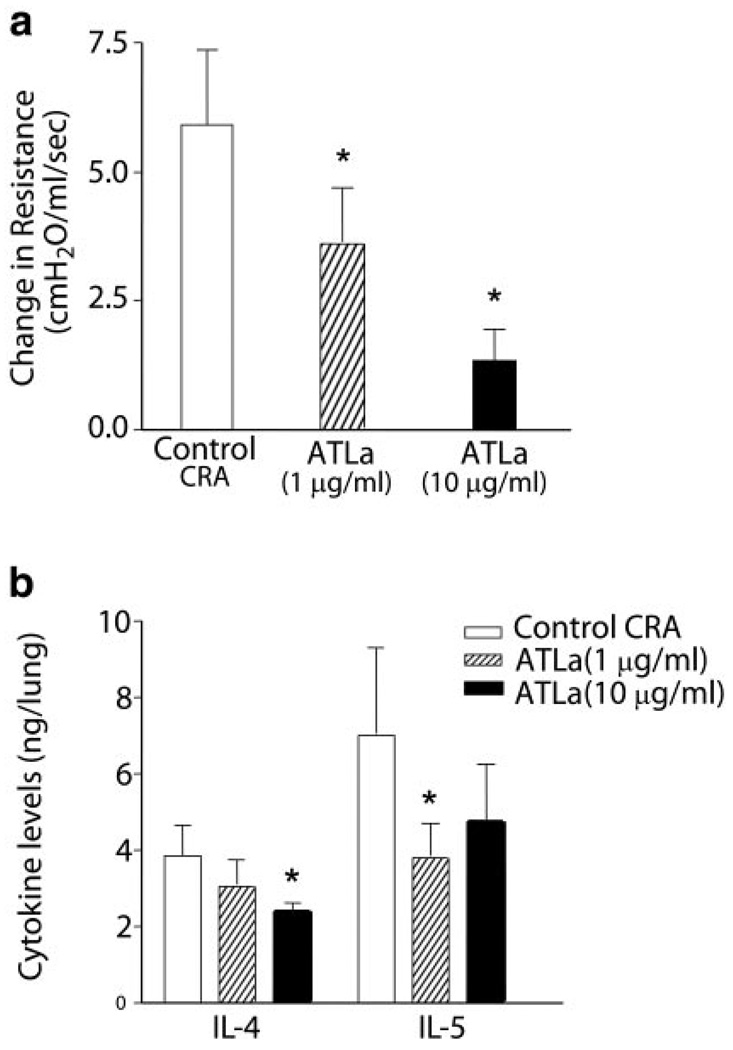
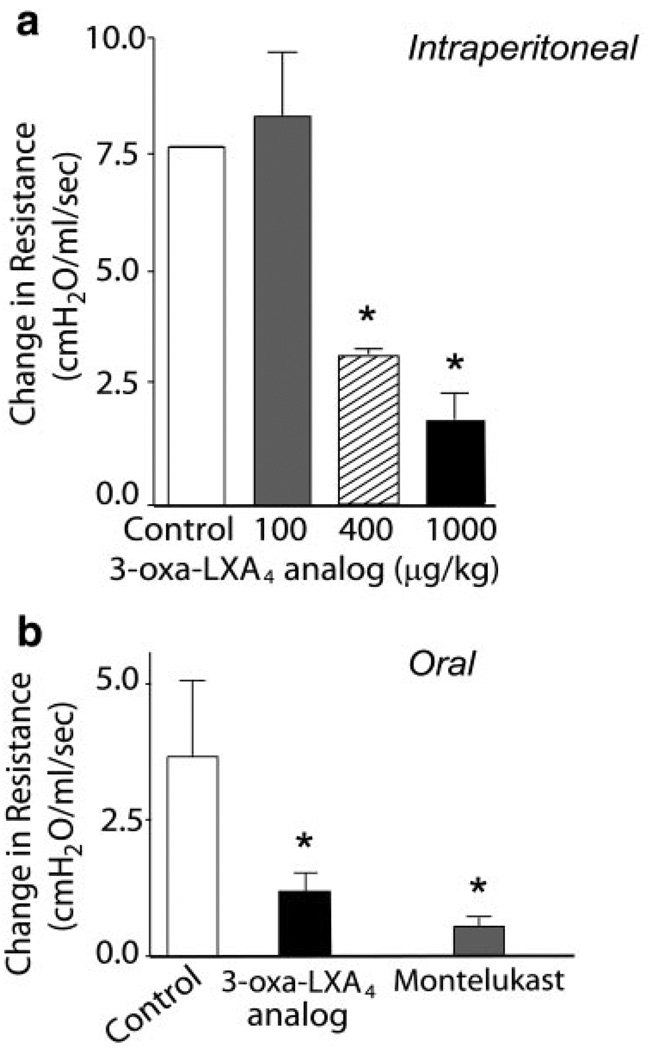
The cell population that has been most closely associated with asthma is the Eos (17) and, unlike the Eos-rich alveolitis generated by OVA aerosol challenge, direct airway challenge with CRA, a common clinical trigger for human asthma (17), leads principally to peribronchial Eos accumulation, which is required for the development of airway hyper-reactivity (18). To determine the effect of LXA4 on allergic airway responses in this second experimental model of asthma, ATLa was prepared at two different concentrations, 1 µg/ml H2O or 10 µg/ml H2O, administered in the animals’ drinking water just before intranasal CRA and continued throughout the entire allergen challenge period (see Materials and Methods). In the 3 days (72 h) of ATLa oral administration, the animals consumed an average of 13 ml/mouse and 14 ml/mouse of the 1 and 10 µg/ml, respectively; 24 h after the final allergen challenge, the animals tested for airway hyper-reactivity or lung tissues were processed for cytokine analysis. In animals challenged with allergen and given ATLa orally in the drinking water, airway hyper-reactivity to methacholine was significantly decreased in a dose-dependent manner (Fig. 4a). The decreased airway responsiveness with ATLa was also associated with decrements in lung IL-4 and IL-5 levels (Fig. 4b). Together, these findings indicate that ATLa is orally active and that treatment with ATLa potently regulates allergic airway responses.
Figure 4.
Oral ATLa stable analog reduces both airway hyper-responsiveness and inflammation. Mice were sensitized and challenged with CRA in the absence (white) or presence of ATLa [1 µg/ml (hatched) or 10 µg/ml (black)] in the drinking water. a) Airway reactivity was determined by methacholine-dependent change in lung resistance. Results are expressed as mean ± se (n≥5). b) Lungs were homogenized and levels of peptide mediators in the aqueous extracts were determined by sensitive and specific EIAs. Results are expressed as the mean ± se (n≥4, d≥2). *P < 0.05 by Student’s t test vs. vehicle control.
ZK-994 decreases allergic airway responses to CRA
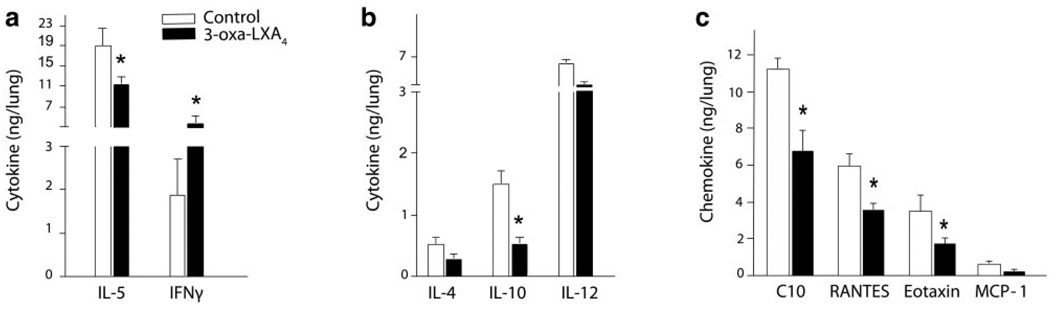
To determine whether the ZK-994 LX/ATL analog could reduce CRA-driven airway inflammation, the compound (100–1000 µg/kg) or a vehicle control was administered intraperitoneally 2 h before intratracheal CRA challenge. Although the lowest dose of ZK-994 (100 µg/kg) showed no significant effect on the development of airway inflammation or hyper-reactivity (vide infra), administration of ZK-994 at 400 µg/kg and higher led to reductions in airway responses. Lung histology revealed reduced peribronchial inflammatory infilitrates (Fig. 5a), and tissue morphometry demonstrated significantly reduced peribronchial Eos accumulation in those animals treated with ZK-994 (Fig. 5b). In murine lung extracts, levels were determined for select cytokines, including IL-4, IL-5, IL-10, IL-12, and IFNγ (Fig. 6a, b), and chemokines, including C10, RANTES, eotaxin, and MCP-1 (Fig. 6c). In this experimental model, the only cytokine levels that were significantly altered by ZK-994 were pulmonary IL-5, IFNγ, and IL-10 (Fig. 6a, b). Significant decrements in all the chemokines except MCP-1 were also present with ZK-994 (Fig. 6c). Decreased IL-5, IL-10, and chemokine levels with increased amounts of IFNγ indicate a central regulatory effect of this analog on adaptive immunity that, in addition to LXA4’s direct cellular actions, served to reduce leukocyte activation and trafficking. Besides these parameters of inflammation, systemic administration of ZK-994 by i.p. injection gave a concentration-dependent inhibition of airway hyper-reactivity to methacholine (Fig. 7a). This protection from airway hyper-responsiveness to methacholine was also present when ZK-994 was administered by oral gavage. This reduction in bronchial hyper-reactivity was similar in potency to that observed with equivalent doses of montelukast (Fig. 7b). Together, these data indicate that ZK-994 carries potent counter-regulatory properties that dampen allergic airway responses; it is orally bioavailable, and either oral administration or a single systemic administration of ZK-994 has protective biological effects that persist for several days.
Figure 5.
3-Oxa, trienyne-containing LXA4 stable analog reduces airway inflammation induced by cockroach allergy. Mice were sensitized and challenged with CRA in the absence (white) or presence (black) of ZK-994 (100–1000 µg/kg, i.p.). a) Lung tissue sections were obtained from formalin-fixed, paraffin-embedded lung tissue prepared and stained with H&E, and b) peribronchial eosinophil accumulation was determined by morphometry (see Materials and Methods). Results are expressed as mean ± se (n≥6, d≥2), *P < 0.05 by Student’s t test vs. vehicle control.
Figure 6.
ZK-994 regulates cytokine and chemokine mediators of Th2 adaptive immune responses to CRA. Mice were sensitized and challenged with CRA in the absence (white) or presence (black) of 3-oxa-LXA4. a–c) Lungs from animals receiving ZK-994 (1000 µg/kg, i.p.) or vehicle control were homogenized and levels of peptide mediators in the aqueous extracts were determined by sensitive and specific EIAs. Results are expressed as the mean ± se (n≥6, d≥2), *P < 0.05 by Student’s t test vs. vehicle control.
Figure 7.
Systemic and oral administration of ZK-994 reduces airway hyper-responsiveness. Cockroach-sensitized mice were treated by a) i.p. injection of vehicle (white) or ZK-994 (100 (gray), 400 (hatched), or 1000 (black) µg/kg, i.p.) or b) 10 µg/ml of either ZK-994 (black) or montelukast (gray), in a blinded manner, in the drinking water before allergen challenge. Airway reactivity was determined by a methacholine-dependent change in lung resistance. Results are expressed as mean ± se (n>5). *P < 0.05 by Student’s t test compared with control animals.
DISCUSSION
Asthmatic lungs are characterized by leukocyte accumulation and hyper-reactive airway responses. Mediators that regulate both leukocyte activation and functional responses of lung resident cells have the potential to resolve the disordered milieu of asthmatic lung and promote a return to homeostasis. Lipoxins display such properties. For example, LXA4 carries counter-regulatory actions that inhibit neutrophil, Eos, dendritic cell, Th2 lymphocyte, and natural killer cell activation, block airway epithelial cell cytokine release, modulate cytokine-induced metalloprotease activity, and stimulate macrophage clearance of apoptotic cells (reviewed in ref. 3). We recently uncovered the multi-pronged inhibition of airway hyper-responsiveness and inflammation by intravenous ATLa in a murine model of OVA-induced allergic airway responses (8). Animals transgenic for CD11b-targeted human ALX receptor expression were also protected, presumably by a heightened sensitivity to endogenous levels of LXA4, during the inflammatory response of these mice. Here we present a structure activity relationship for the protective actions of two LX analogs and the CysLT1 receptor antagonist montelukast in two mechanistically distinct experimental models of allergic airway responses.
Cysteinyl LTs are generated during allergic airway inflammation and serve as potent bronchoconstrictors (19). CysLT1 receptor activation has been associated with chronic airway inflammation and airway remodeling (20). Because LXA4 and select analogs can interact with both ALX and CysLT1, we directly compared responses of LXA4 stable analogs to montelukast, a pharmacological CysLT1 receptor antagonist, in allergen-induced airway inflammation. Unlike montelukast, LXA4 stable analogs significantly decreased Eos and Lymph trafficking as monitored by lung histology and enumeration of cells in BAL fluids. Both montelukast and LXA4 analogs reduced IL-13 levels, yet only the LX analogs dampened IL-4 and IL-5 levels. Of interest, ATLa also blocked CysLT levels. These findings indicate that LXA4 stable analogs transduce their protective effects via sites of action, such as ALX, that are distinct from CysLT1. It is still possible that decrements in IL-13 levels relate in part to regulation of CysLT1 signaling, but the LXA4 stable analogs were substantially more potent regulators of acute inflammation than montelukast.
In two different experimental models of asthma, systemic administration of LXA4 analogs modulated local immune responses, including Eos accumulation, Th2 type cytokine levels, and airway hyper-reactivity. The approach used to elicit inflammation and airway hyper-reactivity can be a critical determinant of the cells and mediators that are responsible for the allergic responses in murine lung (21) as well as the lung compartment involved (18). Systemic sensitization by i.p. injection of OVA adsorbed to an adjuvant, followed by OVA aerosol challenge, induces airway eosinophilia and hyper-reactivity with increased levels of Th2 cytokines and chemokines; however, mast cells and IgE are not required for these responses. In contrast, direct administration of allergen, such as CRA, without adjuvant to the airway leads to mast cell-dependent responses (21) and amore focused peribronchial eosinophilia that better reflects the pathobiology of human asthma (18). Here, ATLa and ZK-994 provided protection in both models. LXs and LX analogs are potent regulators of adaptive immunity, including dendritic and epithelial cell responses (22, 23), as well as cellular effectors of both innate and adaptive immunity such as neutrophils, macrophages, Eos, and Lymphs (8, 9, 23, 24).
Several potential links between LX signaling and cytokines and chemokines in regulating cellular responses have been uncovered (25). Activation of ALX in monocytes leads to phosphorylation of the chemokine receptor CCR5 and attenuates cell responses to select chemokines (26). In addition, HIV gp120 binds to ALX and alters the expression and function of CCR5 and CxCR4, again suggesting that this activation event, via ligation of ALX receptors, actively alters the chemotactic potential of leukocytes (27). In the OVA challenge model, ATLa and ZK-994 both inhibited airway Eos but utilized slightly different mechanisms. ATLa led to decrements in CysLTs and IL-5 whereas ZK-994 reduced RANTES. Specific cytokines, such as IL-5, and chemokines, including C10, eotaxin, and RANTES, play crucial roles in recruiting Eos to the site of allergic reactions (28). In the CRA model, ZK-994 significantly decreased all of these peptide mediators. Lipoxins also regulate structural cells in the airway (29) and inflammatory pain (30). Because inflammatory and neuropeptide mediators have also been associated with airway remodeling in asthma (28), LXA4 stable analog alteration of the mediator phenotype within the lung has the potential to prevent chronic damage and lessen severity in asthma.
LXA4 is generated during human asthmatic responses (31). More severe variants of asthma, including aspirin-exacerbated respiratory disease, are associated with diminished lipoxin biosynthesis compared with more mild asthma (32, 33). The relative conversion of arachidonic acid to LXA4 vs. CysLTs correlates with airflow obstruction (32), and administration of LXA4 to asthmatic subjects challenged with LTC4 significantly attenuates airway responsiveness (34). In conjunction with results presented here in experimental murine models of asthma, these findings indicate that endogenous LXA4 can counter asthmatic inflammation and airway responses.
In summary, our results demonstrate that LXA4 analogs administered by gavage, i.p. injection, or orally in drinking water regulate allergic airway responses in two different experimental models with a mode of therapeutic immunomodulation that is distinct from the currently clinically used montelukast. Thus, the potent counter-regulatory actions of LXA4 stable analogs display promise as needed therapies for allergic airway diseases and as a new therapeutic strategy in asthma.
Acknowledgments
The authors thank Christy Schneider for expert assistance in manuscript preparation. This work was supported in part by National Institutes of Health grants HL68669 (B.D.L.), DE016191 (B.D.L., C.N.S.), AI0608084 (B.D.L.), and GM38765 (C.N.S.).
REFERENCES
- 1.Barnes PJ. New drugs for asthma. Nat. Rev. Drug Discov. 2004;3:831–844. doi: 10.1038/nrd1524. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel SE, Covar R. Update in asthma 2005. Am. J. Respir. Crit. Care Med. 2006;173:698–706. doi: 10.1164/rccm.2601007. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN. Selectivity of recombinant human leukotriene D(4), leukotriene B(4), and lipoxin A(4) receptors with aspirin-triggered 15-epi-LXA(4) and regulation of vascular and inflammatory responses. Am. J. Pathol. 2001;158:3–9. doi: 10.1016/S0002-9440(10)63937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clish CB, Levy BD, Chiang N, Tai HH, Serhan CN. Oxidoreductases in lipoxin A4 metabolic inactivation: a novel role for 15-onoprostaglandin 13-reductase/leukotriene B4 12-hydroxydehydrogenase in inflammation. J. Biol. Chem. 2000;275:25372–25380. doi: 10.1074/jbc.M002863200. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 7.Bandeira-Melo C, Bozza PT, Diaz BL, Cordeiro RS, Jose PJ, Martins MA, Serhan CN. Cutting edge: lipoxin (LX) A4 and aspirin-triggered 15-epi-LXA4 block allergen-induced eosinophil trafficking. J. Immunol. 2000;164:2267–2271. doi: 10.4049/jimmunol.164.5.2267. [DOI] [PubMed] [Google Scholar]
- 8.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat. Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 9.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J. Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto A, Murakami Y, Kitasato H, Hayashi I, Endo H. Glucocorticoids co-interact with lipoxin A4 via lipoxin A4 receptor (ALX) up-regulation. Biomed. Pharmacother. 2007;61:81–85. doi: 10.1016/j.biopha.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat. Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petasis NA, Akritopoulou-Zanze I, Fokin VV, Bernasconi G, Keledjian R, Yang R, Uddin J, Nagulapalli KC, Serhan CN. Design, synthesis and bioactions of novel stable mimetics of lipoxins and aspirin-triggered lipoxins. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:301–321. doi: 10.1016/j.plefa.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Guilford WJ, Bauman JG, Skuballa W, Bauer S, Wei GP, Davey D, Schaefer C, Mallari C, Terkelsen J, Tseng JL, et al. Novel 3-oxa lipoxin A4 analogues with enhanced chemical and metabolic stability have anti-inflammatory activity in vivo. J. Med. Chem. 2004;47:2157–2165. doi: 10.1021/jm030569l. [DOI] [PubMed] [Google Scholar]
- 14.Fiorucci S, Wallace JL, Mencarelli A, Distrutti E, Rizzo G, Farneti S, Morelli A, Tseng JL, Suramanyam B, Guilford WJ, Parkinson JF. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15736–15741. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilford WJ, Parkinson JF. Second-generation beta-oxidation resistant 3-oxa-lipoxin A4 analogs. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:245–250. doi: 10.1016/j.plefa.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Berlin AA, Lukacs NW. Treatment of cockroach allergen asthma model with imatinib attenuates airway responses. Am. J. Respir. Crit. Care Med. 2005;171:35–39. doi: 10.1164/rccm.200403-385OC. [DOI] [PubMed] [Google Scholar]
- 17.Busse WW, Lemanske RF., Jr Asthma. N. Engl. J. Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 18.Campbell EM, Charo IF, Kunkel SL, Strieter RM, Boring L, Gosling J, Lukacs NW. Monocyte chemoattractant protein-1 mediates cockroach allergen-induced bronchial hyperreactivity in normal but not CCR2−/− mice: the role of mast cells. J. Immunol. 1999;163:2160–2167. [PubMed] [Google Scholar]
- 19.Dahlen SE, Hedqvist P, Hammarstrom S, Samuelsson B. Leukotrienes are potent constrictors of human bronchi. Nature. 1980;288:484–486. doi: 10.1038/288484a0. [DOI] [PubMed] [Google Scholar]
- 20.Henderson WR, Jr, Chiang GK, Tien YT, Chi EY. Reversal of allergen-induced airway remodeling by CysLT1 receptor blockade. Am. J. Respir. Crit. Care Med. 2006;173:718–728. doi: 10.1164/rccm.200501-088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taube C, Dakhama A, Gelfand EW. Insights into the pathogenesis of asthma utilizing murine models. Int. Arch. Allergy Immunol. 2004;135:173–186. doi: 10.1159/000080899. [DOI] [PubMed] [Google Scholar]
- 22.Bonnans C, Vachier I, Chavis C, Godard P, Bousquet J, Chanez P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am. J. Respir. Crit. Care Med. 2002;165:1531–1535. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- 23.Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J. Anti-inflammatory actions of lipoxin A(4) and aspirin-triggered lipoxin are SOCS-2 dependent. Nat. Med. 2006;12:330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 24.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J. Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 25.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 26.Li BQ, Wetzel MA, Mikovits JA, Henderson EE, Rogers TJ, Gong W, Le Y, Ruscetti FW, Wang JM. The synthetic peptide WKYMVm attenuates the function of the chemokine receptors CCR5 and CXCR4 through activation of formyl peptide receptor-like 1. Blood. 2001;97:2941–2947. doi: 10.1182/blood.v97.10.2941. [DOI] [PubMed] [Google Scholar]
- 27.Deng X, Ueda H, Su SB, Gong W, Dunlop NM, Gao JL, Murphy PM, Wang JM. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating the 7-transmembrane G-protein-coupled receptor FPRL1/LXA4R. Blood. 1999;94:1165–1173. [PubMed] [Google Scholar]
- 28.Smit JJ, Lukacs NW. A closer look at chemokines and their role in asthmatic responses. Eur. J. Pharmacol. 2006;533:277–288. doi: 10.1016/j.ejphar.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 29.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A4 regulates bronchial epithelial cell responses to acid injury. Am. J. Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J. Exp. Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee TH, Crea AE, Gant V, Spur BW, Marron BE, Nicolaou KC, Reardon E, Brezinski M, Serhan CN. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. Am. Rev. Respir. Dis. 1990;141:1453–1458. doi: 10.1164/ajrccm/141.6.1453. [DOI] [PubMed] [Google Scholar]
- 32.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Diminished lipoxin biosynthesis in severe asthma. Am. J. Respir. Crit. Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, Serhan CN, Szczeklik A. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur. Respir. J. 2000;16:44–49. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- 34.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am. Rev. Respir. Dis. 1992;145:1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]