Summary
A widely discussed hypothesis in neuroscience is that transiently active ensembles of neurons, known as ‘cell assemblies’, underlie numerous operations of the brain, from encoding memories to reasoning. However, the mechanisms responsible for the formation and disbanding of cell assemblies and temporal evolution of cell assembly sequences are not well understood. I introduce and review three interconnected topics, which could facilitate progress in defining cell assemblies, identifying their neuronal organization and revealing causal relationships between assembly organization and behavior. First, I hypothesize that cell assemblies are best understood in light of their output product, as detected by ‘reader-actuator’ mechanisms. Second, I suggest that the hierarchical organization of cell assemblies may be regarded as a neural syntax. Third, constituents of the neural syntax are linked together by dynamically changing constellations of synaptic weights (‘synapsembles’). Existing support for this tripartite framework is reviewed and strategies for experimental testing of its predictions are discussed.
Keywords: inhibition, integrators, synaptic plasticity, oscillations, gamma, theta, cognition, planning, memory, recall, place cells
"If a tree falls in a forest and no one is around to hear it, does it make a sound?"
(attributed to George Berkeley)1
Introduction
Donald Hebb was among the first thinkers who explicitly stated that the brain’s ability to generate coherent thoughts derives from the spatiotemporal orchestration of neuronal activity (Hebb, 1949). Hebb hypothesized that a discrete, strongly interconnected group of active neurons, the ‘cell assembly’, represents a distinct cognitive entity. Because of their high interconnectivity, the stimulation of a sufficient number of assembly members can activate the entire assembly (Legendy, 1967; Palm, 1982, 1987). The chaining of such assemblies by some internal mechanisms (Hebb’s ‘phase sequences’), in turn, would provide the basis by which complex cognitive processes, such as memory recall, thinking, planning and decision making could flow independent of direct control from the environment or the body (Churchland and Sejnowski, 1992; Harris, 2005; John 1967; Kelso 1997; Laurent 1999; Palm, 1982; Pouget et al., 2000; Pulvermüller 2003; Sakurai 1999; Singer 1990; Varela 2005; Varela et al., 2001; Wickelgren, 1999; Yuste et al., 2005). With Hebb’s cell assembly hypothesis, it appeared that cognitive neuroscience had established a comprehensive research program to link psychological and physiological processes. The expectation was that the program would demonstrate that (1) the spiking activity of a strongly connected collection of neurons is the basic unit for neuronal coding and (2) activation of a (sufficiently large) part of the assembly can reconstitute activity in the entire cell assembly, similar to our subjective ability to reconstruct wholes from fragments. However, experimental identification of the hypothesized cell assemblies has proven notoriously difficult (Gernstein et al., 1989; Grossberg 1969; Ikegaya et al., 2004; Lansner 2009; Milner, 1957; 1996; Palm, 1982, 1990; Pouget et al., 2000; Pulvermüller 2003; Singer 1999; Varela 2005; Wallace and Kern, 2010; Wennekers et al., 2003). For the past several decades, the limitations were primarily technical, namely the lack of appropriate methods to record simultaneously from large enough numbers of neurons in behaving animals (Abeles 1991; Strangman et al., 1996; Edelman, 1987; Hebb, 1949; Palm, 1982). However, the recent rapid progress in large-scale recording of individual neurons in multiple brain regions (Buzsáki, 2004; Buzsáki et al., 1992; Eichenbaum and Davis, 1998; Nicolelis 1999; Wilson and McNaughton, 1993) and the initial attempts to track down and experimentally define putative cell assemblies (Harris et al., 2003; Harris, 2005; Truccolo et al., 2010) led to the recognition of another level of difficulties of a more conceptual nature.
How large is a cell assembly, what is its duration (‘life time’) and what, exactly, does it represent in the cognitive or output domain? Does an assembly represent a feature, a figure or background, an object or concept, a thought process, a plan for immediate action, or even more complex processes? 2 Unfortunately, the very idea of identifying the neuronal correlates of such psychological constructs on the presumption that they must have clear boundaries, in correspondence with the neuronal substrates of their representation, is questionable. According to the ‘representational framework’ (Engel et al., 2001; Hebb, 1949; James 1890; Milner 1996; von Malsburg 1994), the way to identify cell assemblies is to present various stimuli to the brain (e.g., an object or aspects of an object) and examine the spatio-temporal distribution of the evoked neuronal responses (Hubel and Wiesel, 1962; Rieke et al., 1997).3 An implicit goal of such a strategy is to eventually explain how elementary attributes that are believed to comprise an object (e.g., color, shape, odor, sound, motion, etc) are bound together at the neuronal level so that the object is identified as an entity with segregated boundaries from its background (von der Malsburg, 1994). However, a paradox inherent in this strategy is that the ‘essential attributes’ necessary for the identification of an object, thing or idea are not universal properties of the external world but are created by the observing brain (Llinás, 2001; Buzsáki, 2006). Therefore, a fundamental question is how the cell assembly concept helps us to track down brain mechanisms of classification and categorization, exemplified by the often used antonym terms such as integration vs. segregation, differentiation vs. generalization, pattern separation vs. pattern completion or parsing vs. grouping (Edelman, 1987; Tononi et al., 1994).
I suggest an alternative strategy to the representational approach of neuronal assembly identification. The main hypothesis is that the cell assembly concept is most useful from the point of view of downstream ‘observer-reader-classifier-integrator’ mechanisms (referred to as readers hereafter) because the biological relevance of a particular constellation of active neurons (i.e., a presumed cell assembly or assembly sequence) can only be judged from the perspective of explicit outputs. An elementary classifier mechanism is the action potential of a reader neuron, which reflects the integration of the activity of an upstream assembly. The action potential is caused by the assembly activity. At the most complex level, such ‘caused’ effects may be motor outputs, decisions, plans, recalls and thoughts.
Sequences of unique assemblies (Figure 1A) evolve in both neuronal space and in time (Rabinovich et al., 2008a,b). My second hypothesis is that, analogous to words and sentences in language, neuronal assemblies are organized by syntactical rules that define their first order and higher order relationships. Chunking information into smaller packages by syntactical rules, known to both sender and receiver, makes communication more straightforward than interpreting long uninterrupted messages (Figure 1B; Wickelgren, 1999). Furthermore, without syntactical rules that can silence assembly activity, an input would generate a perpetual reverberation of excitatory activity (Figure 1A; Lorente de Nó, 1938), potentially involving the entire brain.
Figure 1.
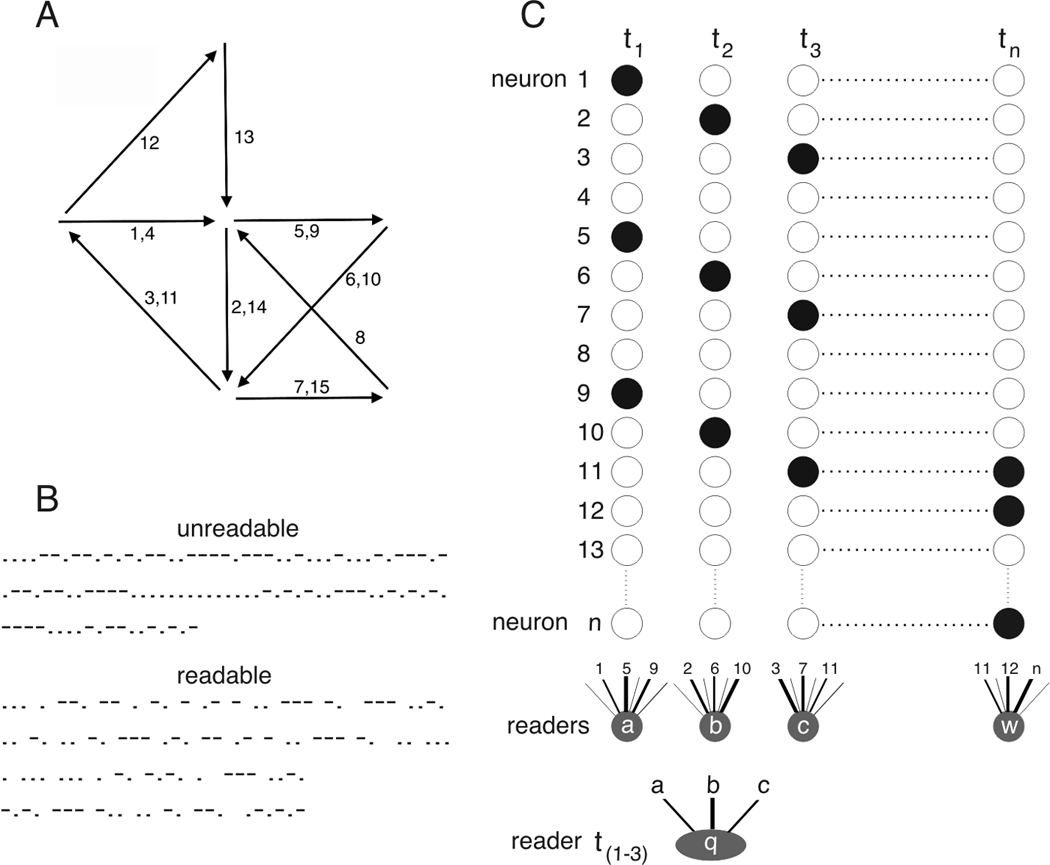
Cell assembly and assembly sequences. A. Hebb’s reverberating cell assembly sequences (‘assembly phases’; after Fig.10 of Hebb, 1949). Arrows represent transitions between individual assemblies. The direction of activity flow across assemblies (edges) is determined by the stronger synaptic strengths among assembly members relative to other connections (not shown). The same assembly can participate in a sequence more than once (e.g. pathway 1, 4 indicates recurring transitions). No mechanism is postulated to explain why activity does not spread to all parts of the network and reverberate forever. B. Top, long sequence of two characters (e.g., dot and dash). Its embedded information is virtually impossible to recover. Bottom, same exact sequence as above after adding syntactic segmentation (space= stop-start punctuation) between the short strings of characters. The Morse code message reads: ‘segmentation of information is essence of coding’. By analogy, segmentation or ‘chunking’ of neuronal assemblies can be brought about by salient external stimulus sequences, brain-initiated, modality-specific synchronizing-blanking mechanisms (such as saccadic eye movement, sniffing, whisking, active touch, licking, contraction of middle ear muscles, etc.), internally generated oscillations or other syntactical mechanisms. C. Reader-defined cell assemblies. Neurons that fire within the time integrating window of a reader mechanism (e.g., the ability of an reader neuron to integrate its inputs within the time frame of its membrane time constant) define an assembly (irrespective whether assembly members are connected synaptically or not). Readers a, b, c and w may receive inputs from many neurons (1 to n) by way of synapses differing in strength but respond only to a combination of spiking neurons to which they are most strongly connected (e.g., reader a responds preferentially to co-firing of neurons 1, 5 and 9 at t1, even though it may be synaptically innervated by neurons 2, 6 and 10 as well; at t2, neuron b fires in response to the discharge of neurons 2, 6 and 10). Synaptic strengths between neurons vary as a function of the spiking history of both postsynaptic and presynaptic neuron (short-term plasticity). The response of the reader neuron, therefore, depends on both the identity of the spiking upstream neurons and the constellation of current synaptic weights (‘synapsembles’). Reader mechanism q has a longer time integrator and, therefore, can link together assemblies to neural ‘words’, reading out a new quality not present in the individual representations of a, b and c.
If indeed cell assemblies and assembly sequences are parsed and separated in time, there must be mechanisms that bridge them across time even in the absence of spiking activity (Buenomano and Maass, 2009). Therefore, the third hypothesis I advance is that the constituents of the neural syntax are linked together by dynamically changing constellations of synaptic weights (von der Malsburg, 1994), which I refer to as ‘synapsembles’.
Reader-centric definition of cell assembly
I suggest that an objective identification of the cell assembly requires two key conditions: a reader-classifier and a temporal frame. Neurons come together in transient time frames to produce a composite downstream effect, which cannot be achieved by single neurons alone. The most important modus operandi in this process is synchrony of events (Abeles 1991; Engel et al., 2001; Fries et al., 2007; Hansel and Sompolinsky, 1992; Singer 1999). In its broad definition, synchrony refers to the concurrence of events in time. However, this definition of synchrony is meaningful only from the perspective of a reader mechanism with the ability to integrate upstream events over time (Buzsáki 2006). Thus, whether events are synchronous or not can be determined only by their impact on a reader-actuator. Similarly, I suggest that the cell assembly can only be defined from the perspective of a reader mechanism.
Even the simplest neural networks can give rise to multiple combinations of firing patterns (Abeles, 1991). Whether one or several of the possible combinations of firing patterns are meaningful can be determined only by a reader-classifier mechanism. If multiple combinations elicit the same output in one reader, they are interpreted as identical from the point of view of the reader. Another reader mechanism may respond to another set of combination of firing patterns. A simple and ubiquitous example of a reader mechanism in the brain is the integration of presynaptic spikes by neurons, constrained by their membrane time constant τ.4 A group of upstream neurons, whose spike discharges occur within the window of the membrane time constant of the reader-integrator neuron, and trigger an action potential, can be regarded as a meaningful neuronal assembly from the viewpoint of the reader neuron. Action potentials of other upstream neurons, which fire outside this critical time window (i.e., non-synchronously), can only be part of another assembly. The reader-integrator mechanism can therefore objectively determine whether neurons are part of the same assembly and serve the same goal (i.e., the discharge of the reader neuron) or belong to different assemblies (Figure 1C). The length of τ is affected by a number of factors, including the background activity in the network and availability of subcortical neuromodulators (cf., Destexhe et al., 2003). In the intact waking cerebral cortex, τ of principal cells is approximately 10–30 msec (Koch et al., 1996).
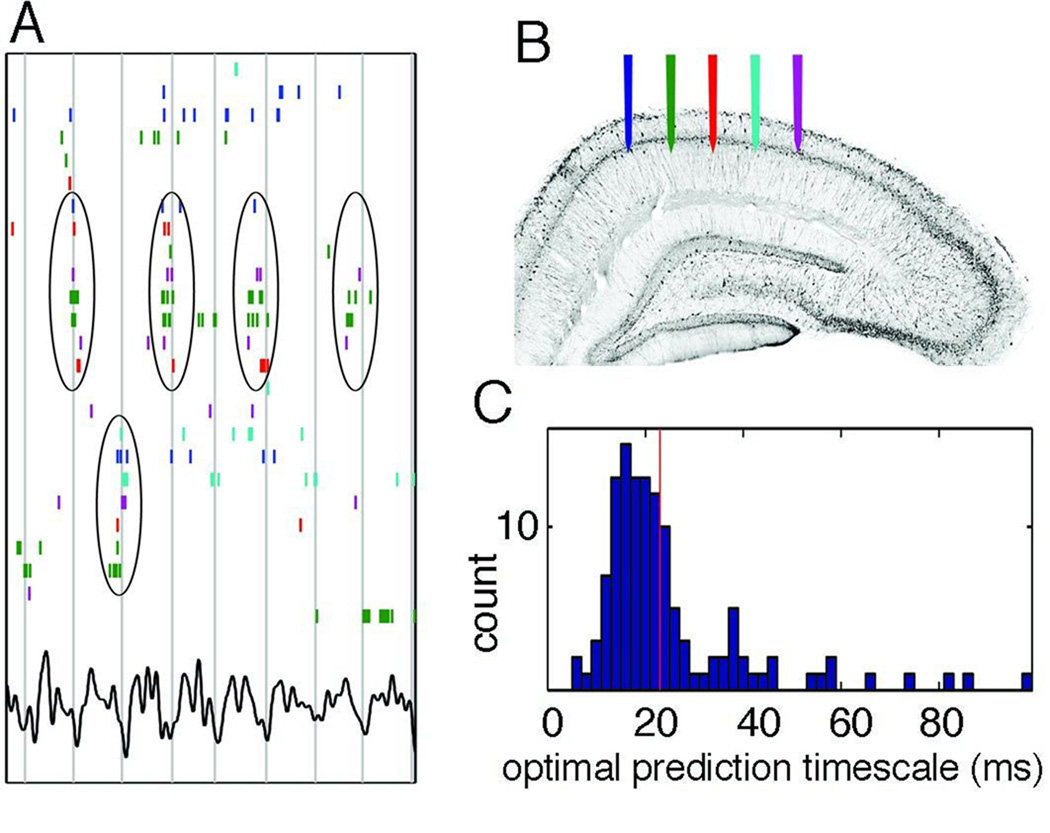
Using the analogy of a musical assembly, in which the tempo of one member can be reasonably predicted from the activity of the other members of the orchestra, the spike occurrence of a neuron taking part in a cell assembly should be reliably predicted from the activity of its peer neurons. To illustrate such assembly cooperation, I draw an example from the hippocampus (for neocortex, see Truccolo et al., 2010).5 Spike timing of hippocampal pyramidal cells can be related to the position of the animal (O’Keefe and Nadel, 1978), to the phase of the local field potential (LFP) theta cycle (O’Keefe and Recce, 1993) and the spiking of other neurons. Each of these variables is correlated with the spiking activity of single neurons but with different temporal resolutions. Since spiking activity refers to events that occur in time, the best prediction of spike timing from the other variables should have an optimum time window. By varying the analysis window experimentally, the best prediction of the spike timing of single hippocampal neurons from the activity of other neurons was found when spiking of peer neurons was assessed in 10 to 30 msec epochs (Figure 2; Jensen and Lisman, 1996; 2000; Harris et al., 2003; Kelemen and Fenton, 2010; Lansner, 2009). When two cells with distinct place fields (O’Keefe and Nadel, 1978) were examined their activity was associated with the spiking of distinct peers and the formed assemblies could alternate in a fast sequence (Figure 2A). The participation of individual assembly members from trial-to-trial can vary much more than the whole assembly (Pouget et al., 2000). Given the similarity between the temporal window of the assembly life time and the time constant of pyramidal cells, the postulated physiological goal of the cell assembly is to mobilize enough peer neurons so that their collective spiking activity can discharge a target (reader) neuron(s). Due to anatomical constraints, various combinations of upstream cells, active in a short time window, converge onto different reader neurons in the target layer (Figure 1C). Whether different constellations of spiking upstream neurons are regarded parts of the same assembly, or rather as different assemblies is not inherent but requires the specification of the downstream classifier-reader neuron(s). Because of the all-or-none spike response of the reader neuron, the reader neuron-defined cell assembly denotes a discrete, collective unitary event, which I refer to as the fundamental cell assembly or assembly τ.
Figure 2.
Cell assembly: the fundamental unit of neural syntax. (A) Raster plot of a subset of hippocampal pyramidal cells that were active during a 1-s period of spatial exploration on an open field out of a larger set of simultaneously recorded neurons, ordered by stochastic search over all possible orderings to highlight the temporal relationship between anatomically distributed neurons. Color-coded ticks (spikes) refer to recording locations shown in (B). Vertical lines indicate troughs of theta waves (bottom trace). ‘Cell assembly’ organization is visible, with repeatedly synchronous firing of some subpopulations (circled). Note that assemblies can alternate (top and bottom sets) rapidly across theta cycles. (C) Spike timing is predictable from peer activity. Distribution of time scales at which peer activity optimally improved spike time prediction of a given cell, shown for all cells. The median optimal timescale is 23 ms (red line). Modified after Harris et al. (2003).
The physiological importance of the cell assembly’s typical ephemeral lifetime is also supported by the fact that this time window temporally overlaps with the duration of AMPA receptor-mediated EPSPs and GABAA receptor-mediated IPSPs (Johnston and Wu, 1995). Furthermore, the temporal interaction between these opposing postsynaptic effects largely determines the period of gamma frequency oscillations observable extracellularly as a local field potential (LFP; Atallah and Scanziani, 2009; Bartos et al., 2007; Bragin et al., 1995; Buzsáki et al., 1983; Csicsvari et al., 2003; Leung, 2004; Mann et al., 2005; Whittington et al., 2000). Finally, this time scale also corresponds to the temporal window of spike timing-dependent plasticity (Magee and Johnston, 1997; Markram et al., 1997; cf., Bi and Poo, 2001). Given the temporal similarity of these basic physiological effects and their functional interactions, the integration time window of τ is therefore a critical reader mechanism that can define the content of gamma wave packet as the fundamental cell assembly. (Reader mechanisms with wider time integration windows can combine several assemblies; see below).
The reader-centric definition of the cell assembly differs from representation-based descriptions (Abeles 1991; Braitenberg and Schuz, 1991; Gernstein et al., 1989; Hebb, 1949; Hopfield and Tank, 1986; Palm 1982; Wickelgren, 1999) in some key aspects. Hebb’s cell assembly is essentially a graph of synaptically interconnected excitatory neurons (Abeles, 1991; Hopfield and Tank, 1986; Palm, 1982; 1987; Wennekers et al., 2003). However, unless the active neurons produce an interpretable output, connectedness is not sufficient to define an assembly. For the reader-centric definition of the assembly, direct excitatory connections among assembly members are optional but not obligatory because what matters is that neurons of an upstream assembly fire within the integrating time window of the reader mechanism (Fig. 1C). For example, in a prominent model of assembly sequences (‘synfire chain’) what matters is that at least one neuron in the target layer responds to the inputs from the upstream layer, irrespective of whether neurons in the upstream layer are strongly connected or not (Abeles, 1991). Naturally, if the transiently formed assembly members are interconnected anatomically, their co-activation can strengthen their membership and facilitate their future joint recurrence.6 Therefore, while the reader-centric definition of a cell assembly incorporates key features of Hebb’s definition it also provides a functional meaning.7
I use the term ‘reader’ as a metaphor to refer to a classifier-actuator mechanism. The reader is both an observer-integrator and a decision maker in the sense that it generates a tangible, measurable, and interpretable output. In the simplest case, the output is binary, such as an action potential of a neuron. The reader is not necessarily an independent, isolated unit but it can be part of the assembly itself, much like members of an orchestra, where each member is a reader of others’ actions. Separation of the reader mechanism from the assembly concept is needed only for a disciplined definition of neuronal alliances serving well-defined goals.
Neural syntax: rules that integrate and parse fundamental assemblies
In general, syntax (grammar) is a set of principles that govern the transformation and temporal progression of discrete elements (e.g., letters or musical notes) into ordered and hierarchical relations (e.g., words, phrases, sentences or chords, chord progression and keys) that allow for a congruous interpretation of the meaning of language or music by the brain (Pulvermüller, 2010). In addition to language and music, grouping or chunking the fundamentals by syntax allows for the generation of a virtually infinite number of combinations from a finite number of lexical elements using a minimal number of rules in sign, body, artificial and computer languages and mathematical logic (Port and Van Gelder, 1995; Wickelgren, 1999). Syntax is exploited in almost all systems where information is coded, transmitted and decoded (Figure 1B). By analogy, I suggest that in the brain distinct time-integrating (reader) mechanisms define the syntax of cell assembly organization and form assembly sequences of various lengths, compiled from strings of the fundamentals (i.e., from τ assemblies).8 As in language, the meaning of various strings of assemblies (or neuronal ‘trajectories; see below) depends on how the fundamentals are ordered and parsed (Pulvermüller 2003). I suggest that neural syntax facilitates the formation of ordered hierarchies of trajectories from the fundamental cell assemblies (Figure 1C).
Using assembly τ as opposed to a single neuron as the fundamental unit of syntax has several advantages. Neuronal trajectories involving only a single or too few neurons at each step would be vulnerable, due to synaptic or spike transmission failures and neuronal damage. Assembly partnership tolerates spike rate variation of individual cells effectively since it is the intensity of assembly activity that matters for the reader. Furthermore, minor differences in synaptic weights between the leading neuron and followers would divert the trajectory in multiple directions in the presence of noise. In contrast, interacting assembly members can compute probabilities, rather than deterministic information, amplify inputs and robustly tolerate noise even if the individual members respond probabilistically (Fiete et al., 2010; Geisler et al., 2007).
Neural words and sentences
The second hypothesis of this review is that temporal sequencing of discrete assemblies by neural syntax can generate neural words and sentences. Although strings of assemblies can be regarded simply as a larger assembly, and indeed assemblies of different length and size refer to many things in neuroscience, I chose the term ‘neural word’ to emphasize that words consist of multiples of the fundamental assemblies. Gamma oscillation episodes, containing a string of assemblies, are typically short lasting (Engel et al., 2001; Fries 2005; Gray and Singer, 1989; Whittington et al., 2000; Sirota et al., 2008) and often grouped by slower oscillations (Bragin et al., 1995; Canolty et al., 2006; Chrobak and Buzsáki, 1998; Hasenstaub et al., 2005; Sirota et al., 2008; Steriade 2006). Such a relatively short sequence of cell assemblies may be regarded as a neural word (Jensen and Lisman, 1996, 2000; Lee and Wilson, 2002; Lisman 1999; Skaggs et al., 1996).
Linking strings of fundamental assemblies requires readers with longer time integration abilities. In addition to the membrane time constant of single neurons, multiple other time integrators are present in the brain. NMDA receptors operate at the time scale of tens to hundreds of milliseconds (Monyer et al., 1992). Time-integration of cell assemblies at the subsecond to seconds time scale can be performed by metabotropic glutamate receptors (Nakanishi, 1994; Conn and Pinn, 1999), GABAB receptors (Deisz and Prince, 1989) and slow afterhyperpolarization-associated conductances (Lancaster and Adams, 1986). Another time integration mechanism at this time scale, and at the level of a single neuron rather than a synapse, is the spiking history-dependence of spike threshold. After a burst or train of spikes but even after a single spike, the spike threshold increases measurably for tens to hundreds of milliseconds, independent of the synaptic inputs (Henze and Buzsáki, 2001; Mickus et al., 1999). Reader mechanisms of spiking activity at longer time scales may be exemplified e.g., by the autonomic nervous system and the 0.1 Hz periodicity of the brain’s ‘default networks’ (Raichle et al., 2001).
Perhaps the most versatile class of reader-integrator mechanisms of neuronal assemblies is oscillations. Neuronal oscillators belong to the family of relaxation oscillators, with separable input (charging or receiving) and output (discharging, transmitting or duty cycle) phases (Buzsáki, 2006; Pikovsky et al., 2001). This asymmetry is due mainly to the within-cycle offset of inhibition and excitation (Buzsáki et al., 1983; Csicsvari et al., 1999). The charging or accrual phase of the oscillator is a typical time integrator (‘reader’) mechanism of upstream activity. Oscillators are also natural parsing and chunking mechanisms of neuronal activity because they have well-defined onsets and offsets with characteristic maximum and minimum spiking activity of the information-transmitting principal cells (Masquelier et al., 2009). This stop-start parsing function of neuronal oscillators can determine the length of an information unit (‘neural word’ or assembly sequence), and multiple cycles can combine word sequences into ‘neural sentences’. Since oscillator readers are a collective product of neuronal cooperation, their occurrence is reflected in the LFP. Therefore, along with other intermittent population events, such as K-complexes, ponto-geniculo-occipital (PGO) spikes and hippocampal sharp waves, LFP rhythms can be used conveniently as mesoscopic reader mechanisms by the experimenter. Assemblies active within a given classifier pattern, such as an oscillation cycle, can represent an integrated entity (e.g., a neural word).
A well-studied and understood example of a neural word is the spatio-temporal pattern of neuronal activity in the antennal lobe (AL) of insects in response to odor stimuli (Figure 3A–C; Laurent 2002; Laurent et al., 2001; MacLeod and Laurent, 1996). When an odor is presented, it induces a transient gamma frequency oscillation in the AL neuronal population, with different small subsets of AL neurons firing in each oscillation cycle. The odor is thus represented (or ‘coded’) by an evolving sequence of activity vectors (a neural word or trajectory), lasting for a few hundred milliseconds. Successive presentations of the same stimuli evoke similar trajectories (Figure 3A, inset), whereas different odors are associated with uniquely different sequences of projection neurons (Broome et al., 2006; Mazor and Laurent, 2005).
Figure 3.
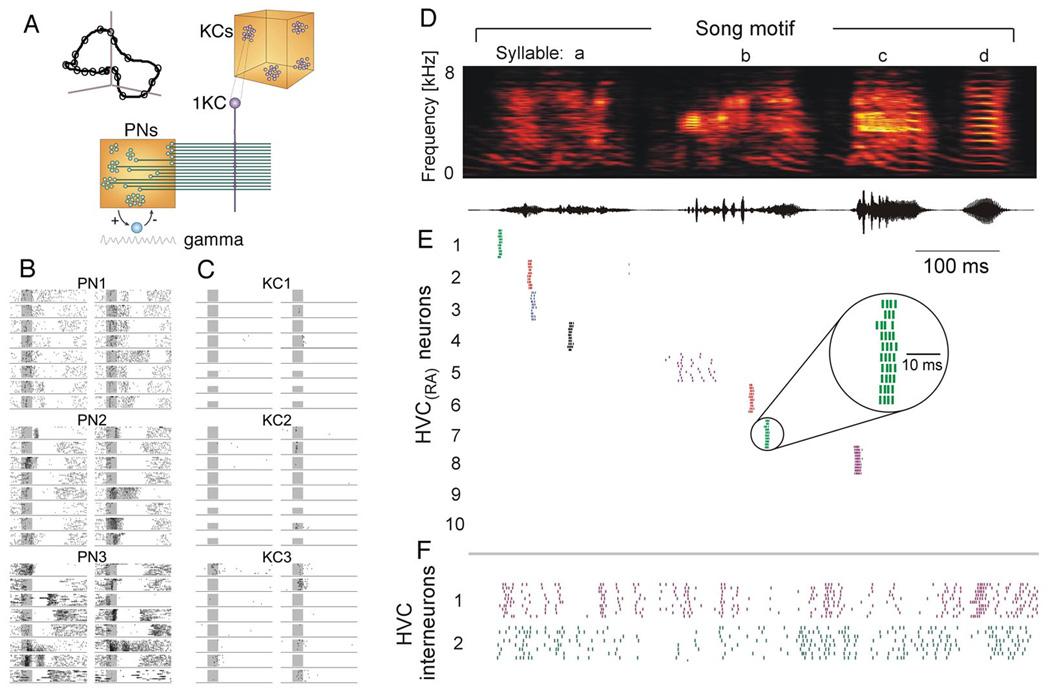
Externally-triggered and internally-generated assembly sequences. A. Wiring diagram of the early olfactory system of the locust. An odorant evokes an odor-specific temporal pattern in several of recurrently connected antennal lobe (AL) neurons, coordinated by a 20–30 Hz (gamma) oscillation. Kenyon cells (KC) of the mushroom body (MB) are the readers of the activity of AL projection neurons (PNs) and integrate their spikes. B. Firing patterns of 3 AL neurons (PN1–3) in response to 16 different odors. In each segment of time (e.g., a gamma oscillation cycle), a different constellation of AL neurons fires. This constellation is referred to as the ‘population vector’ or ‘state’ of the network, and the time-varying population vector (i.e., the shifting states) is described as a trajectory. Although each state of the trajectory codes for the same odor, the state evolves over a few hundred milliseconds before relaxing back to baseline activity (illustrated by the curve in the inset in A). C. Activity of 3 KCs. Each KC carries out a pattern matching operation between its connection vector and the PN population activity vector. The AL output synchrony is strongest at its early phase of evolution and evokes a single burst in the reader KC (‘sparse coding’). D. Time-frequency spectrum of a zebra finch song and its amplitude envelope. E. Spike raster plot of eight projection neurons in the high vocal center (HVC). There is not a one-to-one correspondence between the song syntax and cell assemblies (neural ‘letters’ in HVC). Rather, neurons in HVC generate a temporal ‘state’ sequence (i.e., a trajectory), allowing the target neuronal activity and consequent motor actions unfold. F. Interneuronal activity is also temporally organized and relates to the syntactic structure of the song (F). A to C, modified after Laurent (2002). D to F, modified after Hahnloser et al. (2002).
Another well understood example of neural words is birdsongs. Birdsongs are induced internally rather than triggered by external stimuli. The song consists of distinct bursts of sounds (syllables), separated by silent intervals (Fig. 3D).9 In the zebra finch, the syllable sequences are stereotypical and last for several seconds. The song is controlled by a set of nuclei, which form a mostly feed-forward excitatory pathway (Nottebohm et al., 1976). The critical brain area in song production is the high vocal center (HVC), which projects to the robust nucleus of the arcopallium (RA), which, in turn, drives the hypoglossal motor neurons innervating the vocal organ (syrinx). Experiments have demonstrated that the temporal structure of the song is generated by sparse sequential bursts of RA-projecting HVC neurons (Fee et al., 2004,Hahnloser et al., 2002; Long and Fee, 2008). Each neuron typically emits a single brief burst of spikes only at one time in the song (Figure 3D and E). It is assumed that each of the sequentially activated neurons is a part of an assembly of approximately 200 neurons, whose other members remain unseen to the experimenter (Hahnloser et al., 2002). The sequential activation of the assemblies in approximately 600 msec can be conceived as a word and the same word is repeated numerous times in a singing episode.10
When sequentially activated neural words are different, they can be conceptualized as a neural sentence. Numerous complex behavioral patterns, grouped under the term ‘fixed action patterns’11 or ‘action syntax’ (Lashley, 1951), can be elicited by a relevant cue or emerge without explicit cues. A well-studied fixed action pattern in rodents is grooming, a sequence of face washing, followed by bilateral strokes and the grooming sentence concludes with a postural turn and body licking. Although the neuronal mechanisms underlying the sequential patterns of grooming are largely unknown, the dorsolateral neostriatum may be involved in generating its syntax (Berridge and Whishaw, 1992).
Stereotypical actions can be generated by relatively simple feed-forward excitatory mechanisms (such as a ‘synfire’ chain; Abeles 1991; Hahnloser et al., 2002; Sompolinsky and Kanter 1986;). However, generating multiple neuronal trajectories (i.e., neural sentence structures) serving different action sequences requires more sophisticated solutions. For example, nightingales or marsh warblers can sing dozens of unique songs. In this more complex case, the activation probability of a given assembly in the network likely depends not only on the immediately preceding but also on the previous sequence of a few (or several) assemblies. In strongly recurrently connected systems of large size, equipped with appropriate syntactical rules, very large numbers of trajectories (neural sentences) can be generated. In such model systems the evolution of the assembly sequences (i.e., the uniquely different neural sentences) can be described by a transition rule where the future sequence is probabilistically defined by the previous ordering of assemblies (Jin 2009; Rabinovich et al., 2008a, b; Sakata and Brainard, 2006; Woolley and Rubel, 1997). Indeed, the ability of the brain to sweep through sequences of neuronal assemblies is expected to support our ability to reminisce, think, reason and plan ahead.
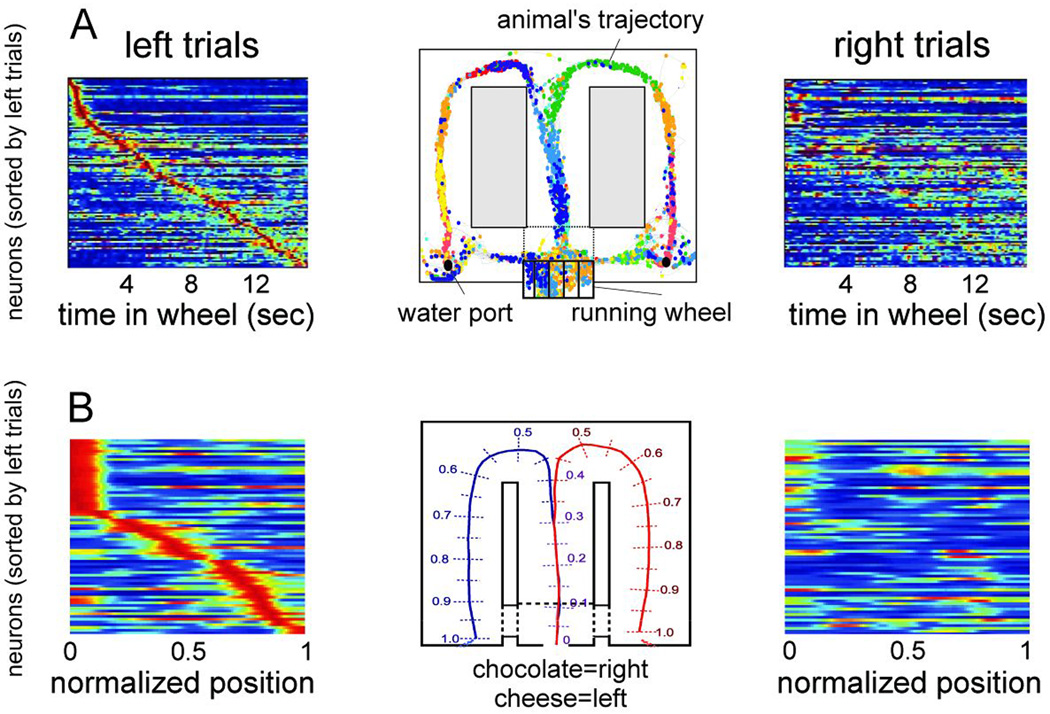
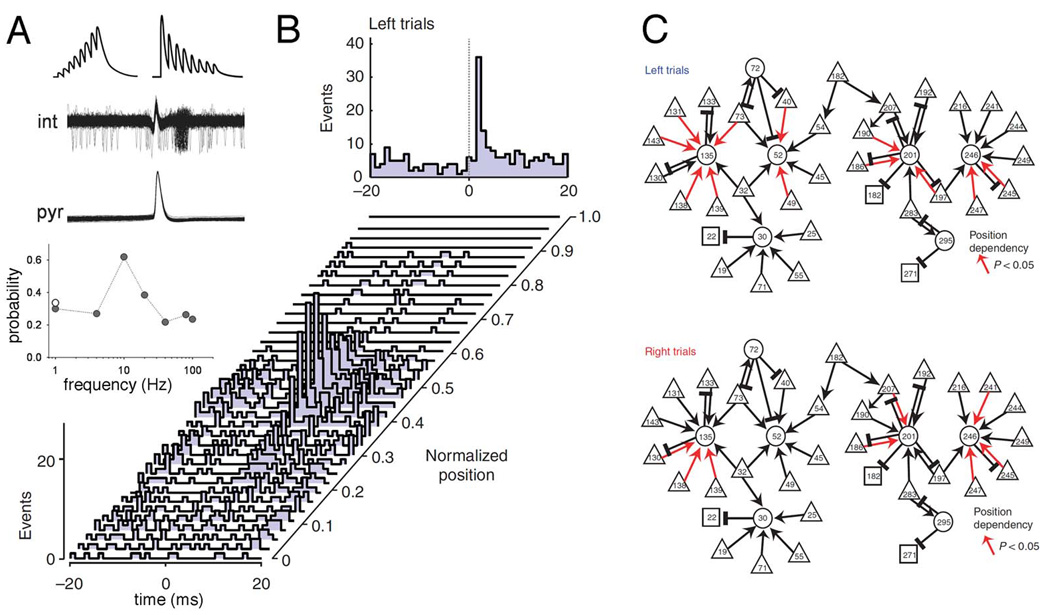
Using large-scale recordings of neuronal spiking activity, self-organized cell assembly sequences, serving mnemonic and planning functions, have recently also been described in the mammalian brain and shown how they move the cognitive content forward or back in time (Pastalkova et al., 2008). Numerous experiments have demonstrated that hippocampal neurons show place-related firing while the rat explores or traverses its environment so that each assembly of hippocampal principal cells defines a particular position of space (O’Keefe and Nadel, 1978; Wilson and McNaughton, 1993). It has been assumed that sequential activity of hippocampal ‘place cell assemblies’ emerges in response to the changing constellation of environmental inputs (O’Keefe and Burgess, 1996) or to body motion-derived cues (McNaughton et al., 1996) that is that they are ‘driven’ by sensory inputs. However, perpetually changing hippocampal assembly sequences could also be observed during the delay part of a memory task in the absence of changing sensory or feedback cues (Fig. 4A). Importantly, several measures of the place cell metric, including the duration of activity episodes of the neurons and the temporal relationship of their spikes relative to the reference theta oscillation cycle during translational behavior (O’Keefe and Recce, 1993) were similar in the internally organized sequences during the delay period, when the rats were required to run steadily in a wheel and remember a previously made choice (Pastalkova et al., 2008). The implication of these observations is that the physiological mechanisms which govern the progression of cell assembly sequences in the hippocampus during navigation and cognitive behaviors are quite similar. The behavioral relevance of self-organized sequential activity is emphasized by the observation that identical initial conditions (e.g., a left choice was rewarded) induced a similar assembly sequence each time, whereas different conditions (i.e., different memories) gave rise to uniquely different trajectories, which accurately predicted upcoming choices in the maze, including erroneous turns (Figure 4A). In situations when keeping track of two concurrent information streams (local and distant cues) were required for correct behavioral performance, two distinct assemblies toggled between representations of the two spatial frames (Johnson et al., 2009; Kelemen and Fenton, 2010). In accordance with experiments in rodents, single unit studies in human patients showed that the hippocampus and entorhinal cortex can generate numerous trajectories corresponding to different memory episodes, and, importantly, that the neurons which fire during free recall are part of the same cell assembly sequences that were activated while watching the cinematic episodes in the learning phase (Gelbard-Sagiv et al., 2008).
Figure 4.
Internally generated assembly sequences during cognitive activity. A. Sequential firing patterns of hippocampal neurons in a memory task. Center, color-coded spikes (dots) of simultaneously recorded hippocampal CA1 pyramidal neurons. The rat was required to run in the wheel facing to the left during the delay between the runs in the maze. Left, normalized firing rate profiles of neurons during wheel running, ordered by the latency of their peak firing rates during left trials (each line is a single cell). Right, normalized firing rates of the same neurons during right trials. B. Sequential firing patterns of prefrontal pyramidal cells in a working memory task. Middle, cheese odor or chocolate odor in the start area signals the availability of cheese or chocolate reward in the left or right goal area (position 1), respectively. Travel trajectories were linearized (0 to 1). Left, neurons were ordered by the location of their peak firing rates relative to the rat’s position in the maze during left trials. Each row represents the position-dependent normalized firing rate of a single neuron. Right, normalized firing rates of the same neurons during right trials. A, reprinted from Pastalkova et al. (2008). B, reprinted from Fujisawa et al. (2008).
Generation of neural sentences is not confined to the hippocampal system. In the medial prefrontal cortex of the rat, neuronal sequences reliably differentiate between right and left trajectories in the maze prior to making a choice, with individual neurons active only for a short duration (Figure 4B; Baeg et al., 2003; Fujisawa et al., 2008). In summary, in contrast to the olfactory network and the birdsong system, cortical circuits can produce multitudes of unfolding assembly sequences in two different ways: either by responding to environmental/idiothetic stimuli, when such inputs are available, or generating them internally.
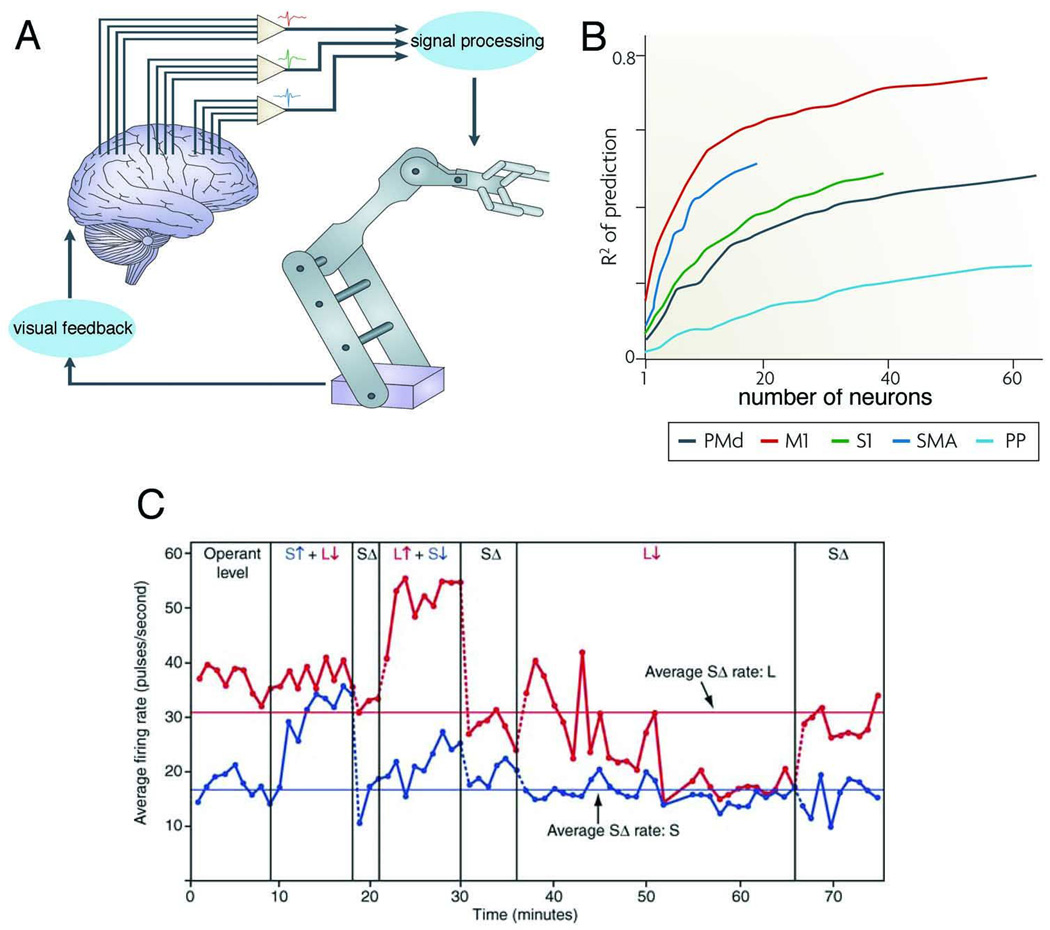
Despite the robust correlation between assembly sequences and behavior, only limited evidence is available to support their critical importance in guiding overt behavior. Perhaps the best examples of the reader-centric definition of cell assembly sequences come from ‘brain-machine interface’ (BMI) studies, where the reader-actuator mechanisms are explicitly defined. There are fundamentally two approaches to control cursors, robotic arms or other actuators by volitional control. In the first approach, large numbers of multiple units or LFP patterns from various cortical areas are recorded from and their assembly sequence activity is first correlated with a chosen natural behavior (e.g., arm movement). In this process, various statistical extraction methods are used to identify the conversion parameters that best describe the executed movement (Carmena et al., 2003; Chapin et al., 1999; Hochberg et al., 2006; Taylor et al, 2002). Spiking patterns of neurons that significantly contribute to the conversion parameters constitute the assembly sentence (Figure 5). In the next stage, these extracted parameters are used as a ‘transform algorithm’ (i.e., a ‘statistical reader’) to control an actuator by brain activity. In the second approach, one or more neurons are chosen and their spiking activity is used to define the various degrees of freedom of the actuator (e.g., 2 neurons for 2-D cursor). These effector neurons are then ‘trained’ to generate the desired spike patterns needed to move the cursor. In this latter approach, it is left to upstream networks to ‘figure out’ the successful, intention-controlled neuronal trajectories, without the need of an experimenter-designed complex transformation algorithm (Donoghue 2002; Fetz, 1969; 2007; Kennedy and Bakay, 1998; Legenstein et al., 2010). The readers, in this case, are the effector neurons and their spiking activity defines the cell assembly sentences that lead to their patterned discharge. During the course of training, the natural proprioceptive feedback is substituted by visual observation of the movements of the effector device. By assigning a new goal, the relationship among the recorded neurons is modified and the muscular movements previously elicited by the firing patterns of the neurons can disappear (e.g., Fetz, 2007; Nicolelis and Lebedev, 2009), an explicit demonstration that different readers (muscles vs actuators) gain control over the coordinated assembly activity of neurons. The success of BMI experiments demonstrates that arbitrarily chosen reader-actuator mechanisms (goals) can rapidly reshuffle assembly members and neural sentences can be composed with remarkable ease.
Figure 5.
A. Schematic of a brain-machine interface (BMI). Activity from multiple ensembles of neurons in several brain areas is recorded and their movement-related information is extracted (‘signal processing’). In the robot-control phase the derived algorithm is used to convert ongoing neuronal activity to generate the desired movements of the robot (reader-actuator). B. Relationship between the numbers of neurons used to predict arm movement position in a monkey and accuracy of the algorithm’s prediction. Note that relatively few neurons are needed to achieve ‘good enough’ performance, whereas very large numbers of neurons may be needed to achieve 100% accuracy. PMd, dorsal premotor cortex, M1, primary motor cortex, S1, primary somatosensory cortex, SMA, supplementary motor area and PP, parietal cortex. C. Differential control of neighboring neurons in the motor cortex by visual feedback in monkey. The firing rate of the one of the neurons (S, blue or L, red) controlled the displacement of a meter arm (Operant Level, upper row). Responses during epochs indicated by the red and blue arrows (upper row) were rewarded on the basis of an arbitrary increase or decrease of firing rate of the chosen reader neuron. The ‘reader’ neurons (S or L) learned to respond differentially to the activity of (hidden) upstream assemblies within minutes. A and B, reprinted from Nicolelis and Lebedev (2010). C, reprinted from Fetz (2007).
Interleaved cell assembly sequences give rise to higher-order connections
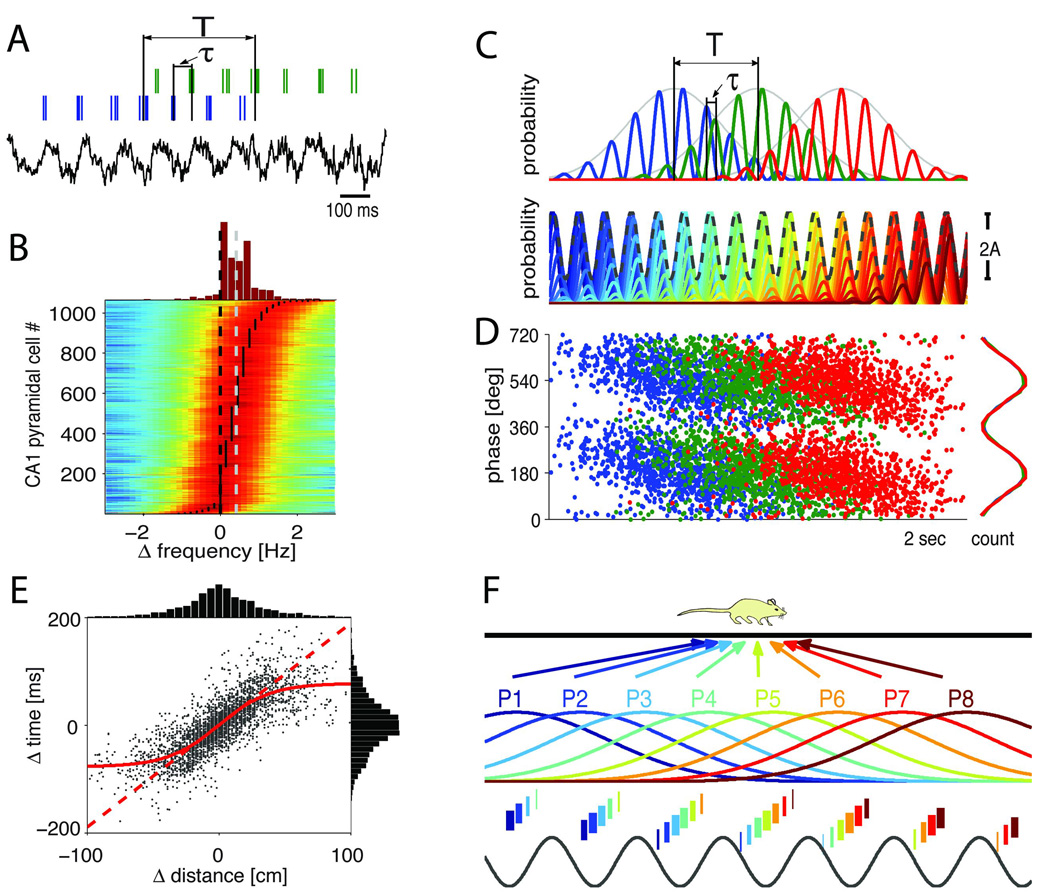
Unlike in written language syntax, where contiguous series of fundamentals (letters) constitute words and sentences, in neural syntax multiple words can overlap and generate both first order and higher order patterns, much like in music. Figure 6 illustrates the genesis and syntactic structure of such interleaved assemblies. The spiking patterns of hippocampal place cells can be approximated by a Gaussian spatial field, modulated by the theta frequency oscillation (Fig. 6A, C; Samsonovich and McNaughton, 1997). The Gaussian fields of different place cells, representing upcoming places or items, can overlap and their temporal relationships are governed by a ‘compression’ rule: within the theta cycle, the spike timing sequence of neurons predicts the upcoming sequence of locations in the path of the rat, with larger time lags representing proportionally larger distances (Fig. 6A, C; Dragoi and Buzsáki, 2006; Skaggs et al., 1996).12 The consequence of the time lags between the spikes of the transiently oscillating neurons is that the oscillation frequency of their population output, also reflected by the local LFP, is slower than the mean of the oscillating frequencies of the constituent neurons (Fig. 6B and C bottom part). The longer the theta time-scale delays between the neurons, the slower the frequency of the population oscillation.
Figure 6.
Interleaved cell assemblies. A. Spiking activity of two hippocampal neurons (blue and green ticks) and LFP theta in a single run (1 second is shown). Temporal distance T is the time needed for the rat to run the distance between the peaks of the two place fields (‘real time’). Tau, time offset between the two neurons within the theta cycle (‘theta time’). B. Distribution of oscillation frequencies of CA1 pyramidal cells (n>1000) during running on the maze relative to the reference LFP theta (8.09 Hz=0 Hz). Gray dashed line: mean oscillation frequency of pyramidal cells (8.61 Hz=0.52 Hz). Note that nearly all place cells oscillate faster than the frequency of the concurrent LFP theta. C. Three example model neurons (color-coded) with identical oscillation frequency but different phase onset, according to their maximal discharge location. Bottom, the summed activity of the entire population of model neurons (black dashed line) oscillates slower than each transiently active individual neuron (color-coded). D. The phase of the three example neurons with respect to the oscillation of the population is plotted against time. Note that the neuronal spikes phase-precess approximately 360° (O’Keefe and Recce, 1993). Right: spike density for the example neurons. E. Correlation between the distances of place fields peaks and theta-scale time lag for >3000 pairs of neurons (as in A). Above and right: histograms of distance and time lag, respectively. F. Interleaved neuron sequences represent position and distance relationships. The width of the bars indicates firing intensity of the hypothesized assemblies while the theta-time scale temporal differences between assemblies reflect their respective distance representations. In successive theta cycles, assemblies representing overlapping place fields (P1 to P8) shift together in time and sustain a temporal order relationship with each other so that the assembly that fires on the earliest phase represents a place field whose center the animal traverses first. The temporal compression mechanism (Skaggs et al., 1996) allows distances to be translated into time. Approximately, 7±2 assemblies/gamma cycles, are present in a given theta period (Bragin et al., 1995; Lisman and Idiart, 1995). The assembly sequences within theta cycles could be conceived as a neural word. Note that neighboring overlapping words differ only by one assembly. The rat has to travel 7±2 theta cycles until a word with entirely different assemblies appear. A to E, modified after Geisler et al. (2010). F, modified after Dragoi and Buzsáki (2006).
The tripartite relationship between global theta frequency ftheta, the oscillation frequency of single neurons fo and the distance-related, theta time-scale temporal lags of spikes (time ‘compressed’ sequences) has important consequences on the assembly organization of hippocampal neurons. First, the difference in oscillation frequency between the population (ftheta) and active single neurons generates an interference pattern, known as ‘phase precession’ of place cells (O’Keefe and Recce, 1993) so that the distance traveled from the beginning of the place field can be instantly inferred from the theta phase of the place cell spikes (Fig. 6D; Dragoi and Buzsáki, 2006; Skaggs et al., 1996). Second, the slope of the phase precession defines the size of the place field (O’Keefe and Recce, 1993; Maurer et al., 2005). Neurons with identical place fields will fire at the same phase, thus the observer neurons will classify them as members of the same assembly. Third, the field size (i.e., the ‘lifetime’ of activity) is inversely related to the oscillation frequency of the neuron. As a result, neurons which oscillate faster have smaller place fields and display steeper phase-precession slopes, as is the case in the septal portion of the hippocampus, compared to neurons in more caudal (temporal) parts of the structure, which oscillate slower, have larger place fields and less steep phase-precession slopes (Kjelsrup et al., 2008; Maurer et al., 2005; 2006a; Royer et al., 2010; Wiener et al., 1994). The dynamic local adjustment of these interdependent parameters is responsible for the globally coherent theta oscillation in the hippocampal system (Bullock et al., 1990; Buzsáki 2002; Geisler et al., 2010; Lubenov and Siapas, 2009).
Owing to the bidirectionally-constrained relationship between single neurons and their population product, the time lags between spikes of neurons have important functional consequences. First, despite variable running speed of the rat, place cells continue to represent the same positions and distances in the same environment because the oscillation frequency of place cells increases in proportion with the velocity, while time lags remain essentially the same (Diba and Buzsáki, 2008; Geisler et al., 2007). Second, the duration of the theta cycle (120–150 msec in the rat) sets a natural upper limit of distance coding by theta-scale time lags (~50 cm for neurons in the dorsal hippocampus; Dragoi and Buzsáki, 2006; Maurer et al., 2005), as reflected by the sigmoid relationship between the theta time lags of neuronal spikes and distance representations (Fig. 6E; Diba and Buzsáki, 2008). The behavioral consequence of the sigmoid relationship is that objects and locations > 50 cm ahead of the rat are initially less distinguishable from more distant landmarks, but as the animal approaches, they are progressively better resolved by the interleaved cell assemblies. Third, the number of cell assemblies that can nest in a given theta period (7 to 9, as reflected by the number of gamma cycles/theta; Bragin et al., 1995; Buzsáki et al., 2003; Chrobak and Buzsáki, 1998), determines the spatial resolution distance representation (approximately 5 cm/theta cycle). A consequence of the limited number of theta-nested assemblies is that distance resolution scales with the size of the environment; temporal lags that represent fine spatial resolution in small enclosures correspond to coarser distance representations in larger environments (Diba and Buzsáki, 2009; Fenton et al., 2008; O’Keefe and Burgess, 1996).13
Assuming that locations can be regarded analogous to discrete items (Fig. 6F; Dragoi and Buzsáki, 2006; Lisman and Idiart, 1995), the temporal compression mechanism can limit the “attention span” and the “register capacity” of the memory “buffer” of the gamma-nested theta-cycle to 7 to 9 items (Lisman 1999; Lisman and Idiart, 1995; Jensen and Lisman, 1996; Hasselmo et al., 2002).14 In this latter context, the sigmoid relationship suggests that the spatiotemporal resolution of an episodic recall is high for the conditions/context that surround a recalled event, whereas the relationships among items representing the far past or far future, relative to the recalled event, are progressively less resolved (Diba and Buzsáki, 2008). However, as the content of the recall moves forward in perceived time, subsequent events gain high contextual resolution (Dragoi and Buzsáki, 2006). The theta dynamic-controlled delays imply that the speed of recall is generic and independent of the temporal relations of the items presented during encoding.
In strongly recurrent systems, such as the hippocampal CA3 region, the temporal compression mechanism (Skaggs et al., 1996) can ensure that in a neural word not only adjacent assemblies but also next-neighbor and more distant assemblies can be linked, as long as they consistently co-occur in the same theta cycles. These higher order connections, in turn, can provide a substrate for alternative routes in the evolution of neuronal trajectories and for combination of different assembly sequences, mechanisms necessary e.g., for solving detour and transitive inference problems (Dusek and Eichenbaum, 1997; Muller et al., 1996) and for higher-order associations in episodic memory (Polyn and Kahana, 2008). Thus, if the recall of a learned chain of fundamental assemblies a, b, c and d is blocked at c, the trajectory may jump to assembly d, i.e., to the second-order partner of assembly b (Gerstner and Kistler, 2002; Kiebel et al., 2009; Rabinovich et al., 2008a).
Since a similar temporal dynamic is at play in the entorhinal cortex (Burgess et al., 2007; Chrobak and Buzsáki, 1998; Hasselmo et al. 2009; Mizuseki et al., 2009; Moser et al., 2008), prefrontal cortex and other structures (Benchenane et al., 2010; Berke et al., 2004; DeCoteau et al., 2007; Jones and Wilson, 2005; Siapas et al., 2005; Sirota et al., 2008; Tort et al., 2008), the mechanisms explored in the hippocampus may apply to these structures as well.
Offline replay of assembly sequences
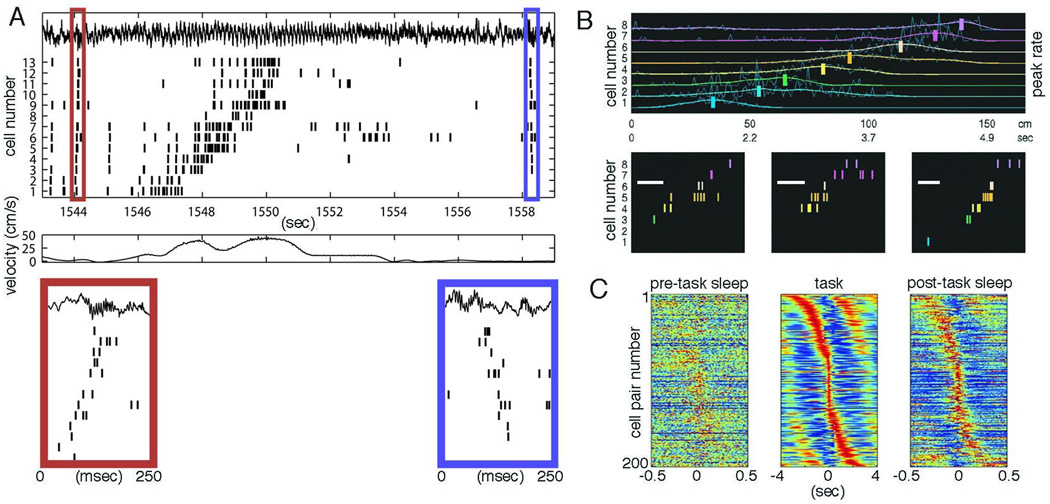
While the time lags between assemblies in the hippocampus depend on theta-nested gamma waves during exploration, assembly sequences can occur both in the absence of theta (or other) oscillations and environmental inputs. During consummatory behaviors, immobility and non-REM sleep, the hippocampal theta rhythm is replaced by irregular sharp waves (Buzsáki et al., 1983). Self-organized population bursts of the hippocampal CA3 pyramidal cells induce a strong depolarization in the apical dendrites of CA1 pyramidal cells, reflected by an LFP sharp wave of negative polarity, accompanied by a transient fast-field oscillation (140–200 Hz) or “ripple” confined to the cell body layer of CA1 pyramidal cells (Buzsáki et al., 1992; O’Keefe and Nadel, 1978). SPW-Rs are the most synchronous assembly pattern in the mammalian brain (Chrobak and Buzsáki, 1994), characterized by a three- to five-fold gain of network excitability (Csicsvari et al., 1999). SPW-Rs have been hypothesized to play a critical role in transferring transient memories from the hippocampus to the neocortex for permanent storage (Buzsáki, 1989; McClelland et al., 1995). In line with this postulated role, both place cell sequences and the distances between the place fields experienced during exploration are reflected in the temporal structure of neuronal sequences during SPW-Rs (Figure 7A, B; Kudrimoti et al., 1999; Lee and Wilson, 2002; Nádasdy et al., 1998; O’Neill et al., 2008; Skaggs and McNaughton, 1996; Wilson and McNaughton, 1994) and their selective elimination after learning interferes with memory consolidation (Ego-Stengel and Wilson, 2010; Girardeau et al., 2009). In the waking animal, SPW-R-related sequences can be replayed in either a forward manner, typically prior to initiating a journey, or in a reverse order after reaching the goal (Fig. 7A; Diba and Buzsáki, 2007; Foster and Wilson, 2006). This bidirectional re-enactment of temporal sequences may also contribute to the establishment of higher-order associations in episodic memory.
Figure 7.
Time-compressed ‘off-line’ replay of learned neural patterns. (A) Forward and reverse pre- and replay of place-cell sequences. (a) Spike trains of 13 neurons during a single lap (CA1 local field potential shown on top). Bottom panels magnify 250-ms sections of the spike train, depicting forward preplay and reverse replay, respectively. Each place cell is assumed to be a member of an assembly of distributed hippocampal neurons defining a particular position, and the ensemble sequence constitutes a neural word. (B) Replay of waking neural words during sleep in hippocampus. Smoothed place fields (colored lines) of 8 place cells during runs from left to right on a track (average of 30 trials). Vertical bars mark the positions of the normalized peaks of the smoothed fields. Non-uniform time axis below shows time within an average lap when above positions were passed. Bottom panels, three SPW-R-related sequences from slow wave sleep after the waking session. Note similar sequences during SPW-Rs and run. Note also difference in timescale. Bar = 50 ms. (C) Time-compressed replay of waking assembly sequences during sleep in mPFC. Sorted cross-correlations from simultaneously recorded cell pairs. Each row in each subpanel shows the cross-correlation between a single pair of cells, sorted according to the temporal offset of the maximum peak during the task. Red indicates the highest coincidence rate and blue, the lowest. The time axis during sleep epochs is magnified. Note similar sequences during the task and post-task sleep. Part A is modified after Diba and Buzsáki (2007) with permission; (B) modified after Lee and Wilson (2002); (C) modified after Euston et al., (2007).
Off-line replay of waking experience-dependent activity has been also observed in the neocortex (Figure 7C; Euston et al., 2007; Hoffmann and McNaughton, 2002; Huber et al., 2004; Johnson et al., 2010; Takehara-Nishiuchi and McNaughton, 2008) and striatum (Lansing et al., 2008; Pennarz et al., 2004), as well as across structures (Ji and Wilson, 2007; Lansing et al., 2009), illustrating that it is a general phenomenon in the brain. Sleep-related assembly sequences are perhaps the strongest evidence for the occurrence of complex self-organized patterns in the brain independent from the influence of the environment. However, in contrast to the internally generated neuronal sentences underlying cognitive operations, such as recall, imagination, decision making or action planning, which occur in real (clock) time, assembly replay during rest and sleep occurs in snippets and is faster, often compressed by at least a factor of ten compared to the behavioral time scale of neuronal activation (Davidson et al., 2009; Diba and Buzsáki, 2007; Euston et al., 2007; Foster and Wilson, 2006; Nádasdy et al., 1999). Although this time compression is only slightly faster than that generated by the theta-scale compression of distances, the main difference is that in contrast to the waking brain, there are no concurrent real time assembly sequences present during slow wave sleep. Thus, while neuronal processing is perpetual in all brain states, conscious experience of such processing may require real time neural words and sentences.15
Since there are no immediate behavioral consequences of the ‘off-line’ state-related cell assembly sequences, one can only assume that the utility of such self-organized patterns is to strengthen or consolidate the synaptic changes initiated during the waking experience and to link assembly representations, which never or rarely overlapped during behavior. The responding reader-integrator neurons of such novel replay patterns will be different from the readers representing each experience separately. As a result, such off-line linking of experiences may facilitate their associations in future waking states.16
Synapsembles link spiking cell assemblies
According to Hebb’s definition (1949), an assembly is characterized by the stronger synaptic connectivity among assembly members than with other neurons. In principle, chains of slow firing neurons, connected with predetermined and fixed synaptic weights can form groups and propagate activity (Abeles, 1991). However, strong, ‘fixed’ connectivity may not be a good model for segregating neuronal groups since synaptic weight distributions are perpetually changing in an activity-dependent fashion in the working brain. In fact, the dynamic range of short-term synaptic plasticity is large and similar to that of long-term plasticity (Marder and Buonomano, 2003), posing problems for the synaptic connection-based definition of cell assemblies. It follows that knowledge of spiking activity is insufficient to properly describe the state of the cortical network unless the distribution of momentary synaptic weights, i.e., the instantaneous functional connection matrix, is also known.
While spikes are generally regarded as the common currency of neuronal communication, experimental and theoretical studies over the past decade have accumulated compelling evidence that short-term synaptic plasticity can also serve related functions (Abbott and Regehr, 2004; Abbott et al., 1997; Maass and Markram, 2002; Mongillo et al., 2008; Sussillo et al., 2007; von der Malsburg, 2004; Zucker and Regehr, 2002). Connectivity in the cortex is characterized by a large range of variation of synaptic weights (Gloveli et al., 1997; Holmgren et al., 2003; Markram et al. 1998; Reyes et al., 1998; Wang et al., 2006), which can change dynamically by both presynaptic and postsynaptic mechanisms (Chung et al., 2002; Deisz and Prince, 1989; Gupta et al., 2000; Markram et al., 1998; Thomson et al., 2002). The fraction of potentiating and depressing synapses is approximately the same in the intact neocortex (Fujisawa et al., 2009; Markram et al. 1998). Indeed, a balance between depressing and potentiating synapses in model networks is needed for stability. At the same time, networks with dynamic synapses can respond robustly to external inputs yet return to baseline activity shortly after the perturbation (Sussillo et al., 2007). Analogous to the assembly of spiking neurons, a particular constellation of synaptic weights in a defined time window can be conceived of as an assembly of synapses or ‘synapsemble’. There are orders of more synapses in the brain than the number of its neurons. In addition, dynamic synapses signal a continuous relationship between neurons, offering a much richer source of communication by synapsembles than by the all or none spikes or discharge rates.
Despite the expected critical role of synapsembles in neural syntax, experimental evidence supporting the role of synapsembles in combining and separating neuronal assemblies is scarce, largely because of the lack of tools to directly measure synaptic connectivity in the behaving animal. An indirect measure of short-term plasticity can be obtained by examining the fine-timescale spike transmission probabilities between simultaneously recorded neurons (Baeg et al., 2007; Constantinidis and Goldman-Rakic, 2002; Fujisawa et al., 2008; Hirabayashi and Miyashita; 2005). Even with this indirect method, only connections between principal cells and interneurons can be studied reliably with current methods (Fig. 8A). As Figure 8 illustrates, synaptic efficacy (defined operationally as the magnitude of excess coincidental spikes at <3 msec latencies between the pre- and postsynaptic neuron; Fujisawa et al., 2008) between connected pairs is not constant but varies both as a function of the animal’s position in the maze (Fig. 8B) and as a function of left versus right trajectories (Fig. 8C). Remarkably, the temporal span of the effective spike transmission between pyramidal cell-interneuron pairs is comparable to the activity lifetime of the principal cells in both hippocampus and prefrontal cortex (Fig. 4), implying that synaptic plasticity may play a role in limiting the duration of cell assemblies by controlling their temporal and spatial evolution.
Figure 8.
Short-term synaptic plasticity in a working memory task. A. Top: Illustration of facilitating and depressing synaptic connections between pyramidal cell and interneuron, as reflected by the changes of EPSPs in the postsynaptic neuron. Middle: superimposed traces (10 msec) of intracellular recording from a hippocampal CA1 pyramidal cell (pyr) and extracellular recording from a putative interneuron (int), aligned by the intracellular action potential. Note short-latency (<2 msec) discharge of the interneuron after the spike of the presynaptic pyramidal cell (visible also as an artifact on the extracellular trace). Bottom, dependence of spike transmission probability on the frequency of the presynaptic pyramidal cell spikes. Note that the highest spike transmission probability occurs at approximately 10 Hz. B. Short-term cross-correlograms between a putative pyramidal cell-interneuron pair in the prefrontal cortex as a function of the rat’s position during left-turn trajectories. The most effective transmission occurred near the choice point (positions 0.3–0.5). Top right, session mean. C. Task-dependent changes of synaptic strengths (red arrows) between putative pyramidal cells (triangles) and interneurons (circles) in a small prefrontal network. A, reprinted from Marshall et al. (2002). B and C, reprinted from Fujisawa et al. (2008).
I hypothesize that synapsembles may serve a dual role. First, they limit the lifetime of neural words to subsecond to seconds time scales. Such self-tuned synapses are likely critical in the build up and termination of assembly activity. This process may be brought about by the depressing excitatory synapses among the active assembly members and/or by potentiated inhibition of the recruited interneurons, assisted by intrinsic neuronal mechanisms, such as firing history-dependence of spike threshold (Henze and Buzsáki, 2001). Second, synapsembles link neuronal words separated by cessation of spiking activity (Buonomano and Maass, 2009). Depressing the inhibitory connections and/or potentiating excitatory synapses between members of the receding and trailing cell assemblies (Wang et al., 2006) may achieve such linking. Clearly, the postulated contribution of self-tuned synaptic plasticity to neural syntax could benefit from future experimental and computational analyses.
Segregation of cell assemblies by inhibition
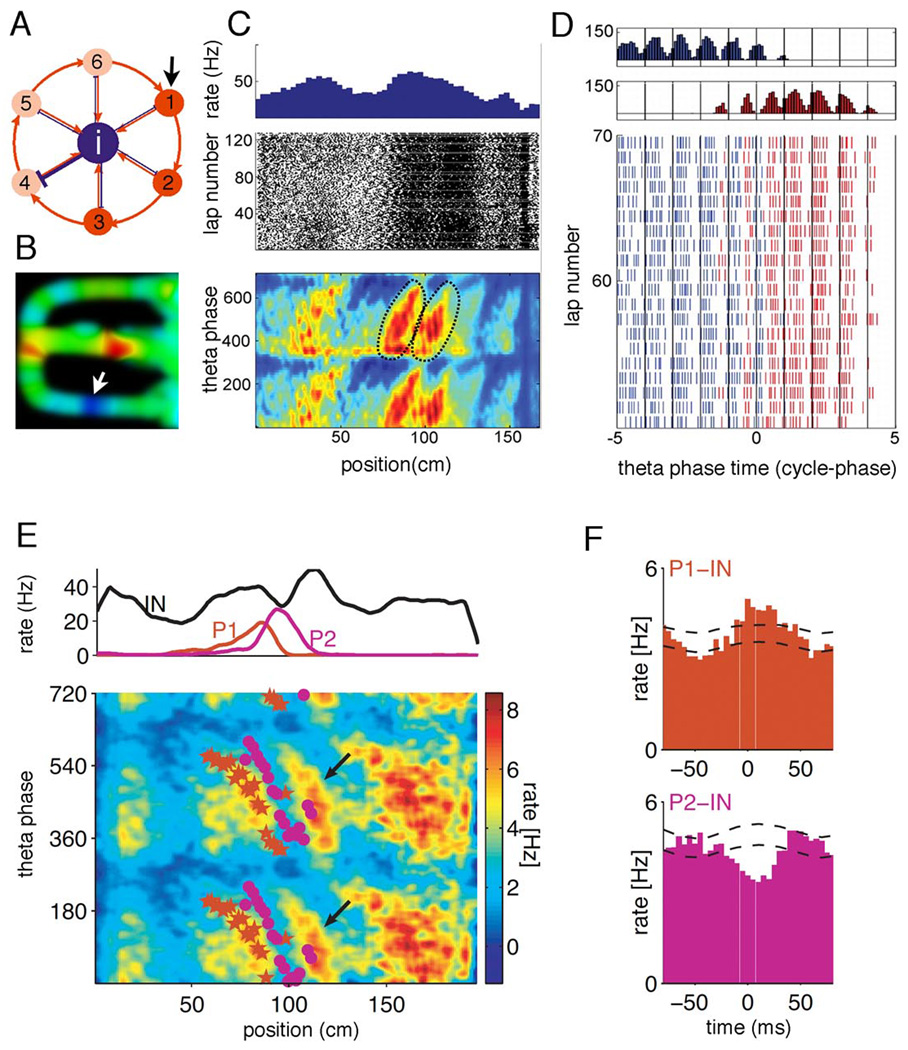
Segregation of excitatory principal cells into functional groups is made possible by inhibition, and this grouping-parsing function is perhaps the most fundamental task performed by the large family of interneuronal classes in the cortex (Freund and Buzsáki, 1996; Klausberger and Somogyi, 2008). As an illustration, consider a ring of excitatory neurons with just one inhibitory interneuron in the middle, reciprocally connected to the excitatory cells (Figure 9A). An external input to any of the neurons may activate a subset of the ring neurons, while silencing others. The interneuron-guided grouping (i.e., formation of a candidate assembly) depends on the location of the input in the ring and, critically, on the fine details of synaptic strengths (i.e., the structure of the synapsemble). With different initial conditions, the interneuron can be ‘enslaved’ to different constellations of excitatory neurons. This example also shows that there is a temporally exquisite relationship between the active assembly, interneurons, and the silenced population. The assembly forming/segregating ability of interneurons may be due to the efficient synapses formed between pyramidal cells and interneurons (Csicsvari et al., 1998; Galarreta and Hestrin, 2001; Geiger et al., 1997; Gulyas et al., 1993; Maurer et al., 2006b; Miles 1990; Thomson et al., 2002) and strong inhibitory interneuron-pyramidal cell connections (Cobb et al., 1995; Pouille and Scanziani, 2001), relative to the typically weak synapses linking principal cells (Miles 1990).
Figure 9.
Segregation of cell assemblies by inhibition. A. A ring of pyramidal neurons (1–6), mutually innervating an interneuron (i). The synaptic strength between the interneuron and pyramidal cell 4 is stronger than between other pairs. When pyramidal cell one receives an input (arrow), cells 1 to 3 are activated while 4 to 6 remain silent (segregated). B. CA1 hippocampal interneuron carrying spatial information. Firing rate map of the neuron in a figure 8-shape maze. Red, 56 Hz, dark blue ~ 0 Hz. Note silent ‘place field’ (arrow) in the lower arm. C. An interneuron can belong to two assemblies within the same theta cycle. Firing rate, spikes in each lap and theta phase of spikes of the interneuron as a function of position. Phase distributions are shown twice for better visibility. Place field boundaries were assigned according to the phase precession of the interneuron (black ellipsoids). D. Event-triggered average of spike times (top), aligned on the time of the theta peak that occurred nearest (in time) to the point of maximum spatial overlap of the two fields (blue, first field encountered; red, second field encountered). Time is in units of phase relative to the trigger point in the middle of the field. Bottom, spikes of the interneurons in successive laps. Note that the systematic phase precession of spikes uncovers two independent but overlapping fields (blue and red spikes), an indication that the interneuron can switch assembly partnerships even within the same theta cycle. E. Firing rates of two pyramidal cells (red, P1; magenta, P2) and a putative basket cell (black, IN) as a function of position (top). Bottom, mean phase precession of P1 (stars) and P2 (dots), superimposed interneuron’s color-coded smoothed density of firing. Note the similar phase slope of P1 and the interneuron. F, Temporal cross-correlation between P1 and interneuron IN and P2 and the interneuron shown in E. Note positive temporal correlation between P1 and IN and negative correlation between P2 and IN. The dashed lines indicate the 95% confidence interval for shuffled spike trains. B, courtesy of K. Mizuseki. C and D are reprinted from Maurer et al., 2006b; E and F are reprinted from Geisler et al. (2007).
In the neocortex, inhibition can have either a positive or inverse correlation with excitatory thalamic input (Ferster 1986; Gentet et al., 2010; Wehr and Zador, 2003;). Excitatory and inhibitory inputs interact in a complex manner to shape the response to On and Off transitions of the stimulus (Borg-Graham et al., 1998) or to affect the tuning properties of the principal cells (Monier et al., 2003; Wilent and Contreras, 2005). Similarly, the firing rates of interneurons in the hippocampus often vary as a function of the animal’s position (Figure 9B; McNaughton et al., 1983) and can mimic several signatures of place cells, including positional information, field size, speed modulation of rate and oscillation frequency and phase precession (Ego-Stengel and Wilson, 2007; Marshall et al., 2002; Maurer et al., 2006b; Geisler et al., 2007; Wilent and Nitz, 2007). Importantly, the input-related specific patterns are not only associated with increased but also by selectively decreased firing of inhibitory interneurons in both neocortex and hippocampus (Figure 9B; Gentet et al., 2010; Rao et al., 1999; Wiebe and Staubli 2001; Wilent and Nitz, 2007). Such well-defined suppression of inhibitory neurons in a neural sentence may facilitate the emergence of new assemblies, suppressed by the same interneurons in other parts of the sentence. How can inhibitory neurons play such a two-faced role: to be part of an assembly and also suppress competing assemblies? Since assembly members are typically drawn from sparsely firing neurons of a large neuron network (Fujisawa et al., 2008; Harris et al., 2003; Sakata and Harris, 2009), only a few principal cells are typically active in a given volume of tissue at any given time. Although interneurons are expected to respond to all of their principal cell inputs more or less equally, in a given short time window only one or a few strongly active principal cells discharge them, thereby essentially ‘copying’ the principal cell’s firing pattern. In turn, the transient ally interneuron can suppress the activity of competing principal cells in the vicinity of their (mostly local) axon collaterals. As a result, only a single assembly (the ‘winner’) may be active at a time even in a large neuronal volume.
An example of the firing pattern-mimicking behavior of hippocampal interneurons is the theta phase precession of their spikes. In contrast to pyramidal cells, the spikes of hippocampal interneurons are either locked to a narrow phase of the theta cycle or show broad phase distribution with dominant locking to the trough. However, whenever an interneuron spikes display a transient phase shift, its phase precession slope is similar to that of the pyramidal cell(s) to which the interneuron is monosynaptically connected (Maurer et al., 2006b). Because multiple interleaving place cell assemblies are present in a given theta cycle (Figure 6), it is expected that the active assemblies induce selective firing in their own interneuron targets at discrete theta phases. This is indeed the case (Figure 9C, D). While the firing rate of the example interneuron in Figure 9C gives little indication that it is driven by neurons taking part in two assemblies, two separate phase precession cycles are clearly revealed in ‘phase-space’ (arrows in Figure 9C). Using the spike phase information, two distinct place-related firing patterns of the same interneuron can be readily segregated, each with a monotonic phase dynamic (Figure 9C), an indication that its firing is under the control of two distinct cell assemblies. In addition, Figure 9D shows that some interneurons are not only driven specifically by assemblies but they also actively contribute to the segregation of competing assemblies. In this example, two spatially overlapping place cells were simultaneously recorded with a putative basket interneuron (Geisler et al., 2007). The gamma time-scale positive correlation between one place cell (P1) and the interneuron suggests that both cells belonged to the same cell assembly. In contrast, the spiking activity and phase-precession of the second place cell (P2) was anti-correlated with the discharge of the interneuron (Figure 9D), indicating an enabling mechanism of the interneuron at times when P2 and its assembly peers were active.
In summary, the available research points to the critical roles of interneurons and inhibition in the formation and segregation of cell assemblies, and in organizing their temporal evolution (c.f., Rabinovich et al., 2006). Given the diverse interneuron classes in the cortex (Freund and Buzsáki, 1996; Klausberger and Somogyi, 2008; Markram et al., 1998), it is expected that further research will identify novel mechanisms by which the different classes interact with each other and the principal cells to choreograph the syntactical structures of externally controlled and internally generated neural sentences.
The size of cell assemblies – a hierarchy of importance
Do neuronal assemblies more resemble quartets, chamber orchestras or large philharmonic orchestras? In Hebb’s cell assemblies, membership is defined by connectedness through excitatory synapses (Figure 1A). However, as discussed above, neither a sufficient nor total number of assembly members can be determined without knowing the timeframe and the goal. Since the reader-centric definition of the assembly depends on classifier mechanisms, the question of assembly size should also be approached from this perspective. As discussed above (Figure 2), if the goal of an assembly is to discharge a downstream pyramidal cell in vivo, the number of neurons whose spikes can be integrated in approximately 20 msec (i.e., one gamma cycle) can quantitatively define the size of the effective assembly. Since approximately 1% of hippocampal pyramidal cells fire in a 20-msec time window during theta-related behaviors (Csicsvari et al., 1998; 1999), and 15,000 to 30,000 CA3 pyramidal cells converge on a CA1 pyramidal neuron (Li et al., 1994; Megias et al., 2001), these relationships indicate that, on average, 150 to 300 CA3 pyramidal cells firing within a gamma cycle comprise an assembly (de Almeida et al., 2010); a number similar to the estimate in HVC of the zebra finch (Hahnloser et al., 2002). Under special conditions, when the inputs converge on the same dendritic branch and fire synchronously in <6 msec, as few as 20 neurons may be sufficient to initiate a forward-propagating dendritic spike (Losonczy and Magee, 2006). These conditions may be present in the hippocampus during sharp wave ripples (Csicsvari et al., 2000) and in the geniculo-cortical system during visual transmission (Wang et al., 2010).
In a different approach to estimate the minimum number of spiking neurons to effectively substitute the effect of a sensory input, channelrhodopsin-2 (ChR2)-expressing neurons in the motor cortex were directly stimulated by light. Mice could detect the occurrence of single action potentials in approximately 300 synchronously active neurons. Even fewer neurons (~ 60) were required when the light induced a train of spikes (Huber et al., 2008). Under special conditions, stimulation of a single pyramidal cell or interneuron can recruit a large fraction of neurons in the circuit (Miles, 1990; Bonifazi et al., 2009; Ellender et al., 2010). Intense trains of intracellularly evoked spikes in a single motor cortex neuron were sufficient to evoke or reset whisking movement in the rat (Brecht et al., 2004). However, in these studies the directly discharged neurons likely activated an unknown number of other cells, and without monitoring the entire population the number of neurons that generated the desired behaviors has remained unknown.
An inherent difficulty in determining the size of a neuronal assembly is that without an explicit goal, it is not possible to quantitatively define which neurons belong to the primary assembly and which represent feedback activation of assembly members or newly recruited assemblies, serving other goals. Although many neurons can contribute to a cell assembly, the contribution of individual members is most often strongly skewed, as is the case for musical orchestras. For example, activity of just a few strongly firing hippocampal place cells can be much more informative about the rat’s position than several dozens of simultaneously recorded other neurons from the same volume and with the same total number of spikes (Wilson and McNaughton, 1993). Similarly, neurons that can predict the future choice of the animal in the hippocampus and prefrontal cortex represent only 1 to 10 percent of the recorded active cells yet they are more informative about the behavioral outcome than the entire remaining population (Ferbinteanu and Shapiro, 2003; Frank et al., 2000; Fujisawa et al., 2008; Pastalkova et al., 2008; Quian Quirroga et al., 2005; Wood et al. 2000). In the olfactory bulb, less than 10% of sharply tuned reader-classifier mitral cells are responsible for generating discrete and defined outputs, even though a large fraction of neurons contribute some spikes (Niessing and Friedrich, 2010).
BMI studies, where the reader mechanisms required to control an actuator are well defined by the experimenter, also support the view that assembly member contribution is non-isotropic (Fetz 2007). Multiple laboratories have reported that the most informative subset of 10 to 20 task-related motor cortex neurons can predict as much as 60 to 80% accuracy of limb position or gripping force, and adding further information from the remaining several dozens of simultaneously recorded neurons from either the motor cortex or other areas improve the prediction only by a modest 10 to 15% (Fig. 5b; Carmena et al., 2003; Hochberg et al, 2006; Serruya et al., 2002; Taylor et al., 2002; Wessberg et al., 2000; cf., Nicolelis and Lebedev, 2009). A similar hyperbolic relationship between the number of CA3 neurons and the occurrence of CA1 ‘ripples’ (‘reader pattern’) has been described in the hippocampus (Csicsvari et al., 2000). The diminishing returns obtained from increasing the assembly size in achieving target control in BMI studies can be interpreted in two different ways. First, that coordinated activity by a few or perhaps dozens of neurons comprises an assembly, which can be regarded by a reader mechanism as ‘good enough’ (Fetz 2007; Serruya et al., 2002). Alternatively, if the goal is to achieve 100% accuracy of performance each time then spiking information from very large numbers of neurons in multiple related brain areas may be needed (Nicolelis and Lebedev, 2009).17
The challenges in objectively determining the size of the assembly, neuronal word or sentence, which can lead to an observable output, include not only recording large numbers of neurons simultaneously but also determining the critical brain areas, cortical layers and neuron types which are most relevant in producing the desired output. Adding more neurons from structures not critical for the task would artificially reduce the estimated fraction of participating cells. Finally, if the network in which the assembly is embedded has scale-free features, the assembly size may scale with the network, rather than represent an ‘optimal’ size (Sporns et al., 2010). To date, we can only tentatively conclude that even a small cell assembly in the cortex likely involves tens to hundreds of pyramidal cells and their transient partner interneurons but the exact size depends on the required accuracy of the goal. It appears then that while the cell assembly can be conceived of as a large philharmonic orchestra in which the contribution of each instrument is needed to perform a perfect concert, a small fraction of key assembly members can play a ‘good enough’ recital.
Reading cell assemblies and assembly sequences
Neural messages are only as useful as their readability. Complex assembly sequences acquire meaning only through appropriate reader mechanisms, which can reliably differentiate among the multiple overlapping sequence patterns. While establishing a correlation between various sensory inputs and firing patterns is an important step in brain research, the biological relevance of these statistics-derived ‘representations’ can be verified only through some actuator mechanism. Multiple time-integrator (reader) mechanisms exist in the brain; each with a characteristic temporal window, and integrators with longer time constants can combine neural assemblies into long sequences. Different reader mechanisms may simultaneously monitor the activity of the same assembly patterns and may extract different types of meanings, for example temporal relationships for one feature and spiking intensity for another (Hirase et al., 1999; Huxter et al., 2003; Konishi 1990; Niessing and Friedrich, 2010).
To date, very little experimental evidence is available regarding the exact mechanisms that allow readers to segregate complex trajectories (MacLeod et al., 1998). In the simplest case, a particular temporal pattern of neurons converges on a given reader neuron due to the hard-wired features of a circuit. This simple but non-realistic example assumes that the readers are in a constant ‘alert’ state, ready to integrate. In a more realistic situation, the readers may be influenced by other inputs as well (e.g., subcortical neuromodulators); therefore their pattern segregating may be strongly influenced by the state of the neural network. To forge a special relationship between readers and their assemblies, words or sentences, further learning or selection rules, which can bring about long-term modification of the relationship between neurons, may be needed. Synaptic plasticity, particularly spike timing-dependent plasticity (Levy and Steward, 1979; Magee and Johnston, 1997; Markram et al., 2007), is often exploited in computational models to modify circuit connections. The learning process may be facilitated by some supervisory mechanism and/or feedback modifying mechanisms. Supervision can simply mean just a time constraint, such as oscillation-induced silencing of readers and their potential upstream assemblies or it can refer to other complex top-down effects, which a priori allow some combinations and disallow others. Alternatively, the reader’s ability to identify a unique upstream constellation of neuronal patterns can be strengthened by reinforcers (i.e. goals), which optimize the connectivity of the upstream assembly post-hoc so that it will activate the reader more effectively on future occasions (Izhikevich 2007; Legenstein and Maas, 2007; Maas et al., 2002; Seung, 2003). If multiple readers send their outputs to a downstream integrator/reader, the readers in the input layer become assembly partners from the perspective of the downstream reader (Figure 1C).18 In turn, the links between the ‘hidden layer’ readers (Rumelhart and Zipser, 1986) may be modified by any of the above mechanisms. Although neither the generality nor the biological viability of these hypothetical selection processes is firmly supported by physiological data, the reader-centric perspective of assembly organization provides a disciplined framework to uncover the mechanisms that enhance the relationship between upstream firing patterns and the readers of such patterns.
Simple computational models, using reverse correlations (e.g., Berry et al., 1997; deCharms et al., 1998), can illustrate the pattern classification abilities of reader/actuator mechanisms (Rumelhart and Zipser, 1986). Various population patterns generated by a network of model neurons can evoke spiking responses in one or just a few reader cells. A given reader neuron or assembly of readers can respond to a random pattern of neuronal discharge in the input layer and during the learning process it becomes selective to it and only to it. Thus, only a specific pattern becomes meaningful to this reader. To provide biological meaning to a second pattern, another reader, selectively tuned to the second pattern, is needed (Fig. 1C). Learning to discriminate numerous patterns requires increasing numbers of selective readers (Masquelier et al., 2009). For example, the 50,000 reader KCs in the mushroom body, in principal, can respond to 50,000 odorant combinations (Fig. 3A; Jortner et al., 2007; Perez-Orive et al., 2002). Discriminating between two trajectories (assembly sequences) of hippocampal or prefrontal neurons by downstream readers, corresponding to two different choices, is a relatively simple task (Figure 4). On the other hand, segregating large numbers of trajectories, representing all episodes collected in one’s lifetime, requires complex mechanisms with many dedicated readers. Such system of readers with the ability to effectively orthogonalize upstream patterns is exemplified by the strong divergence of the entorhinal cortex-dentate granule cell connectivity and the sparse responses of granule cells (Jung and McNaughton, 1993; Leutgeb et al., 2007).19 On a larger scale, the entire neocortex can be conceived as a segregating orthogonalizing layer, with its reader mechanisms learning to classify and segregate overlapping hippocampal output patterns and lay them down as memories (McLelland et al., 1995) or translate them to plans and overt behavioral responses.
In addition to their current input connectivity vector, readers may be also sensitive to the preceding states of the assembly sequence (Figure 3B; Gütig and Sompolinsky, 2006; Truccolo et al., 2010). As discussed above for BMI actuators, extracting the most accurate information about e.g., arm position is a daunting task when the statistical classifier mechanisms have to monitor a high-dimensional sample of the active state of neurons. In contrast, computational considerations suggest that the high-dimensionality of the input vector, in fact, can often facilitate the extraction of information by neuronal readers (Cover, 1965; Haeusler et al., 2003; Maass et al., 2002; Pulvermüller and Knoblauch, 2009), and separation of trajectories becomes progressively easier with increasing dimensionality of state space (Legenstein and Maass, 2007; Legenstein et al., 2010; c.f., Buonomano and Maass, 2009). This may explain why natural readers, such as neurons, have a high flexibility and can adapt to very subtle differences between neuronal trajectories (Fetz, 2007; Logothetis and Pauls, 1995; Poggio and Edelman, 1990). These examples indicate that extracting useful information from temporally evolving neuronal trajectories of long series of assemblies by statistical means may be a more formidable task than separating different neuronal trajectories by the response patterns of reader-decoder mechanisms (Laurent 1999)20 because of their ability to drastically reduce the dimensionality of information streams (Nessler et al., 2010).
Reader-initiated transfer of neuronal messages
Transfer of messages from source (sender) to target (reader) is usually considered a unidirectional operation: the source sends the information to an ever-ready recipient network. Brain networks do not appear to work this way. Instead, the reader plays the initiating role by temporally biasing activity in the source networks and creating time windows within which the reader can most effectively receive information (Figure 10; Sirota et al., 2003; 2008). Each sensory system has co-evolved with such a reader-initiated transfer mechanism. Dedicated motor outputs, such as saccadic eye movements, licking, sniffing, whisking, touching, twitching of the inner ear muscles or other gating mechanisms assist their specific sensory systems by ‘resetting’ or synchronizing spiking activity in large parts of the corresponding sensory system and/or creating transient gains, which enhance the reader (sensory) system’s ability to process the inputs (Ahissar and Arieli, 2001; Bremmer et al., 2009; Desimone and Ungerleider, 1989; Guiterrez et al., 2010; Halpern, 1983; Henson 1965; Kepecs et al., 2006; Kleinfeld et al., 2006).
Figure 10.
Reader-initiated transfer of information. (a) The reader sends an output command to optimize the sensor. Brain-initiated synchronizing-blanking mechanisms are used in all modalities (such as eye movement, sniffing, whisking, active touch, licking, contraction of middle ear muscles, etc.), which generate transient ‘gains’. (b) Reader-initiated transfer is used at all levels of the brain. In this example, the hippocampus (reader)-generated theta oscillation synchronizes computations in widespread neocortical areas (reflected by transient gamma oscillations). (c) The duty phase of hippocampal theta (white arrow) biases the timing of neocortical circuits so that the results of the local computations are presented to the reader during the accrual (‘readiness’) phase of the oscillation.
Neuronal networks in the inner parts of the brain have also adopted reader-initiated mechanisms for transient gains. For example, transfer of hippocampal information to the neocortex (the ‘reader’) during slow wave sleep can be initiated by the down-up transition of the neocortical slow oscillation (Buzsáki 1998; Isomura et al., 2006; Sirota and Buzsáki, 2005; Sirota et al., 2003), which can bias the spike content of hippocampal sharp wave-ripples (Battaglia et al., 2004; Ji and Wilson, 2007). In the waking brain, the direction bias works in the opposite direction. Now the dialogue is initiated by the hippocampus via theta-phase control of neocortical network dynamics (Sirota et al., 2008). As a result, the content of the temporally biased, self-organized gamma oscillations at multiple cortical locations can arrive to the hippocampus at the phase of the theta cycle when hippocampal networks (the ‘reader’) are in their most sensitive, plastic state (Huerta and Lisman, 1996). Exchange of information between different stages of the visual system appears to follow similar rules (Fries 2005; Womelsdorf et al., 2007), implicating a general rule for the reader-initiated transfer of neural messages.21
Mimicking and perturbing cell assemblies, neural words and sentences
Event A is believed to cause event B if it regularly precedes B in time and if in its absence B fails to occur. Thus, while the activity of a reader-actuator (event B) may regularly follow a unique trajectory of assemblies (event A), providing circumstantial evidence for a cause-effect relationship, definite evidence requires either artificial recreation or elimination of the cause (event A). Since methods for selective and fast activation and inactivation of multiple single neurons and synapses by light activation are on the horizon (Boyden et al., 2005; Deisseroth et al., 2006; Luo et al., 2008; Miesenböck, 2009; Zhang et al., 2007), discussion of their potential use in identifying assemblies, neural words and sentences are warranted.
Knowledge of the regular features about the spatiotemporal patterns of spiking behavior in an assembly could be used to recreate those patterns artificially and examine whether such synthetic assembly patterns evoke similar behaviors as the native ones (c.f., Cohen and Newsome, 2004). In principle, this approach could provide the long-waited mechanistic understanding of cell assembly organization (c.f., Luo et al., 2008; O’Connor et al., 2009). It may also help extract the essential features of assembly activity, such as the minimum assembly size, the required temporal precision and sequential recruitment effects. While this approach should be attempted, it may not always work effectively. A failure to elicit the desired effect may occur for various reasons. For example, the required assembly to be activated may reside in multiple structures and activation of neurons in a single structure may not be sufficient. Even if one manages to activate all neurons, the imposed synthetic pattern has to compete with an ongoing program because neuronal networks in the brain are spontaneously and perpetually active. The meaning of an artificial pattern for the same reader in the context where the native assembly pattern was originally observed or, say, during sleep therefore might be fundamentally different. Ideally, the imposed pattern should be embedded in the same mesoscopic temporal dynamic as the observed one. This may be facilitated, for example, by detecting LFP or firing patterns of neurons and use their phase or other features for proper timing of the synthetic pattern.
A practical challenge for the successful application of the synthetic assembly method is to selectively activate neurons. This would require an a priori knowledge of the spatiotemporal pattern of the assembly members and selective light delivery only to these member neurons in the appropriate temporal order. Currently used ‘optrodes’ are not up to this task because light is delivered to orders of magnitude more neurons than the few observed (Cardin et al., 2009; Han et al., 2009; Sohal et al., 2009; c.f., Miesenböck, 2009). More localized delivery of light limited to the volume of recorded neurons is a necessary requirement to this end (Royer et al., 2010). An alternative solution is to express light sensitivity in those neurons only, which are active in a given specific task and test whether their subsequent activation elicits the same behavior. While such activity markers may identify the members of neural words (Claridge-Chang et al., 2009), appropriate temporal sequencing may still be necessary since different temporal ordering of the same assemblies may be interpreted differently by the reader-actuator. Finally, even successful elicitation of a desired behavior should also be interpreted with caution because in situations with a limited repertoire of choices many stimulus patterns may elicit the same (or the only available) choice.
Silencing the presumed members of an assembly, normally causally related to an event, may not lead to the absence of an effect. For example, temporary or even permanent silencing of ‘Halle Berry neurons’ in the hippocampus and associated structures (Quian Quiroga et al., 2005) may not erase the semantic representation of the actress. The reason is that specific firing of these explicit neurons (‘grandmother cells’; Barlow, 1972) is a result of a dynamic and hierarchical relationship between winner neurons and their transiently inhibited competing peers. Elimination of winners may be instantaneously replaced by runner-up neurons.
An alternative strategy to native pattern replication or transient neuron elimination for studying cell assemblies is to systematically perturb the on-line monitored native pattern or part of it. For example, properly timed discharge of weakly connected neurons may strengthen their connections and incorporate them into the assembly sequence (Dragoi et al, 2003; King et al., 1999). Conversely, appropriately timed silencing of assembly members may eliminate them from future attendance in the assembly. An equally promising direction is the temporal jittering of spikes by applying statistically defined noise. While temporal jittering of spikes can maintain firing rates and the average spiking behavior of neurons, it can be used to probe the reader’s tolerance for interpreting the relevant information. For studying the behavioral impact of such manipulations, the obvious challenge is to jitter spikes in a large enough volume of neuronal tissue selectively. In addition to engineering efficient optogenetic methods, drugs affecting short-term synaptic plasticity, but less so firing rates, can be used to probe circuits. For example, drug activation of presynatic cannabinoid receptors (Freund et al., 2003) had no effect on the positional representation of hippocampal place cells, field size or their population vector, thus leaving the ‘spatial map’ intact, yet the rats could not solve a spatial task. Since the drug interfered with the timing of neuronal spikes at the gamma-theta time scale, the behavioral impairment may be explained by the inability of the reader mechanisms to properly interpret the hippocampal messages (Robbe and Buzsáki, 2009).
Analysis of synapsembles requires specific manipulations of the connectivity between different neurons types (von Engelhardt et al., 2010; Wulff et al., 2009). Synapsembles can be also targeted by fast optical means (Wang et al., 2007; Szobota et al., 2007) so that transitions from words to words can be affected. While it is impossible to be prophetic in this fast developing area of research (O’Connor et al., 2009; Miesenböck 2009), it is likely that the combination of large-scale multiple single neuron recordings and application of a whole family of perturbation methods will open new possibilities for understanding the content and meaning of assemblies and their sequential organization.
In addition to these invasive and complex methods, studying the temporal evolution of LFPs and other collective features of neurons can provide insights into the organization of neural syntax. If gamma waves or measures of population activity do in fact reflect the fundamental assemblies of the syntax, examining the time course of gamma power and its modulation by the phase of the slower alpha, theta and delta rhythms may be a promising direction in human subjects even if the semantic content shaped by the neural syntax remains invisible (Bastiaansen et al., 2002; 2009; Jacobs and Kahana, 2009; Steinvorth et al., 2010). Interpreting mesoscopic signals will require further exploration since the frequency of LFP gamma transients and their coupling across layers and cortical ‘modules’ vary as a function of behavior (Colgin et al., 2009; Montgomery and Buzsáki, 2007; Sirota et al, 2008). With appropriate methods the temporal dynamics of neuronal recruitment and their LFP reflections can be accelerated or slowed down and their impact on the reader mechanism evaluated (Long and Fee, 2008). One can only speculate that the roots of language and musical syntax do in fact emanate from the neural syntax native to the brain (Pulvermüller, 2010) since it is the neural syntax that secures a match between brains, which both generate and interpret information.
Supplementary Material
Acknowledgments
I would like to thank Moshe Abeles, Asohan Amarisingham, Gerald M. Edelman, Michale Fee, Katalin Gothard, Kenneth D. Harris, Gilles Laurent, John E. Lisman, Wolfgang Maass, Kenji Mizuseki, Eva Pastalkova, Michail Rabinovich and Charles Yokohama for their comments on various versions of the manuscript. Supported by National Institutes of Health (NS034994; MH54671), National Science Foundation (SBE 0542013) and the J.D. McDonnell Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Footnotes are available in the Supplemental Material.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Abeles M. Corticonics: Neural Circuits of the Cerebral Cortex. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Ahissar E, Arieli A. Figuring space by time. Neuron. 2001;32:185–201. doi: 10.1016/s0896-6273(01)00466-4. [DOI] [PubMed] [Google Scholar]
- Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62:566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40:177–188. doi: 10.1016/s0896-6273(03)00597-x. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Kim J, Ghim JW, Kim JJ, Jung M. Learning-induced enduring changes in functional connectivity among prefrontal cortical neurons. J. Neurosci. 2007;27:909–918. doi: 10.1523/JNEUROSCI.4759-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HH. Single units and sensation: A neuron doctrine for perceptual psychology? Perception. 1972;1:371–394. doi: 10.1068/p010371. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, van Berkum JJ, Hagoort P. Syntactic processing modulates the theta rhythm of the human EEG. Neuroimage. 2002;17:1479–1492. doi: 10.1006/nimg.2002.1275. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Magyari L, Hagoort P. Syntactic unification operations are reflected in oscillatory dynamics during on-line sentence comprehension. J Cogn Neurosci. 2009;21:2358–2368. doi: 10.1162/jocn.2009.21283. [DOI] [PubMed] [Google Scholar]
- Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical "up-state" transitions. Learn. Mem. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Whishaw IQ. Cortex, striatum and cerebellum: control of serial order in a grooming sequence. Exp. Brain Res. 1992;90:275–290. doi: 10.1007/BF00227239. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Warland DK, Meister M. The structure and precision of retinal spike trains. Proc. Natl. Acad. Sci. U S A. 1997;94:5411–5416. doi: 10.1073/pnas.94.10.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, Represa A, Ben-Ari Y, Cossart R. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- Borg-Graham LJ, Monier C, Frégnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg V, Schuz A. Anatomy of the cortex: statistics and geometry. Berlin: Springer-Verlag; 1991. [Google Scholar]
- Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004;427:704–710. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Kubischik M, Hoffmann KP, Krekelberg B. Neural dynamics of saccadic suppression. J. Neurosci. 2009;29:12374–12383. doi: 10.1523/JNEUROSCI.2908-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome BM, Jayaraman V, Laurent G. Encoding and decoding of overlapping odor sequences. Neuron. 2006;51:467–482. doi: 10.1016/j.neuron.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Buzsáki G, McClune MC. Coherence of compound field potentials reveals discontinuities in the CA1-subiculum of the hippocampus in freely-moving rats. Neuroscience. 1990;38:609–619. doi: 10.1016/0306-4522(90)90055-9. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Maass W. State-dependent computations: spatiotemporal processing in cortical networks. Nat. Rev. Neurosci. 2009;10:113–125. doi: 10.1038/nrn2558. [DOI] [PubMed] [Google Scholar]
- Burgess N, Barry C, O'Keefe J. An oscillatory interference model of grid cell firing. Hippocampus. 2007;17:801–812. doi: 10.1002/hipo.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for "noisy" brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7 Suppl 1:17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Buzsáki G, Buhl DL, Harris KD, Csicsvari J, Czéh B, Morozov A. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116:201–211. doi: 10.1016/s0306-4522(02)00669-3. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hip- pocampal EEG in the behaving rat. Brain. Res. Rev. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CK. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena JM, Lebedev MA, Crist RE, O'Doherty JE, Santucci DM, Dimitrov DF, Patil PG, Henriquez CS, Nicolelis MA. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol. 2003;1:E42. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin JK, Moxon KA, Markowitz RS, Nicolelis MAL. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999;2:664–670. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J. Neurosci. 1994;14:6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Churchland P, Sejnowski T. The Computational Brain. Cambridge MA: MIT Press; 1992. [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenböck G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Newsome WT. What electrical microstimulation has revealed about the neural basis of cognition. Curr. Opin. Neurobiol. 2004;14:1–9. doi: 10.1016/j.conb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Ann. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J. Neurophysiol. 2002;88:3487–3497. doi: 10.1152/jn.00188.2002. [DOI] [PubMed] [Google Scholar]
- Cover TM. Geometrical and statistical properties of systems of linear inequalities with applications in pattern recognition. IEEE Trans. Electronic Computers. 1965;14:326–334. [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Buzsáki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsáki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron. 2000;28:585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida L, Idiart M, Lisman JE. Memory retrieval time and memory capacity of the CA3 network: role of gamma frequency oscillations. Learn Mem. 2007;14:795–806. doi: 10.1101/lm.730207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCharms RC, Blake TD, Merzenich MM. Optimizing sound features for cortical neurons. Science. 1998;280:1439–1444. doi: 10.1126/science.280.5368.1439. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, et al. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc. Natl. Acad. Sci. U S A. 2007;104:5644–5649. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Feng G, Majewska AK, Miesenböck G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA, Prince DA. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. Spatial and temporal effects of spatial attention on human saccadic eye movements. Vision Research. 1989;32:293–304. doi: 10.1016/0042-6989(92)90140-e. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Paré D. The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat. Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K, Buzsáki G. Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments. J. Neurosci. 2008;28:13448–13456. doi: 10.1523/JNEUROSCI.3824-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP. Connecting cortex to machines: Recent advances in brain interfaces. Nat. Neurosci. 2002;5:1085–1088. doi: 10.1038/nn947. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Buzsáki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Harris KD, Buzsáki G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron. 2003;39:843–853. doi: 10.1016/s0896-6273(03)00465-3. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc. Natl. Acad. Sci. USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM. Neural Darwinism: The theory of neuronal group selection. New York: Basic Books; 1987. [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Spatial selectivity and theta phase precession in CA1 interneurons. Hippocampus. 2007;17:161–174. doi: 10.1002/hipo.20253. [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum HB, Davis JL. Neuronal ensembles: strategies for recording and decoding. New York: Wiley; 1998. [Google Scholar]
- Ellender TJ, Nissen W, Colgin LL, Mann EO, Paulsen O. Priming of hippocampal population bursts by individual perisomatic-targeting interneurons. J Neurosci. 2010;30:5979–5991. doi: 10.1523/JNEUROSCI.3962-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Euston DR, Tatsuno M, McNaughton BL. Fast-forward playback of recent memory sequences in prefrontal cortex during sleep. Science. 2007;318:1147–1150. doi: 10.1126/science.1148979. [DOI] [PubMed] [Google Scholar]
- Fee MS, Kozhevnikov AA, Hahnloser RH. Neural mechanisms of vocal sequence generation in the songbird. Ann N Y Acad Sci. 2004;1016:153–170. doi: 10.1196/annals.1298.022. [DOI] [PubMed] [Google Scholar]
- Fenton AA, Kao HY, Neymotin SA, Olypher A, Vayntrub Y, Lytton WW, Ludvig N. Unmasking the CA1 ensemble place code by exposures to small and large environments: more place cells and multiple, irregularly arranged, and expanded place fields in the larger space. J Neurosci. 2008;28:11250–11262. doi: 10.1523/JNEUROSCI.2862-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Ferster D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J Neurosci. 1986;6:1284–1301. doi: 10.1523/JNEUROSCI.06-05-01284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE. Operant conditioning of cortical unit activity. Science. 1969;163:955–957. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- Fetz EE. Volitional control of neural activity: implications for brain-computer interfaces. J Physiol. 2007;579:571–579. doi: 10.1113/jphysiol.2006.127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete IR, Senn W, Wang CZ, Hahnloser RH. Spike-Time-Dependent Plasticity and Heterosynaptic Competition Organize Networks to Produce Long Scale-Free Sequences of Neural Activity. Neuron. 2010;65:563–576. doi: 10.1016/j.neuron.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson MA. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Lubke J, Roth A, Frotscher M, Jonas P. Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron. 1997;18:1009–1023. doi: 10.1016/s0896-6273(00)80339-6. [DOI] [PubMed] [Google Scholar]
- Geisler C, Robbe D, Zugaro M, Sirota A, Buzsáki G. Hippocampal place cell assemblies are speed-controlled oscillators. Proc Natl Acad Sci U S A. 2007;104:8149–8154. doi: 10.1073/pnas.0610121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CCH. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gerstein GL, Bedenbaugh P, Aertsen AMHJ. Neuronal assemblies. Biomedical Engineering, IEEE Transactions. 1989;36:4–14. doi: 10.1109/10.16444. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Schmitz D, Empson RM, Heinemann U. Frequency-dependent information flow from the entorhinal cortex to the hippocampus. J Neurophysiol. 1997;78:3444–3449. doi: 10.1152/jn.1997.78.6.3444. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. U S A. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg S. Some networks that can learn, remember, and reproduce any number of complicated space-time patterns. J. Matt. Mechanics. 1969;19:53–91. [Google Scholar]
- Gulyas AI, Miles R, Sik A, Toth K, Tamamaki N, Freund TF. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993;366:683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Simon SA, Nicolelis MA. Licking-induced synchrony in the taste-reward circuit improves cue discrimination during learning. J. Neurosci. 2010;30:287–303. doi: 10.1523/JNEUROSCI.0855-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gütig R, Sompolinsky H. The tempotron: a neuron that learns spike timing-based decisions. Nat. Neurosci. 2006;9:420–428. doi: 10.1038/nn1643. [DOI] [PubMed] [Google Scholar]
- Haeusler S, Markram H, Maass W. Perspectives of the high dimensional dynamics of neural microcircuits from the point of view of low dimensional readouts. Complexity (Special Issue on Complex Adaptive Systems) 2003;8:39–50. [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. Sleep-related neural activity in a premotor and a basal-ganglia pathway of the songbird. J. Neurophysiol. 2006;96:794–812. doi: 10.1152/jn.01064.2005. [DOI] [PubMed] [Google Scholar]
- Halpern BP. Tasting and smelling as active, exploratory sensory processes. Am. J. Otolaryngol. 1983;4:246–249. doi: 10.1016/s0196-0709(83)80066-0. [DOI] [PubMed] [Google Scholar]
- Han X, Qian X, Bernstein JG, Zhou HH, Talei-Franzesi G, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel D, Sompolinsky H. Synchrony and computation in a chaotic neural network. Phys. Rev. Lett. 1992;68:718–721. doi: 10.1103/PhysRevLett.68.718. [DOI] [PubMed] [Google Scholar]
- Harris KD. Neural signatures of cell assembly organization. Nat. Rev. Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsáki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Hay J, Ilyn M, Gorchetchnikov A. Neuromodulation, theta rhythm and rat spatial navigation. Neural Netw. 2002;15:689–707. doi: 10.1016/s0893-6080(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Brandon MP, Yoshida M, Giocomo LM, Heys JG, Fransen E, Newman EL, Zilli EA. A phase code for memory could arise from circuit mechanisms in entorhinal cortex. Neural Netw. 2009;22:1129–1138. doi: 10.1016/j.neunet.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. New York: JohnWiley & Sons; 1949. [Google Scholar]
- Henson OW., Jr The activity and function of the middle-ear muscles in echo-locating bats. J. Physiol. 1965;180:871–887. doi: 10.1113/jphysiol.1965.sp007737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Buzsáki G. Action potential threshold of hippocampal pyramidal cells in vivo is increased by recent spiking activity. Neuroscience. 2001;105:121–130. doi: 10.1016/s0306-4522(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Hirabayashi T, Miyashita Y. Dynamically modulated spike correlation in monkey inferior temporal cortex depending on the feature configuration within a whole object. J. Neurosci. 2005;25:10299–10307. doi: 10.1523/JNEUROSCI.3036-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Czurkó A, Csicsvari J, Buzsáki G. Firing rate and theta-phase coding by hippocampal pyramidal neurons during 'space clamping. Eur. J. Neurosci. 1999;11:4373–4380. doi: 10.1046/j.1460-9568.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL. Coordinated reactivation of distributed memory traces in primate neocortex. Science. 2002;297:2070–2073. doi: 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J. Physiol. (Lond.) 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ, Tank DW. Computing with neural circuits: A model. Science. 1986;233:625–633. doi: 10.1126/science.3755256. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromádka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Low-frequency stimulation at the troughs of theta-oscillation induces long-term depression of previously potentiated CA1 synapses. J. Neurophysiol. 1996;75:877–884. doi: 10.1152/jn.1996.75.2.877. [DOI] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O'Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: Temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsáki G. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. Solving the distal reward problem through linkage of STDP and dopamine signaling. Cereb. Cortex. 2007;17:2443–2452. doi: 10.1093/cercor/bhl152. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Harvard University Press; 1890/1983. p. 1328. [Google Scholar]
- Jacobs J, Kahana MJ. Neural representations of individual stimuli in humans revealed by gamma-band electrocorticographic activity. J Neurosci. 2009;29:10203–10214. doi: 10.1523/JNEUROSCI.2187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal CA3 region predicts memory sequences: accounting for the phase precession of place cells. Learn. Mem. 1996;3:279–287. doi: 10.1101/lm.3.2-3.279. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Position reconstruction from an ensemble of hippocampal place cells: contribution of theta phase coding. J. Neurophysiol. 2000;83:2602–2609. doi: 10.1152/jn.2000.83.5.2602. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Jin DZ. Generating variable birdsong syllable sequences with branching chain networks in avian premotor nucleus HVC. Phys. Rev. E. 2009;80:051902. doi: 10.1103/PhysRevE.80.051902. [DOI] [PubMed] [Google Scholar]
- John ER. Mechanisms of memory. Oxford, England: Academic Press; 1967. [Google Scholar]
- Johnson A, Fenton AA, Kentros C, Redish AD. Looking for cognition in the structure within the noise. Trends Cogn. Sci. 2009;13:55–64. doi: 10.1016/j.tics.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Euston DR, Tatsuno M, McNaughton BL. Stored-trace reactivation in rat prefrontal cortex is correlated with down-to-up state fluctuation density. J. Neurosci. 2010;30 doi: 10.1523/JNEUROSCI.1617-09.2010. 2650-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Wu SM. Foundations of cellular neurophysiology. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jortner RA, Farivar SS, Laurent G. A Simple connectivity scheme for sparse coding in an olfactory system. J. Neurosci. 2007;27:1659–1669. doi: 10.1523/JNEUROSCI.4171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kelemen E, Fenton AA. Dynamic grouping of hippocampal neural activity during cognitive control of two spatial frames. PLoS Biol. 2010;8(6):e1000403. doi: 10.1371/journal.pbio.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso JAS. Dynamic patterns: The self-organization of brain and behavior. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Kennedy PR, Bakay RA. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998;9:1707–1711. doi: 10.1097/00001756-199806010-00007. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem. Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Kiebel SJ, von Kriegstein K, Daunizeau J, Friston KJ. Recognizing sequences of sequences. PLoS Compu Biol. 2009;5(8):e1000464. doi: 10.1371/journal.pcbi.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Henze DA, Leinekugel X, Buzsáki G. Hebbian modification of a hippocampal population pattern in the rat. J. Physiol. 1999;521:159–167. doi: 10.1111/j.1469-7793.1999.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321 doi: 10.1126/science.1157086. 140-114. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr. Opin. Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Koch C, Rapp M, Segev I. A Brief History of Time (Constants) Cereb. Cortex. 1996;6:93–101. doi: 10.1093/cercor/6.2.93. [DOI] [PubMed] [Google Scholar]
- Konishi M. Similar algorithms in different sensory systems and animals. Cold Spring Harb Symp Quant Biol. 1990;55:575–584. doi: 10.1101/sqb.1990.055.01.055. [DOI] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J. Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J. Neurophysiol. 1986;55 doi: 10.1152/jn.1986.55.6.1268. 1268-1128. [DOI] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, Joosten RN, McNaughton BL, Pennartz CM. Preferential reactivation of motivationally relevant information in the ventral striatum. J. Neurosci. 2008;28:6372–6382. doi: 10.1523/JNEUROSCI.1054-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansner A. Associative memory models: from cell assembly theory to biophysically detailed cortex simulations. Trends Neurosci. 2009;32:178–186. doi: 10.1016/j.tins.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Lashley KS. The problem of serial order in behavior. In: Jeffress LA, editor. In Cerebral Mechanisms in Behavior, The Hixon Symposium. New York: Wiley; 1951. pp. 112–136. [Google Scholar]
- Laurent G. A systems perspective on early olfactory coding. Science. 1999;286(5440):723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat. Rev. Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD. Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu. Rev. Neurosci. 2001;24:263–297. doi: 10.1146/annurev.neuro.24.1.263. [DOI] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Legendy CR. On the scheme by which the human brain stores information. Math. Biosci. 1967;1:555–597. [Google Scholar]
- Legenstein R, Maass W. Edge of chaos and prediction of computational performance for neural circuit models. Neural Netw. 2007;20:323–334. doi: 10.1016/j.neunet.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Legenstein R, Chase SM, Schwartz AB, Maass W. A reward-modulated hebbian learning rule can explain experimentally observed network reorganization in a brain control task. J. Neurosci. 2010;30:8400–8410. doi: 10.1523/JNEUROSCI.4284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS. Fast (beta) rhythms in the hippocampus: A review. Hippocampus. 2004;2:293–298. doi: 10.1002/hipo.450020202. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Levy WB, Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979;175:233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J. Comp. Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J. Psychophysical and physiological evidence for viewer-centered object representations in the primate. Cereb. Cortex. 1995;5:270–288. doi: 10.1093/cercor/5.3.270. [DOI] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyze temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente De Nó R. Analysis of the activity of the chains of internuncial neurons. J. Neurophysiol. 1938;1:207–244. [Google Scholar]
- Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–539. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass W, Markram H. Synapses as dynamic memory buffers. Neural Networks. 2002;15:155–161. doi: 10.1016/s0893-6080(01)00144-7. [DOI] [PubMed] [Google Scholar]
- Maass W, Natschlaeger T, Markram H. Real-time computing without stable states: A new framework for neural computation based on perturbations. Neural Compu. 2002;14:2531–2560. doi: 10.1162/089976602760407955. [DOI] [PubMed] [Google Scholar]
- MacLeod K, Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- MacLeod K, Bäcker A, Laurent G. Who reads temporal information contained across synchronized and oscillatory spike trains? Nature. 1998;395:693–698. doi: 10.1038/27201. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Marder CP, Buonomano DV. Differential effects of short- and long-term potentiation on cell firing in the CA1 region of the hippocampus. J. Neurosci. 2003;23:112–121. doi: 10.1523/JNEUROSCI.23-01-00112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc. Natl. Acad. Sci. USA. 1998;95:5323–5328. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 2007;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Marshall L, Henze DA, Hirase H, Leinekugel X, Dragoi G, Buzsáki G. Hippocampal pyramidal cell-interneuron spike transmission is frequency dependent and responsible for place modulation of interneuron discharge. J Neurosci. 2002;22:RC197. doi: 10.1523/JNEUROSCI.22-02-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masquelier T, Guyonneau R, Thorpe SJ. Competitive STDP-based spike pattern learning. Neural Comput. 2009;21:1259–1276. doi: 10.1162/neco.2008.06-08-804. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Vanrhoads SR, Sutherland GR, Lipa P, McNaughton BL. Self-motion and the origin of differential spatial scaling along the septo-temporal axis of the hippocampus. Hippocampus. 2005;15:841–852. doi: 10.1002/hipo.20114. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Organization of hippocampal cell assemblies based on theta phase precession. Hippocampus. 2006a;16:785–794. doi: 10.1002/hipo.20202. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Phase precession in hippocampal interneurons showing strong functional coupling to individual pyramidal cells. J. Neurosci. 2006b;26:13485–13492. doi: 10.1523/JNEUROSCI.2882-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor O, Laurent G. Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron. 2005;48:661–673. doi: 10.1016/j.neuron.2005.09.032. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McNaughton BL. Long-term synaptic enhancement and short-term potentiation in rat fascia dentata act through different mechanisms. J. Physiol. 1982;324:249–262. doi: 10.1113/jphysiol.1982.sp014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp. Brain Res. 1983;52:41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, Weaver KL. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J. Exp. Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- Megias Z, Emri TF, Freund, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Mickus T, Jung H, Spruston N. Properties of slow, cumulative sodium channel inactivation in rat hippocampal CA1 pyramidal neurons. Biophys. J. 1999;76:846–860. doi: 10.1016/S0006-3495(99)77248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G. The optogenetic catechism. Science. 2009;326:395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol (Lond) 1990;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner PM. The cell assembly: Mark II. Psychol. Rev. 1957;64:242–252. doi: 10.1037/h0042287. [DOI] [PubMed] [Google Scholar]
- Milner PM. Neural representations: some old problems revisited. J. Cog. Neurosci. 1996;8:69–77. doi: 10.1162/jocn.1996.8.1.69. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Sirota A, Pastalkova E, Buzsáki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, Fregnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–680. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Buzsáki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc. Natl. Acad. Sci. U S A. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu. Rev. Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Muller RU, Stead M, Pach J. The hippocampus as a cognitive graph. J Gen Physiol. 1996;107:663–694. doi: 10.1085/jgp.107.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, Buzsáki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Nessler B, Pfeiffer M, Maass W. In Proc. of NIPS 2009: Advances in Neural Information Processing Systems. Vol. 22. MIT Press; 2010. STDP enables spiking neurons to detect hidden causes of their inputs; pp. 1357–1365. [Google Scholar]
- Nicolelis MAL. Methods for neural ensemble recordings. CRC Press; 1999. [PubMed] [Google Scholar]
- Nicolelis MA, Lebedev MA. Principles of neural ensemble physiology underlying the operation of brain-machine interfaces. Nat. Rev. Neurosci. 2009;10:530–540. doi: 10.1038/nrn2653. [DOI] [PubMed] [Google Scholar]
- Niessing J, Friedrich RW. Olfactory pattern classification by discrete neuronal network states. Nature. 2010;465:47–52. doi: 10.1038/nature08961. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Huber D, Svoboda K. Reverse engineering the mouse brain. Nature. 2009;461:923–929. doi: 10.1038/nature08539. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; 1978. [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Senior TJ, Allen K, Huxter JR, Csicsvari J. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nat. Neurosci. 2008;11:209–215. doi: 10.1038/nn2037. [DOI] [PubMed] [Google Scholar]
- Palm G. Neural assemblies, an alternative approach to artificial intelligence. Secaucus, NJ: Springer-Verlag New York, Inc; 1982. [Google Scholar]
- Palm G. Computing with neural networks. Science. 1987;235:1227–1228. doi: 10.1126/science.235.4793.1227b. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL. The ventral striatum in off-line processing: ensemble reactivation during sleep and modulation by hippocampal ripples. J. Neurosci. 2004;24:6446–6456. doi: 10.1523/JNEUROSCI.0575-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297 doi: 10.1126/science.1070502. 359-356. [DOI] [PubMed] [Google Scholar]
- Pikovsky M, Rosenblum, Kurths J. Synchronization: a unified approach to nonlinear science. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Poggio T, Edelman S. A network that learns to recognize three-dimensional objects. Nature. 1990;343:263–266. doi: 10.1038/343263a0. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Kahana MJ. Memory search and the neural representation of context. Trends Cogn Sci. 2008;12:24–30. doi: 10.1016/j.tics.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port RF, Van Gelder T. Mind As Motion. Cambridge, MA: The MIT Press; 1995. p. 608. [Google Scholar]
- Pouget A, Dayan P, Zemel R. Information processing with population codes. Nat Rev Neurosci. 2000;1:125–132. doi: 10.1038/35039062. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. The neuroscience of language. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- Pulvermüller F. Brain embodiment of syntax and grammar: Discrete combinatorial mechanisms spelt out in neuronal circuits. Brain Lang. 2010;112:167–179. doi: 10.1016/j.bandl.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Knoblauch A. Discrete combinatorial circuits emerging in neural networks: A mechanism for rules of grammar in the human brain? Neural Networks. 2009;22:161–172. doi: 10.1016/j.neunet.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Quian Quiroga R, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single-neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Rabinovich MI, Varona P, Selverston AI, Abarbanel HDI. Dynamical principles in neuroscience. Rev.Mod. Physics. 2006;78:1213–1265. [Google Scholar]
- Rabinovich M, Huerta R, Laurent G. Transient dynamics for neural processing. Science. 2008a;321:48–50. doi: 10.1126/science.1155564. [DOI] [PubMed] [Google Scholar]
- Rabinovich MI, Huerta R, Varona P, Afraimovich VS. Transient cognitive dynamics, metastability, and decision making. PLoS Compu. Biol. 2008b;4:e1000072. doi: 10.1371/journal.pcbi.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function". Proc. Natl. Acad. Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J. Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J. Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat. Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rieke F, Warland D, de Ruyter van Steveninck R, Bialek W. Spikes: Exploring the neural code. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Robbe D, Buzsáki G. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. J. Neurosci. 2009;29:12597–12605. doi: 10.1523/JNEUROSCI.2407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumelhart DE, McLelland JL the PDP Research Group. Foundations. Cambridge, MA: MIT Press; 1986. Parallel Distributed Processing: Explorations in the Microstructure of cognition - Vol. 1. [Google Scholar]
- Rumelhart DE, Zipser D. the PDP Research Group. Feature discovery by competitive learning. In: Rumelhart DE, McClelland JL, editors. Parallel Distributed Processing: Explorations in the Microstructure of cognition, Vol. 1: Foundations. Cambridge, MA: MIT Press; 1986. pp. 151–193. [Google Scholar]
- Sakata JT, Brainard MS. Real-time contributions of auditory feedback to avian vocal motor control. J. Neurosci. 2006;26:9619–9628. doi: 10.1523/JNEUROSCI.2027-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64 doi: 10.1016/j.neuron.2009.09.020. 404-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y. How do cell assemblies encode information in the brain?”. Neurosci. Biobehav. Rev. 1999;23:785–796. doi: 10.1016/s0149-7634(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416:141–142. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- Seung HS. Learning in spiking neural networks by reinforcement of stochastic synaptic transmission. Neuron. 2003;40:1063–1073. doi: 10.1016/s0896-6273(03)00761-x. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Singer W. The formation of cooperative cell assemblies in the visual cortex. J. Exp. Biol. 1990;153:177–197. doi: 10.1242/jeb.153.1.177. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Sirota A, Buzsáki G. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat. Syst. 2005;3:245–259. doi: 10.1017/S1472928807000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky H, Kanter II. Temporal association in asymmetric neural networks. Phys. Rev. Lett. 1986;57:2861–2864. doi: 10.1103/PhysRevLett.57.2861. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, Kötter R. Identification and Classification of Hubs in Brain Networks. PLoS ONE. 2007:e1049. doi: 10.1371/journal.pone.0001049. doi:10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Wang C, Ulbert I, Schomer D, Halgren E. Human entorhinal gamma and theta oscillations selective for remote autobiographical memory. Hippocampus. 2010;20:166–173. doi: 10.1002/hipo.20597. [DOI] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Strangman G. Searching for cell assemblies: How many electrodes do I need? J. Compu. Neurosci. 1996;3:111–124. doi: 10.1007/BF00160807. [DOI] [PubMed] [Google Scholar]
- Sussillo D, Toyoizumi T, Maass W. Self-tuning of neural circuits through short-term synaptic plasticity. J. Neurophysiol. 2007;97:4079–4095. doi: 10.1152/jn.01357.2006. [DOI] [PubMed] [Google Scholar]
- Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, Scott EK, Kramer RH, Baier H, Trauner D, Isacoff EY. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54:535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science. 2008;322:960–963. doi: 10.1126/science.1161299. [DOI] [PubMed] [Google Scholar]
- Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP, Mercer A, Morris OT. Target and temporal pattern selection at neocortical synapses. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2002;357:1781–1791. doi: 10.1098/rstb.2002.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. U S A. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc. Natl. Acad. Sci. U S A. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, Hochberg LR, Donoghue JP. Collective dynamics in human and monkey sensorimotor cortex: Predicting single neuron spikes. Nat. Neurosci. 2010;13:105–111. doi: 10.1038/nn.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela FJ. Resonant cell assemblies: a new approach to cognitive functions and neuronal synchrony. Biol Res. 1995;28:81–95. [PubMed] [Google Scholar]
- Varela FJ, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Mack V, Sprengel R, Kavenstock N, Li KW, Stern-Bach Y, Smit AB, Seeburg PH, Monyer H. CKAMP44: A brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science. 2010;327:1518–1522. doi: 10.1126/science.1184178. [DOI] [PubMed] [Google Scholar]
- von der Malsburg C. The correlation theory of brain function. In: Domany E, van Hemmen JL, Schulten K, editors. Models of neural networks II: temporal aspects of coding and information. New York: Springer; 1994. [Google Scholar]
- Wallace DJ, Kerr JN. Chasing the cell assembly. Curr. Opin. Neurobiol. 2010;20:296–305. [PubMed] [Google Scholar]
- Wang Y, Markram H, Goodman PH, Berger TK, Ma J, Goldman-Rakic PS. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat. Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- Wang H, Peca J, Matsusaki M, Matsusaki K, Noguchi J, Qiu L, Wang D, Zhang F, Boyden ES, Deisseroth K, Kasai H, Hall WC, Feng G, Augustine GJ. High-speed mapping of synaptic connectivity using photostimulation in channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci. 2007;104:8143–8848. doi: 10.1073/pnas.0700384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HP, Spencer D, Fellous JM, Sejnowski TJ. Synchrony of thalamocortical inputs maximizes cortical reliability. Science. 2010;328:106–109. doi: 10.1126/science.1183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wennekers T, Sommer F, Aertsen A. Neuronal assemblies. Theory Biosci. 2003;122:1–104. [Google Scholar]
- Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408:361–365. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int. J. Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Webs, cell assemblies, and chunking in neural nets. Can. J. Exp. Psychol. 1999;53:118–131. doi: 10.1037/h0087304. [DOI] [PubMed] [Google Scholar]
- Wiebe SP, Staubli UV. Recognition memory correlates of hippocampal theta cells. J. Neurosci. 2001;21:3955–3967. doi: 10.1523/JNEUROSCI.21-11-03955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci. 2005;8:1364–1370. doi: 10.1038/nn1545. [DOI] [PubMed] [Google Scholar]
- Wilent WB, Nitz DA. Discrete place fields of hippocampal formation interneurons. J Neurophysiol. 2007;97:4152–4161. doi: 10.1152/jn.01200.2006. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bähner F, Both M, Tort AB, Kopell NJ, Wisden W, Monyer H. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106:3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, MacLean JN, Smith J, Lansner A. The cortex as a central pattern generator. Nat. Rev. Neurosci. 2005;6:477–483. doi: 10.1038/nrn1686. [DOI] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nature Rev. Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.