Summary
Ibalizumab is a humanized, anti-CD4 monoclonal antibody. It potently blocks HIV-1 infection and targets an epitope in the second domain of CD4 without interfering with immune functions mediated by interaction of CD4 with major histocompatibility complex (MHC) class II molecules. We report here the crystal structure of ibalizumab Fab fragment in complex with the first two domains (D1-D2) of CD4 at 2.2 Å resolution. Ibalizumab grips CD4 primarily by the BC-loop (residues 121-125) of D2, sitting on the opposite side of gp120 and MHC-II binding sites. No major conformational change in CD4 accompanies binding to ibalizumab. Both monovalent and bivalent forms of ibalizumab effectively block viral infection, suggesting that it does not need to crosslink CD4 to exert antiviral activity. While gp120-induced structural rearrangements in CD4 are probably minimal, CD4 structural rigidity is dispensable for ibalizumab inhibition. These results could guide CD4-based immunogen design and lead to a better understanding of HIV-1 entry.
Introduction
CD4, a cell-surface glycoprotein containing four immunoglobulin domains (D1 to D4), has an essential role in both T cell activation and HIV-1 (human immunodeficiency virus type-1) infection. As a T-cell coreceptor, CD4 interacts directly with major histocompatibility complex (MHC) class II molecules on the surface of antigen presenting cells and helps recruit a tyrosine kinase, p56lck, to facilitate activation of helper T cells, thereby modulating adaptive immune responses (Doyle and Strominger, 1987; Xu and Littman, 1993). The interaction of CD4 with MHC II has been defined by structural studies of a complex containing 2D CD4 and the murine I-Ak class II MHC molecule with a bound peptide (Wang et al., 2001). The first domain (D1) of CD4 makes direct contacts with the α2 and β2 domains of MHC II, suggesting that T-cell receptor and CD4 communicate with each other through the peptide-MHC complex (Wang et al., 2001). HIV-1 exploits CD4 as its primary receptor for initial attachment and for inducing formation of the coreceptor binding site on the HIV-1 envelope glycoprotein gp120 (Dalgleish et al., 1984; Wyatt and Sodroski, 1998). Encounter of the coreceptor by gp120 is believed to be the crucial trigger for dissociation of gp120 and a cascade of refolding events in the viral fusion protein gp41, leading to fusion of viral and host cell membranes (Harrison, 2008). Comparison of crystal structures of gp120 core in the CD4-bound and the unliganded conformations reveals molecular details of the interaction of CD4 with gp120 and the accompanying structural rearrangements in the viral protein (Chen et al., 2005; Kwong et al., 1998). The gp120 core contains two closely associated domains, named “inner” and “outer”. In the CD4-bound conformation, the relative orientations of the two domains are fixed by a four-strand β-sheet, the “bridging sheet”, formed by two β hairpins, the stem of the V1-V2 variable loop from the inner domain and a hairpin that projects from the outer domain. D1 of CD4 interacts, primarily through the C” edge, with both the inner and outer domains, as well as with the bridging sheet (Kwong et al., 1998). CD4 binding results in large rearrangements of the inner domain, leading to formation of the bridging sheet (Chen et al., 2005). Together with the V3 variable loop, the bridging sheet makes up the binding site for coreceptor (Huang et al., 2007; Rizzuto et al., 1998). The ectodomain of CD4 could project up to ~115 Å from the cell surface, with D1 at the membrane-distal end (Wu et al., 1997). Conformational changes in gp120, CD4 or both would be needed to bring the bound gp120 (or the virus) close to the coreceptor, a seven-transmembrane receptor embedded in cell membrane.
Ibalizumab (also known as Hu5A8 or TNX-355), is a humanized IgG4 monoclonal antibody (mAb) derived from a mouse antibody, 5A8, which binds D2 of CD4 and blocks HIV-1 infection in vitro (Burkly et al., 1992; Dimitrov, 2007; Reimann et al., 1997). Ibalizumab has also been shown to neutralize a broad spectrum of HIV-1 primary isolates in vitro, and to effectively reduce plasma viral loads and increase CD4+ T cell counts in both SIV (simian immunodeficiency virus)-infected rhesus monkeys and HIV-1 infected patients (Jacobson et al., 2009; Kuritzkes et al., 2004; Reimann et al., 2002). Because of its potency and breadth, ibalizumab is currently being evaluated as a potential therapeutic antibody in a phase IIb clinical trial conducted by TaiMed Biologics, Inc. (Jacobson et al., 2009). Previous mutagenesis studies suggest that the epitope of ibalizumab on CD4 does not overlap with the binding site of gp120 or MHC class II molecules, consistent with the observations that the antibody does not interfere with viral attachment and that it is well tolerated when infused into patients (Burkly et al., 1992; Moore et al., 1992; Song et al., 2010). Residues 121-124 and 127-134 in D2 of CD4 were found to be critical for 5A8 binding, and it was reported that only the bivalent form of the antibody was effective in blocking HIV-1 infection (Burkly et al., 1992). These results implied that the antibody might exert its antiviral activity by crosslinking CD4, thereby preventing certain post-attachment events required for HIV-1 entry.
To define the ibalizumab epitope at the atomic level and to reveal potential structural changes in CD4 induced by binding to the antibody, we have determined the crystal structure of the Fab fragment of ibalizumab in complex with the two N-terminal domains of human CD4 at 2.2 Å resolution. The antibody primarily contacts the BC-loop in D2 of CD4, at the D1-D2 junction on the opposite side of both gp120 and MHC class II binding sites. Small, local conformational changes in CD4 occur at the binding interface, but they do not propagate to other regions to have a significant impact on the receptor function. Both the IgG and Fab forms of ibalizumab effectively block viral entry by certain HIV-1 isolates, ruling out the possibility that crosslinking of CD4 is required for its antiviral activity. We have also shown that interaction with gp120 does not lead to major structural rearrangements in the ectodomain of CD4, and that structural rigidity in the membrane-bound CD4 does not play a role in ibalizumab inhibition. These results could guide CD4-based immunogen design for eliciting ibalizumab-like antibody responses against HIV-1 infection, and lay a foundation for further investigations to better understand HIV-1 entry.
Results
Inhibition of HIV-1 infection by Ibalizumab IgG and Fab
It was previously reported that only bivalent forms of mouse mAb 5A8 were effective in blocking HIV-1 entry (Burkly et al., 1992). To verify this observation using ibalizumab, we chose a panel of HIV-1 Env pseudoviruses, including 3 laboratory-adapted isolates and 9 primary isolates from clades A, B and C, to test inhibition by its bivalent IgG and monovalent Fab fragment. The antigenic diversity of HIV-1 is also well represented by the selected viruses, as their sensitivities to neutralization by soluble CD4 or a broadly neutralizing, anti-gp41 antibody 4E10 differ significantly (Table 1). Contrary to previous findings, both IgG and Fab of ibalizumab can block infection by most viruses, however, inhibition strengths of bivalent IgG and monovalent Fab vary greatly among different isolates (Figures 1 and S1, Table 1). In general, the three laboratory-adapted strains, SF162.LS, MN-3 and HxB2.DG, are less sensitive to ibalizumab than the primary isolates. Two of the patient isolates, Du156.12 and Du422.1, both from clade C, are the most sensitive ones among all the viruses tested, with almost equal sensitivity to the IgG and to the Fab. For all other primary strains, the IgG is indeed more potent than the Fab, but the latter does reduce viral infection of all isolates tested. In addition, Fab and bivalent F(ab’)2 also show comparable inhibitory activity against several HIV-1 isolates (Table S2). Thus, we conclude that the bivalency of ibalizumab is not required for its antiviral activity, but its size may have an impact. The discrepancy between this work and the published results is likely caused by the limited number of laboratory-adapted strains and the cell-cell fusion assay used for the previous study (Burkly et al., 1992). Ibalizumab exhibits a relatively unusual, strain-dependent inhibition pattern, where the neutralization curve plateaus below 100% inhibition with increase of the antibody concentration (Figures 1 and S1; designated as maximum percent inhibition (MPI) in Table 1). The reason for this phenomenon is unknown, as the viruses used were all pseudotyped with homogenous Env molecular clones and CD4-independent HIV-1 isolates are extremely rare (Kolchinsky et al., 2001). Regardless, viruses with MPI less than 100% could potentially escape from ibalizumab inhibition.
Table 1.
Neutralization of 12 selected HIV-1 isolates by Ibalizumab IgG and Fab, soluble CD4 and 4E10
| Ibalizumab IgG | Ibalizumab FAb | sCD4 | 4E10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Virus | Clade | IC50 Titera | MPI (%)b | IC50 Titer | MPI (%) | IC50 Titer | MPI (%) | IC50 Titer | MPI (%) |
| SF162.LS | B | >100 | 33 | >100 | 24 | 0.13 | 100 | 11.10 | 73 |
| MN | B | 0.24 | 71 | >100 | 39 | <0.02 | 100 | 0.14 | 100 |
| HxB2.DG | B | 2.20 | 52 | >100 | 26 | <0.02 | 100 | 0.19 | 100 |
| SC422661.8 | B | 0.03 | 81 | 0.90 | 59 | 8.80 | 75 | 1.20 | 95 |
| PVO.4 | B | 0.02 | 92 | 0.18 | 67 | 8.50 | 90 | 50.00 | 50 |
| QH0692.42 | B | 0.14 | 78 | 3.40 | 59 | 2.10 | 100 | 7.40 | 78 |
| ZM53M.PB12 | C | 0.13 | 78 | >100 | 47 | 6.80 | 98 | 10.10 | 89 |
| Du156.12 | C | 0.01 | 100 | 0.02 | 99 | 22.30 | 71 | 0.30 | 100 |
| Du422.1 | C | 0.02 | 97 | 0.09 | 91 | 9.30 | 85 | 10.20 | 88 |
| Q769.d22 | A | >100 | 48 | >100 | 27 | 1.10 | 100 | 3.70 | 88 |
| Q461.e2 | A | 0.10 | 78 | 53.80 | 57 | 17.80 | 90 | 28.50 | 65 |
| Q259.d2.17 | A | 0.02 | 90 | 0.32 | 74 | 8.80 | 97 | 11.10 | 74 |
Figure 1. Inhibition of representative HIV-1 isolates by ibalizumab IgG and Fab.
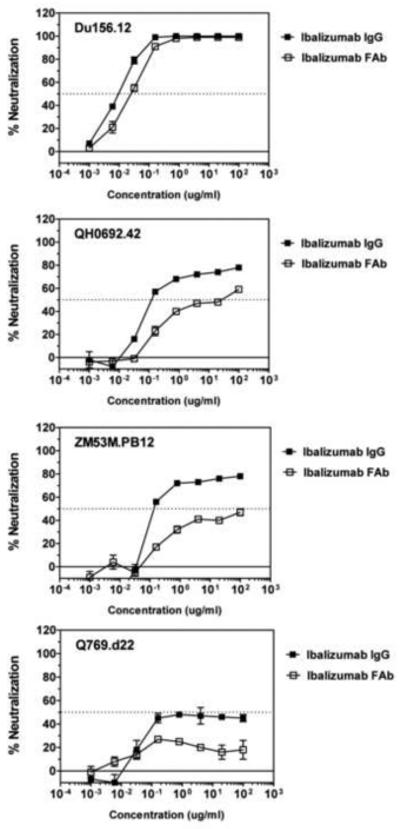
HIV-1 primary isolates, Du156.12 (clade C), QH0692.42 (clade B), ZM53M.PB12 (clade C) and Q769.d22 (clade A) were analyzed for inhibition by the anti-CD4 monoclonal antibody ibalizumab using a luciferase-based virus neutralization assay with TZM.bl cells. Serial dilutions of ibalizumab in the bivalent IgG form (filled square) or the monovalent Fab form (open square) were tested for neutralization against each virus. 50% of inhibition is indicated by a dashed, horizontal line. IC50 values and MPI (maximum percent inhibition) are summarized in Table 1. The experiments were repeated twice with similar results. See also Figure S1 and Table S2.
Structure of the complex of ibalizumab Fab and soluble 2 domain CD4
To define the epitope of ibalizumab precisely and to gain molecular insights on whether ibalizumab binding could block CD4 receptor function, we have determined the crystal structure of the complex of its Fab and human CD4 at 2.2 Å resolution. The Fab fragment of ibalizumab was produced by papain digestion and purified by protein A affinity and gel-filtration chromatography. A two-domain fragment of human CD4 (residues 1-182) with a C-terminal His-tag, produced in insect cells, was mixed with ibalizumab Fab, and the complex was further purified by gel-filtration chromatography. The complex was crystallized by hanging-drop vapor diffusion with a mother liquor containing 3 M Na malonate, pH 5.5. The crystals diffract to 2.2 Å resolution and belong to space group P212121 (a=52.2, b=66.3, c=266.6 Å), with one Fab-CD4 complex per crystallographic asymmetric unit. The structure was determined by molecular replacement using 2D CD4 and a library of Fab coordinates as search models by MOLREP and Phaser (Aoki et al., 2009; McCoy et al., 2007; Vagin and Teplyakov, 1997). Complementarity determining regions (CDRs) of both heavy- and light-chains of ibalizumab were rebuilt iteratively in O (Jones and Kjeldgaard, 1997), and the final model was refined with an Rwork of 19.3% and an Rfree of 22.4% (Table S1).
As shown in Figure 2, ibalizumab grips CD4 mainly by direct contacts between both its heavy- and light- chains, and the BC-loop (residues 121-125) in D2 of CD4. These interactions are consistent with the previous mutagenesis results (Burkly et al., 1992; Song et al., 2010), but another segment (residues 127-134) in CD4, previously suggested to be critical for mAb 5A8 binding (Burkly et al., 1992), barely makes contact with the antibody. There is a distorted disulfide (S-S) bond between residues Cys130 and Cys159, which are located in two antiparallel beta strands, respectively, and the geometry required for the S-S bond is not favorable (Wang et al., 1990). Therefore any deletions in the neighboring residues could further disfavor the formation of this S-S bond and in turn affect the conformation of the core epitope - the BC loop. A more recent epitope-mapping study on ibalizumab by point mutation has indicated that the segment (residues 127-134) is less critical (Song et al., 2010). As shown in Figure 3A, the tip of the BC-loop in CD4 is sandwiched between the CDR H3 and CDR L3 of ibalizumab. Pro122 of CD4 is tightly packed against Tyr30A, Tyr32 and Tyr92 of the L-chain. Additional contacts include an extensive hydrogen bond network formed among residues 120-126 of CD4 and ibalizumab, either directly or mediated by water molecules. Clearly, the BC-loop constitutes the core epitope of ibalizumab (Figure 3A). The second major contact involves residues 164-165 (the short FG loop in D2) of CD4 and the ibalizumab H-chain (Figure 3B). The FG loop is surrounded by all three CDRs from the heavy chain. Binding to ibalizumab induces a flip of the peptide bond between Asn164 and Gln165, resulting in a flip of the FG loop (Figure 3B). The induced conformation is stabilized by a number of hydrogen bonds, indicating that the FG loop forms an important part of the epitope. This conformational change in CD4 triggered by ibalizumab binding is nevertheless entirely local and does not propagate to other regions. Thus, it appears to have no significant impact on CD4 function. The third major contact is made by interaction of the tip of CDR L1 with the EF loop in D1 of CD4. The side chains of Tyr30A and Thr30C of L-chain form hydrogen bonds with the main chain carbonyl of Ser79 and Glu77, respectively. Thus, D1 also contributes substantially to the ibalizumab epitope. Overall, there are no major conformational changes within CD4 upon binding to ibalizumab that could propagate to other regions.
Figure 2. Structure of the complex of 2 domain CD4 and the Fab fragment of ibalizumab.
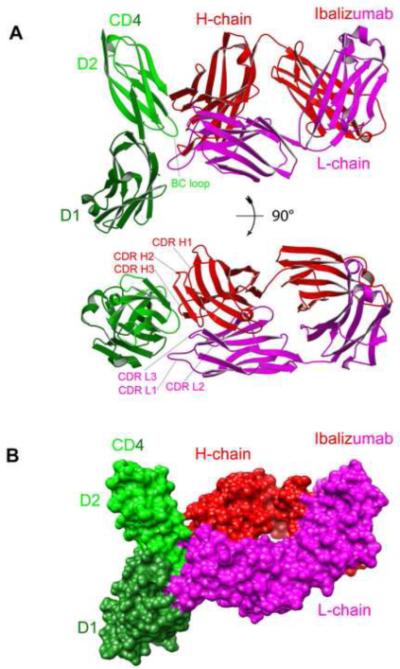
(A) Top and side views of the overall structure of 2D CD4 in complex with ibalizumab Fab, shown in ribbon representation. CD4 is in green (lighter green for D2 and darker green for D1), the heavy chain of ibalizumab in red, and the light chain in magenta. The BC-loop in the second domain of CD4, which constitutes the core epitope, and the CDR loops of ibalizumab are all indicated. (B) Surface representation of the complex. CD4 and ibalizumab are shown in the same color scheme as in (A). See also Figure S2 and Figure S4, and Table S1.
Figure 3. Close-up of major contacts between CD4 and ibalizumab.
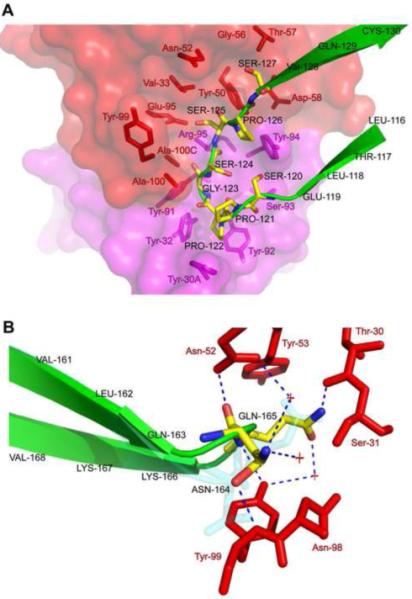
(A) Residues making direct contacts between the heavy- and light- chains of ibalizumab and the BC-loop (residues 121-125) of D2 in CD4. The heavy and light chains of ibalizumab are shown by surface representation in red and magenta, respectively, and the residues that make contacts with CD4 are also shown by stick model in the same color scheme. The BC-loop of CD4 is in green in ribbon diagram and the residues interacting with ibalizumab are shown by stick model with carbon atoms in yellow, nitrogen in blue and oxygen in red. (B) Contacting residues between the FG loop (residues 164, 165) of D2 in CD4 and the ibalizumab heavy-chain. The residues that make direct contacts with the FG loop of CD4 in the heavy chain are shown by stick model in red. The FG loop of CD4 is shown in ribbon diagram in green with residues 164 and 165 in stick model in the same color scheme as in (A). The conformation of the two residues in the unbound CD4 is also shown in cyan; a flip of the loop upon binding to ibalizumab is evident. See also Figure S3 and Table S1.
The binding site of ibalizumab does not overlap with the binding site for MHC II (Figures S2 and S3), in agreement with the observation that ibalizumab is non-immunosuppressive (Boon et al., 2002). Since the gp120 binding site on CD4 does not overlay that of ibalizumab either (Figures S2 and S3), consistent with the previous findings that binding of mAb 5A8 to CD4 does not reduce CD4 affinity to gp120, nor does it block CD4 binding to HIV-1 virions (Moore et al., 1992), and with our result that ibalizumab does not hinder interaction between CD4 and a stable, homogenous HIV-1 gp140 trimer (Figure S4), how does ibalizumab block HIV-1 infection? Earlier studies suggested that ibalizumab may exert its antiviral activity by crosslinking CD4, thereby impeding the postbinding movement of CD4. Our finding that the monovalent form of ibalizumab inhibits at least some HIV-1 isolates has ruled out the possibility that crosslinking of CD4 plays a major role in neutralization by ibalizumab. Another possibility is that the entire ectodomain of CD4 may undergo, upon binding to gp120, certain structural rearrangements that are required for the viral entry and could be blocked by ibalizumab.
Binding by gp120 does not lead to large conformational changes in soluble, 4-domain CD4
Gp120-CD4 interaction triggers large structural rearrangements in gp120 to form the coreceptor binding site. In contrast, gp120 binding does not alter the structure of the first two domains of CD4 (Kwong et al., 1998). Based on reconstructions of 2 domain CD4-gp120 and 4 domain CD4-gp120 complexes derived from small angle x-ray scattering (SAXS) data, Ashish et al. have proposed that interaction with gp120 induces a large conformational change in the ectodomain of CD4, in which the first two domains (D1-D2) fold back along the D2-D3 hinge region onto the third and fourth domains (D3-D4) (Ashish et al., 2008). The two complexes appeared to have a similar Dmax (the largest dimension of the protein or protein complex) or Rg (radius of gyration) in this study. Close examination of the D2-D3 linker region in the crystal structure of 4D CD4 leads us to believe that such a dramatic, jackknife-type rearrangement is highly unlikely, because it would lead to serious side chain clashes between residues in D2 and D3, if CD4 had to bend by ~180 degrees (Wu et al., 1997). Furthermore, in the Ashish et al. study, the use of a full-length gp120 protein and unpurified gp120-CD4 complexes is questionable and could complicate interpretation of the SAXS data (Ashish et al., 2008). We decided to reexamine this issue by using the SAXS technique to analyze the complexes of soluble CD4 with gp120 core, for which the structure is much better understood than that of the full-length gp120. To ensure the conformational homogeneity of the samples before SAXS data collection, all the proteins and protein complexes were purified by gel-filtration chromatography on a S200 column (Figure S5). The Rg of each sample was derived from both Guinier analysis and distance distribution function P(r) analysis (Figure S6). As summarized in Table 2, Rgs obtained from the two different analyses are in excellent agreement for each sample. In particular, Rg for the 2 domain CD4-gp120 core complex is ~36.0 Å while Rg for the 4 domain CD4-gp120 core complex is ~47.0 Å. The derived Dmax values for these two complexes are quite different and consistent with those measured directly from the crystal structure or the modeled 4D CD4-gp120 structure assuming no conformational changes in 4D CD4 when bound to gp120 (Table 2 and Figure S7). We conclude that there are no dramatic, gp120-induced conformational changes in the ectodomain of CD4. It remains possible that CD4 could bend by a few degrees at the D2-D3 junction, which may not be detectable by SAXS.
Table 2.
SAXS (small angle x-ray scattering) analysis of 2D CD4-gp120 core and 4D CD4-gp120 core complexes
| Protein | Mass (kDa) |
Rg (Å) Guinier analysis |
Rg (Å) P(r) analysis |
Dmax (Å) P(r) analysis |
Dmax (Å) crystal structure |
|---|---|---|---|---|---|
| 2D CD4 | 21.0 | 22.10±0.41 | 22.42±0.10 | 70 | 67 |
| 4D CD4 | 41.2 | 34.80±0.14 | 35.35±0.14 | 118 | 115 |
| gp120 core | ~66.0 | 30.90±0.13 | 30.31±0.06 | 90 | 83 |
| 2D CD4-gp120 core | ~87.0 | 36.00±0.27 | 36.71±0.08 | 110 | 102 |
| 4D CD4-gp120 core | ~107.2 | 46.80±0.26 | 47.54±0.17 | 155 | 152 |
See also Figures S5, S6 and S7.
Impact of the structural rigidity in the ectodomain of CD4 on ibalizumab inhibition
Since CD4 does not undergo a jackknife-type change when bound to gp120, its ectodomain may need to swing towards the membrane as a rigid body in order to bring its D1 at the membrane-distal end and the bound gp120 close to the coreceptor. If this large swing of CD4 is required for gp120 to access coreceptor, ibalizumab could potentially block or retard this critical movement by binding to D2. To test this hypothesis, we introduced flexibility into the ectodomain of CD4 by replacing D3-D4 of CD4 with flexible, artificial linkers of various lengths. Ibalizumab would not be able to block HIV-1 infection mediated by these CD4 variants if the structural rigidity of CD4 were necessary for inhibition. We created four CD4 variants with the first two domains (D1-D2) connected directly to the transmembrane (TM) segment and cytoplasmic tail by a flexible linker (CD4-L1, CD4-L2, CD4-L3 and CD4-L4 in Figure 4). Two additional variants were also constructed, with either a 5-residue deletion (residues 367-371; TPVQP) in the linker region between D4 and TM of CD4 (CD4-Δ5) to rigidify the ectodomain or the entire C-terminal half of CD4 replaced by the human CD8α hinge region and TM (CD4-CD8TM). The latter two constructs were previously reported to be able to support HIV-1 infection, albeit at reduced efficiency (Moir et al., 1996; Poulin et al., 1991). When introduced into a 293T cell line stably expressing human CCR5 (ACTOne CCR5 cells) by transient transfection, all CD4 variants except for CD4-Δ5 express at a similar level on the cell surface as does the wildtype CD4 construct, as judged by flow cytometry (Figures 4 and S8). They can also support HIV-1 entry when infected with a recombinant HIV-1 SC422661.8 Env pseudotyped virus expressing a luciferase reporter gene (Figures 4 and S9A), but the efficiency varies and roughly correlates with the length of the flexible linker. All CD4 variants remain sensitive to inhibition by ibalizumab, however, with IC50 and MPI values similar to those of wildtype CD4 (Figures 4 and S9B), suggesting that structural rigidity within the CD4 ectodomain is not essential for either HIV-1 entry or for inhibition by ibalizumab. These results further confirm that D3-D4 of CD4 is dispensable for its receptor function, arguing against the jackknife model proposed previously (Ashish et al., 2008).
Figure 4. Impact of flexibility in the CD4 ectodomain on HIV-1 inhibition by ibalizumab.
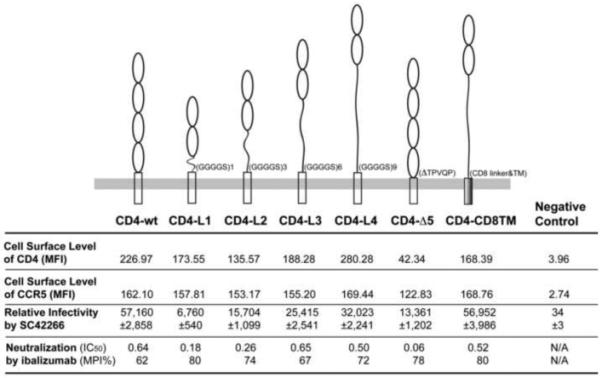
To introduce flexibility in the ectodomain of CD4, four variants with its D3-D4 (residues 179-365) substituted by a flexible linker, GGGGS (CD4-L1), GGGGSGGGGSGGGGS (CD4-L2), GGGGSGGGGSGGGGSGGGGSGGGGSGGGGSGGGGS (CD4-L3) and GGGGSGGGGSGGGGSGGGGSGGGGSGGGGSGGGGSGGGGSGGGGS (CD4-L4) were generated. Two additional CD4 variants were also constructed with either a 5-residue (residues 367-371; TPVQP) deletion (CD4-Δ5) or the entire D3-D4 and TM (residues 179-433) replaced by a human CD8α hinge region and TM (residues 115-214) (CD4-CD8TM). ACTOne CCR5 cells were transfected with wildtype CD4 and six variants. Surface expression levels of CD4 and CCR5 were detected by fluorescence-activated cell sorting (FACS) analyses and the mean fluorescence intensity (MFI) values are summarized. The relative infectivity of these target cells by a recombinant HIV-1 Env pseudovirus, SC422661.8, expressing the firefly luciferase reporter gene is shown by relative luminescence units (RLU). Inhibition of this HIV-1 Env pseudovirus by ibalizumab IgG are reported as IC50 values and MPI (maximum percent inhibition). The experiments were repeated twice with similar results and one set of data is shown. See also Figures S8 and S9.
Modeling of the structure of the complex of ibalizumab, 4-domain CD4 and gp120 core containing the V3 loop suggests that ibalizumab is distant from the coreceptor binding site, which includes the bridging sheet and V3 loop (Figure S3B). It is therefore unlikely that ibalizumab would directly block gp120 binding to the coreceptor on the cell surface.
Discussion
Ibalizumab is a humanized, anti-CD4 monoclonal antibody and is currently being evaluated as a potential anti-HIV-1 therapeutic agent. We have determined the atomic structure of the Fab fragment of ibalizumab in complex with the first two N-terminal domains (D1-D2; residues 1-182) of human CD4 at 2.2 Å resolution. The BC-loop of D2 in CD4 near the D1-D2 junction constitutes the core epitope of ibalizumab, which does not overlap with the binding site of either gp120 or MHC class II. The crystal structure provides a clear explanation, at atomic resolution, of why ibalizumab does not directly block HIV-1 attachment or interfere with immune functions mediated by CD4, in agreement with previous mutagenesis studies (Burkly et al., 1992; Song et al., 2010). Ibalizumab binding induces only a small, local movement of the FG loop in CD4, but this change does not propagate to other regions and does not lead to any significant consequences for receptor function. We have also shown that both IgG and Fab forms of ibalizumab effectively block viral entry by certain HIV-1 isolates, ruling out the possibility that the antibody exerts its antiviral activity by crosslinking CD4. We have further shown that the ectodomain of CD4 does not undergo any dramatic, gp120-induced structural rearrangements, but that its structural rigidity is not required for ibalizumab to inhibit HIV-1 infection. Finally, structure modeling indicates that ibalizumab binding to CD4 should not prevent the envelope from binding to CCR5 on the target-cell surface, suggesting that ibalizumab acts on subsequent steps after gp120 engagement with coreceptor. Our findings could guide strategies for optimizing CD4-based immunogens to induce ibalizumab-like antibodies and thus facilitate development of novel therapeutic agents against HIV-1 infection. Ibalizumab could also be used as a unique reagent to probe CD4 receptor function for a better understanding of HIV-1 entry.
Structure-based CD4 immunogen design to develop therapeutic antibodies
Conventional HIV vaccine strategies focusing on inducing virus-specific humoral and cellular immune responses have failed to protect against HIV-1 infection in clinical trials to date (Pitisuttithum et al., 2006; Rerks-Ngarm et al., 2009), primarily due to the enormous challenge of HIV-1 evolution and global diversity. There has been increasing recognition that conserved host factors could be targeted for antiviral purposes. For instance, viruses using CCR5 (R5 viruses) are responsible for viral transmission, and individuals who express the CCR5Δ32 mutation are healthy but almost completely resistant to HIV-1 infection (Dean et al., 1996; Liu et al., 1996). Thus, CCR5 has been explored as an antiviral target to block HIV-1 transmission for both therapeutic and vaccine development. CCR5 antagonists, such as maraviroc, have proven safe and effective in HIV-1-infected patients, and a humanized anti-CCR5 monoclonal antibody PRO140 is currently in clinical trials (Gulick et al., 2008; Jacobson et al., 2008). Targeting the primary receptor, CD4, might raise serious safety concerns, however, because of its important role in regulating adaptive immune responses. The ongoing clinical trial of ibalizumab has demonstrated the potential utility of a non-immunosuppressive, anti-CD4 antibody in anti-HIV-1 strategies (Jacobson et al., 2009). How can the atomic structure of the complex of ibalizumab and CD4 help this endeavor? With precise knowledge of the ibalizumab epitope, an immediate application would be to modify human CD4 to expose only this unique epitope for inducing ibalizumab-like monoclonal antibodies in animals. The more the candidates like ibalizumab we have for humanization, perhaps with even better potency or pharmacokinetic properties, the more likely will this strategy eventually succeed. Approaches to selectively expose the ibalizumab epitope on CD4 could involve strategically placing N-linked glycans to cover the surface of 2 domain CD4 except at the D1-D2 junction or grafting the epitope onto other Ig domain scaffolds. The high-resolution crystal structure presented here would be indispensible for this type of protein-engineering problem. If such modified immunogens prove effective in eliciting ibalizumab-like antibodies in animals, a possible next step could be to test them directly in humans in conjunction with approaches to break tolerance as a vaccine strategy. However, it could still remain risky since ibalizumab contains IgG4 heavy chain by design to minimize Fc-mediated CD4+ T cell depletion (Reimann et al., 1997), while IgG1 and IgG2 may predominate antibody responses following vaccination and these two subclasses could bind Fc receptors and lead to CD4+ T cell depletion (Clynes et al., 2000; Raghavan and Bjorkman, 1996).
Molecular mechanism of HIV-1 inhibition by ibalizumab
Our current work has yet to lead to a definitive picture of how ibalizumab blocks HIV-1 infection, but it has ruled out several possibilities. First, previous studies have suggested that ibalizumab might exert its antiviral activity by crosslinking CD4 (Burkly et al., 1992), thereby impeding the postbinding movement of CD4 or disrupting certain specific configurations for multiple CD4 molecules - e.g., if three CD4 molecules are required to trigger a gp120 trimer to promote fusion. Our results show that the monovalent form of ibalizumab effectively inhibits most of the HIV-1 isolates tested, ruling out the possibility that crosslinking of CD4 plays a major role in ibalizumab inhibition. Ibalizumab does reduce the surface level of CD4 on the target cells, but the down-regulation is only transient and clearly not sufficient to account for the potent antiviral activity of the antibody (Y. Huang and D. D. Ho, unpublished observations). We further show that soluble, 4D CD4 maintains an extended conformation even in complex with gp120 core, consistent with our structure-based prediction that a jackknife rearrangement of D1-D2 relative to D3-D4, proposed previously (Ashish et al., 2008), is energetically unfavorable and highly unlikely. Thus, we conclude gp120 binding does not lead to any dramatic conformational change in the ectodomain of CD4. It is possible that the D2-D3 junction remains flexible and D1-D2 could bend relative to D3-D4 by a modest extent in the presence of gp120. Ibalizumab would probably not interfere with such a modest movement, even if such a change were required for viral entry. Moreover, ibalizumab does not induce any obvious structural changes in CD4 that could impede receptor function. The length of 4D CD4 is at least 115 Å, the D1-D2 and D3-D4 junctions are very rigid, and the D2-D3 junction has only limited flexibility (Wang et al., 1990; Wu et al., 1997). A large swing of CD4 to bring D1 at the membrane-distal end towards membrane along the hinge region between D4 and TM might be required to bring the bound gp120 to the vicinity of co-receptor. Attachment of ibalizumab to D1-D2 could affect the kinetics of the swing and thus block docking of gp120 onto coreceptor. If this were the case, we predicted that replacing D3-D4 with a flexible linker would allow D1-D2 to bend over freely and would thus confer resistance to ibalizumab. Our results in Figure 4 clearly argue against this possibility, suggesting that a postbinding swing of CD4 cannot be the ibalizumab target. Finally, a model based on the crystal structures of ibalizumab-2D CD4, 4 domain CD4, and gp120 core containing the V3 loop indicates that the coreceptor binding site on gp120 is far away from ibalizumab and the antibody should not directly block gp120 binding to the coreceptor on the cell surface.
Implications for HIV-1 entry
Why, then, is ibalizumab a potent inhibitor of HIV-1 infection? Our current picture of HIV-1 entry has emerged from extensive biochemical and structural studies (Harrison, 2008). It is generally believed that sequential binding of gp120 to viral receptor and coreceptor induces large conformational changes, which then trigger dissociation of gp120 and a cascade of refolding events in gp41. Structural transition of gp41 from its prefusion conformation to the “prehairpin intermediate”, followed by a six-helix bundle, postfusion conformation is the driving force to bring viral and target cell membranes together (Chan et al., 1997; Chan and Kim, 1998; Weissenhorn et al., 1997). Our data strongly suggest that ibalizumab does not act on the steps prior to coreceptor binding by gp120 but rather that it acts on a step before gp120 dissociation or on gp120 dissociation itself. Once gp120 falls off along with CD4 and ibalizumab, the latter could have no influence on membrane fusion. If departure of gp120 is the final trigger for gp41 refolding, ibalizumab could inhibit viral infection by preventing gp120 dissociation. Interestingly, mAb 5A8 does inhibit soluble CD4-induced gp120 dissociation from HIV-1 virions (Moore et al., 1992). It is also possible that gp120 may remain bound even to triggered gp41, as in the cases of HA1 of influenza hemagglutinin (HA) and GP1 of Ebola glycoprotein (GP), both of which are covalently associated with their transmembrane fusion subunit, probably throughout the membrane fusion process (Lee et al., 2008; Wilson et al., 1981). In this scenario, gp120 would have to coordinate with gp41 to allow a smooth transition from the prefusion state to the postfusion conformation via the prehairpin intermediate, in a very crowded space. It is conceivable that ibalizumab might obstruct certain movements of gp120 during the molecular gymnastics required for HIV-1 entry. Many puzzles about HIV-1 membrane fusion thus remain unsolved, and ibalizumab could present an opportunity for answering some of these questions.
Experimental Procedures
Expression constructs, cell lines and protein production
Expression constructs for his-tagged gp120 core derived from HIV-1 92UG037.8, soluble 2D (residues 1-182) and 4D (residues 1-363) human CD4 in pFastBac-1 vector (Invitrogen) were generated by standard PCR techniques. To introduce flexibility in the ectodomain of CD4, four variants with its D3-D4 (residues 179-365) substituted by a flexible linker, GGGGS for pCD4-L1, GGGGSGGGGSGGGGS for pCD4-L2, GGGGSGGGGSGGGGSGGGGSGGGGSGGGGS for pCD4-L3 and GGGGSGGGGSGGGGSGGGGSGGGGSGGGGSGGGGSGGGGSGGGGS for pCD4-L4, were created. Two additional CD4 variants were also constructed with either a 5-residue (residues 367-371; TPVQP) deletion for pCD4-Δ5 or the entire D3-D4, TM and cytoplamic tail (residues 179-433) replaced by a human CD8α hinge region and TM (residues 115-214 from CD8α) for pCD4-CD8TM. Synthetic genes were made for L1 (linker 1)-TM- CT (cytoplamic tail), L2-TM-CT, L3-TM-CT, L4-TM-CT, CD8-hinge-TM (GENEART AG, Regensburg, Germany). These segments were fused with D1-D2 of CD4 amplified by PCR and cloned into pcDNA 3.1(+) (Invitrogen). pCD4-Δ5 was produced by produced by overlapping PCR with primers harboring the 5-residue deletion. TZM.bl cell used for HIV-1 neutralization assays was described previously (Li et al., 2005). ACTOne Chemokine (C-C motif) Receptor 5 cell (Codex Biosolutions, Montgomery Village, MD) is a stable cell line derived from 293T cells expressing human CCR5. Ibalizumab was provided by TaiMed Biologics (Irvine, CA) to D. H.. F(ab’)2 fragment of ibalizumab was prepared using Pierce F(ab’)2, Preparation Kit (Thermo Scientific) according to manufacturer’s instruction. Briefly, 4 mg of ibalizumab IgG was cleaved with 125 μl immobilized pepsin (0.2-0.3 mg; ≥ 600 units) at 37°C for 6 hours, and then purified by protein A affinity chromatography. Anti-CD4 antibody conjugated with Alexa Fluor 488 was purchased from EXBIO Praha, Vestec, Czech Republic; PE mouse anti-human CD195 (CCR5) antibody from BD Biosciences, San Jose, California; and gp120 derived from the isolate Du156.12 from Immune Technology Corp., New York, NY. Gp120 core, soluble 2D and 4D human CD4 were expressed in insect cells and purified by a protocol described previously (Rits-Volloch et al., 2006).
Crystallization and structure determination
Fab fragment of ibalizumab was produced by papain (Sigma) digestion at 37°C for 3 hours with an enzyme to antibody ratio of 1:10,000 by weight, and then purified by protein A affinity and gel-filtration chromatography. 2D CD4 with a C-terminal His-tag was mixed with ibalizumab Fab at a molar ratio of 1:1.2 at room temperature for 1 hour and the complex was further purified by gel-filtration chromatography using a Superdex 200 column. The complex was crystallized by hanging-drop vapor diffusion with a mother liquor containing 3 M sodium malonate, pH 5.5. The crystals were flash-frozen in liquid nitrogen using the mother liquor as a cryoprotectant solution. X-ray diffraction data were collected at 100° K at beamline 24-ID, Advanced Photon Source (Argonne National Laboratory, IL). HKL2000 (HKL Research, Inc.) was used to integrate and scale diffraction data. The crystals diffract to 2.2 Å resolution and belong to space group P212121 (a=52.2, b=66.3, c=266.6 Å), with one Fab-CD4 complex per crystallographic asymmetric unit. Initial phase information was obtained by molecular replacement using MOLREP and Phaser with 2D CD4 and a library of 244 different Fab coordinates as search models (Aoki et al., 2009; McCoy et al., 2007; Vagin and Teplyakov, 1997). Complementarity determining regions (CDRs) of both heavy- and light-chains of ibalizumab were rebuilt iteratively in O (Jones and Kjeldgaard, 1997) and the final model was refined by Refmac (Winn et al., 2001) with an Rwork of 19.3% and an Rfree of 22.4%.
Surface Plasmon Resonance Binding Assays
All experiments were performed with a Biacore 3000 instrument (Biacore Inc) and analyzed following a protocol as described (Frey et al., 2008).
Inhibition assays
Inhibitory activity of ibalizumab was measured using a modified protocol of the luciferase-based HIV-1 neutralization assay in TZM.bl cells as described (Li et al., 2005; Montefiori et al., 2004). Briefly, 5-fold serial dilutions of ibalizumab IgG or Fab were performed in duplicate (96-well flat bottom plate) in 10% DMEM growth medium (100 μl/well). TZM.bl cells were added (1×104/well in 50 μl volume) and the plates were incubated for 1 hour at 37°C. HIV-1 Env pseudovirus was then added (100 μl/well) in 10% DMEM growth medium containing DEAE-Dextran (Sigma, St. Louis, MO) at a final concentration of 11 μg/ml. Assay controls included replicate wells of TZM.bl cells alone (cell control) and TZM.bl cells with virus (virus control). Following a 48 hour incubation at 37°C, 150 μl of assay medium was removed from each well and 100 μl of Bright-Glo luciferase reagent (Promega, Madison, WI) were added and luminescence measured using a Victor 3 luminometer (Perkin Elmer). The 50% inhibitory concentration (IC50) was calculated based on the antibody dilution that caused a 50% reduction in relative luminescence units (RLU) compared to the virus control wells after subtraction of cell control RLUs. For experiments utilizing ACTOne CCR5 cells transfected with different CD4 variants as targets, cells were added at 2×104/well. Assay stocks of molecularly cloned Env-pseudotyped viruses were prepared by co-transfecting 293T/17 cells with an Env-expressing plasmid and an Env-deficient backbone plasmid (SG3ΔEnv), as described (Li et al., 2005), Specifically, recombinant HIV-1 SC422661.8 Env pseudovirus expressing the firefly luciferase reporter gene was generated by co-transfecting 293T/17 cells with an Env-expressing plasmid, and the pNL4-3.Luc ΔEnv backbone plasmid (Raymond et al., 2008).
Small angle x-ray scattering
To ensure conformational homogeneity of protein samples, 2D CD4, 4D CD4, 92UG037.8 gp120 core, 2D CD4-gp120 core complex and 4D CD4-gp120 core complex were further purified by gel-filtration chromatography using a Superdex 200 column in a buffer containing 25 mM Tris pH 7.5, 150 mM NaCl. Small- and wide-angle x-ray scattering (SAXS/WAXS) data were collected using a monochromatic x-ray beam (14.18 keV) by a SAXS detector (Mar-165 CCD) or a WAXS detector (Photonic Science) at a distance of 3.3 and 0.47 m, respectively, at beamline X9, National Synchrotron Light Source (NSLS), Brookhaven National Laboratory. Sample-to-detector distances were calibrated by the diffraction pattern of a standard silver behenate sample using the program Fit2D (Hammersley et al., 1996). Scattering data were circularly averaged as a function of momentum transfer vector, q (q=4πsinθ/λ; λ is the wavelength, and 2θ is the scattering angle) by Fit2D. For each protein sample, scattering data were collected at three different concentrations (1, 3 and 6 mg/ml) in a flow cell. Data reduction includes normalization of the one-dimensional scattered curve to the intensity of the transmitted beam and subtraction of the background scattering of the buffer (25 mM Tris pH 7.5, 150 mM NaCl). A complete scattering plot was obtained by merging SAXS and WAXS data at the overlapping region of q from 0.1 to 0.2 Å−1.
Guinier analysis involves linear fitting the Guinier plot of ln[I(q)] vs q2 within a range where q·Rg <1.3 to give an estimation of the radius of gyration (Rg) and the forward scattering intensity I(0) of the scattering sample (Guinier and Fournet, 1955). The pair-distance distribution function P(r), which is a measure of the frequency of interatomic vector lengths within a molecule, yields information on the shape of the scattering particle. Guinier analysis and P(r) analysis were performed using programs Primus and GNOM, respectively (Konarev et al., 2003; Svergun, 1991). The maximum dimension (Dmax) values, which reflect the largest dimension of the protein or protein complex, were first calculated from the entire scattering curve I(q), in the q range from zero to qmax, by a direct Fourier transform as described (Hong and Hao, 2009), and also analyzed by an indirect Fourier transform using GNOM.
Flow cytometry
Transfected or untransfected 293T or ACTOne CCR5 cells were detached from plates using 0.05% trypsin and 1mM EDTA in PBS, followed by immediate addition of complete medium containing 10% FBS, and then washed with ice-cold PBS containing 1% BSA. 106 cells/ml were stained for 30 min at room temperature with either anti-CD4 antibody conjugated with Alexa Fluor 488 (EXBIO Praha, Vestec, Czech Republic) or PE mouse anti-human CD195 (CCR5) antibody (BD Biosciences, San Jose, California) diluted to 200 μg/ml in PBS containing 1% BSA. Labeled cells were then washed with cold PBS with 1% BSA and either fixed in 2.5% formaldehyde or analyzed immediately using a MoFlo Legacy Cell Sorter and Summit Software v4.3 (Beckman Counter, Brea, California).
Accession code
Coordinates and structure factors have been deposited in Protein Data Bank with accession code 3O2D.
Supplementary Material
Acknowledgments
We thank Stephen Harrison, Andrea Carfi, Gary Frey, Hanqin Peng, Uhn-Soo Cho, Yaoxing Huang for generous advice and assistance, the staff of the Northeastern Collaborative Access Team at Advanced Photon Source, Argonne National Laboratory for assistance with x-ray data collection and the staff at the National Synchrotron Light Source, Brookhaven National Laboratory for assistance with SAXS data collection. We acknowledge support from NIH grants GM083680 (to B.C.) and AI084794 (to B.C. and Dan Barouch), and the Bill and Melinda Gates Foundation Comprehensive Antibody Vaccine Immune Monitoring Consortium Grant 38619 (to M.S.). X.H. was partially supported by the Genomics Science Program, U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki ST, Settembre EC, Trask SD, Greenberg HB, Harrison SC, Dormitzer PR. Structure of rotavirus outer-layer protein VP7 bound with a neutralizing Fab. Science. 2009;324:1444–1447. doi: 10.1126/science.1170481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashish, Juncadella IJ, Garg R, Boone CD, Anguita J, Krueger JK. Conformational rearrangement within the soluble domains of the CD4 receptor is ligand-specific. J Biol Chem. 2008;283:2761–2772. doi: 10.1074/jbc.M708325200. [DOI] [PubMed] [Google Scholar]
- Boon L, Holland B, Gordon W, Liu P, Shiau F, Shanahan W, Reimann KA, Fung M. Development of anti-CD4 MAb hu5A8 for treatment of HIV-1 infection: preclinical assessment in non-human primates. Toxicology. 2002;172:191–203. doi: 10.1016/s0300-483x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- Burkly LC, Olson D, Shapiro R, Winkler G, Rosa JJ, Thomas DW, Williams C, Chisholm P. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J Immunol. 1992;149:1779–1787. [PubMed] [Google Scholar]
- Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- Chen B, Vogan EM, Gong H, Skehel JJ, Wiley DC, Harrison SC. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature. 2005;433:834–841. doi: 10.1038/nature03327. [DOI] [PubMed] [Google Scholar]
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- Dimitrov A. Ibalizumab, a CD4-specific mAb to inhibit HIV-1 infection. Curr Opin Investig Drugs. 2007;8:653–661. [PubMed] [Google Scholar]
- Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987;330:256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci U S A. 2008;105:3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinier AG, Fournet G, editors. Small Angle Scattering X-rays. John Wiley & Sons; New York: 1955. [Google Scholar]
- Gulick RM, Lalezari J, Goodrich J, Clumeck N, DeJesus E, Horban A, Nadler J, Clotet B, Karlsson A, Wohlfeiler M, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammersley AP, Svensson SO, Hanfland M, Fitch AN, Häusermann D. Two-Dimensional Detector Software: From Real Detector to Idealised Image or Two-Theta Scan. High Pressure Research. 1996;14:235–248. [Google Scholar]
- Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Hao Q. High resolution pair-distance distribution function P(r) of protein solutions. Applied Physics Letters. 2009;94:083903–083903. [Google Scholar]
- Huang CC, Lam SN, Acharya P, Tang M, Xiang SH, Hussan SS, Stanfield RL, Robinson J, Sodroski J, Wilson IA, et al. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science. 2007;317:1930–1934. doi: 10.1126/science.1145373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JM, Kuritzkes DR, Godofsky E, DeJesus E, Larson JA, Weinheimer SP, Lewis ST. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 2009;53:450–457. doi: 10.1128/AAC.00942-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JM, Saag MS, Thompson MA, Fischl MA, Liporace R, Reichman RC, Redfield RR, Fichtenbaum CJ, Zingman BS, Patel MC, et al. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J Infect Dis. 2008;198:1345–1352. doi: 10.1086/592169. [DOI] [PubMed] [Google Scholar]
- Jones TA, Kjeldgaard M. Electron-density map interpretation. Methods in Enzymology. 1997;277:173–208. doi: 10.1016/s0076-6879(97)77012-5. [DOI] [PubMed] [Google Scholar]
- Kolchinsky P, Kiprilov E, Bartley P, Rubinstein R, Sodroski J. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J Virol. 2001;75:3435–3443. doi: 10.1128/JVI.75.7.3435-3443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. Journal of Applied Crystallography. 2003;36:1277–1282. [Google Scholar]
- Kuritzkes DR, Jacobson J, Powderly WG, Godofsky E, DeJesus E, Haas F, Reimann KA, Larson JL, Yarbough PO, Curt V, Shanahan WR., Jr. Antiretroviral activity of the anti-CD4 monoclonal antibody TNX-355 in patients infected with HIV type 1. J Infect Dis. 2004;189:286–291. doi: 10.1086/380802. [DOI] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 2008;454:177–182. doi: 10.1038/nature07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Perreault J, Poulin L. Postbinding events mediated by human immunodeficiency virus type 1 are sensitive to modifications in the D4-transmembrane linker region of CD4. J Virol. 1996;70:8019–8028. doi: 10.1128/jvi.70.11.8019-8028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC, Metch B, McElrath MJ, Self S, Weinhold KJ, Corey L. Demographic factors that influence the neutralizing antibody response in recipients of recombinant HIV-1 gp120 vaccines. J Infect Dis. 2004;190:1962–1969. doi: 10.1086/425518. [DOI] [PubMed] [Google Scholar]
- Moore JP, Sattentau QJ, Klasse PJ, Burkly LC. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J Virol. 1992;66:4784–4793. doi: 10.1128/jvi.66.8.4784-4793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- Poulin L, Evans LA, Tang SB, Barboza A, Legg H, Littman DR, Levy JA. Several CD4 domains can play a role in human immunodeficiency virus infection in cells. J Virol. 1991;65:4893–4901. doi: 10.1128/jvi.65.9.4893-4901.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan M, Bjorkman PJ. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 1996;12:181–220. doi: 10.1146/annurev.cellbio.12.1.181. [DOI] [PubMed] [Google Scholar]
- Raymond S, Delobel P, Mavigner M, Cazabat M, Souyris C, Sandres-Saune K, Cuzin L, Marchou B, Massip P, Izopet J. Correlation between genotypic predictions based on V3 sequences and phenotypic determination of HIV-1 tropism. AIDS. 2008;22:F11–16. doi: 10.1097/QAD.0b013e32830ebcd4. [DOI] [PubMed] [Google Scholar]
- Reimann KA, Khunkhun R, Lin W, Gordon W, Fung M. A humanized, nondepleting anti-CD4 antibody that blocks virus entry inhibits virus replication in rhesus monkeys chronically infected with simian immunodeficiency virus. AIDS Res Hum Retroviruses. 2002;18:747–755. doi: 10.1089/08892220260139486. [DOI] [PubMed] [Google Scholar]
- Reimann KA, Lin W, Bixler S, Browning B, Ehrenfels BN, Lucci J, Miatkowski K, Olson D, Parish TH, Rosa MD, et al. A humanized form of a CD4-specific monoclonal antibody exhibits decreased antigenicity and prolonged plasma half-life in rhesus monkeys while retaining its unique biological and antiviral properties. AIDS Res Hum Retroviruses. 1997;13:933–943. doi: 10.1089/aid.1997.13.933. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009 doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Rits-Volloch S, Frey G, Harrison SC, Chen B. Restraining the conformation of HIV-1 gp120 by removing a flexible loop. Embo J. 2006;25:5026–5035. doi: 10.1038/sj.emboj.7601358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto CD, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- Song R, Franco D, Kao CY, Yu F, Huang Y, Ho DD. Epitope Mapping of Ibalizumab, a Humanized Anti-CD4 Monoclonal Antibody with Anti-HIV-1 Activity in Infected Patients. J Virol. 2010;84:6935–6942. doi: 10.1128/JVI.00453-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svergun DI. Mathematical methods in small-angle scattering data analysis. Journal of Applied Crystallography. 1991;24:485–492. [Google Scholar]
- Vagin A, Teplyakov A. Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr. 1997;66:22–25. doi: 10.1107/S0907444909042589. [DOI] [PubMed] [Google Scholar]
- Wang J, Yan Y, Garrett TPJ, Liu J, Rodgers DW, Garlick RL, Tarr GE, Husain Y, Reinherz EL, Harrison SC. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990;348:411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Wang JH, Meijers R, Xiong Y, Liu JH, Sakihama T, Zhang R, Joachimiak A, Reinherz EL. Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule. Proc Natl Acad Sci U S A. 2001;98:10799–10804. doi: 10.1073/pnas.191124098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr D Biol Crystallogr. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- Wu H, Kwong PD, Hendrickson WA. Dimeric association and segmental variability in the structure of human CD4. Nature. 1997;387:527–530. doi: 10.1038/387527a0. [DOI] [PubMed] [Google Scholar]
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: Fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- Xu H, Littman DR. A kinase-independent function of Lck in potentiating antigen-specific T cell activation. Cell. 1993;74:633–643. doi: 10.1016/0092-8674(93)90511-n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


