Abstract
We characterized agonist-induced internalization, recycling and downregulation of each muscarinic receptor subtype (M1 – M5) stably expressed in Chinese hamster ovary (CHO) cells. The radioligands [3H]QNB and [3H]NMS were used to measure the total and plasma membrane populations of muscarinic receptors, respectively. Following carbachol treatment (1 mM), the rank orders for the rate of carbachol-induced internalization of the muscarinic subtypes were M2 > M4 = M5 > M3 = M1, respectively. Unlike the M2 receptor, M1, M3, M4 and M5 receptors recycled back to the plasma membrane after one-hour carbachol treatment. The receptor downregulation elicited to 24-hour carbachol treatment was similar for M2, M3, M4 and M5 receptors, whereas that for the M1 receptor was greater. Our results indicate that there are subtype-specific differences in the rate and extent of agonist-induced muscarinic receptor internalization, recycling and downregulation in CHO cells.
Keywords: muscarinic, internalization, recycling, downregulation, carbachol
1. Introduction
Muscarinic acetylcholine receptors are G protein-coupled receptors and five subtypes (M1-M5) have been cloned (see review, Hulme et al., 1990). When expressed in cells lacking endogenous muscarinic receptors, M1, M3, and M5 receptors mediate stimulation of phosphoinositide hydrolysis, whereas M2 and M4 receptors mediate inhibition of adenylyl cyclase activity (Hammer, 1980; Kashihara et al., 1992). Consistent with the second messenger signaling, M1, M3, and M5 receptors couple with Gq/11 proteins and M2 and M4 receptors to couple with Gi/o proteins (DeLapp et al., 1999; Offermanns et al., 1994; Parker et al., 1991).
Typically, prolonged agonist exposure activates mechanisms that regulate G protein-coupled receptor activity and there are three major mechanisms: desensitization, internalization, and downregulation. Homologous receptor desensitization is thought to be initiated by phosphorylation of G protein-coupled receptors by G protein-coupled receptor kinases (GRKs), which causes the recruitment of β-arrestin and a subsequent reduction in second messenger signaling by uncoupling the receptor from associated G proteins (see review, Shenoy and Lefkowitz, 2003). β-arrestin binding also leads to receptor internalization via clathrin coated vesicles and β-arrestin has binding sites for the heavy chain of clathrin and the clathrin adaptor AP2 (Goodman et al., 1996; Laporte et al., 2000). Once internalized, G protein-coupled receptors may be recycled back to the plasma membrane or degraded in lysosomes in a process referred to as downregulation.
Agonist binding to muscarinic receptors, like other G protein-coupled receptors, leads to receptor desensitization, internalization, and downregulation depending upon the concentration and duration of agonist exposure. Several studies have characterized these agonist-dependent processes and subtype- and cell-specific differences have been observed. For instance, when exposed to the muscarinic agonist carbachol, M1 and M3 receptors internalized to a lesser extent than M2 and M4 receptors in CHO and COS-7 cells (Koenig and Edwardson, 1996; Tsuga et al., 1998b). The rate of carbachol-dependent M3 receptor internalization was approximately 10-fold greater in SH-SY5Y neuroblastoma cells compared to CHO cells transfected with a M3 receptor construct (Koenig and Edwardson, 1996). These are a few of many examples of subtype- and cell-specific differences in the agonist-dependent regulation of muscarinic receptors.
To date, the agonist-dependent internalization, recycling and downregulation of all five subtypes of muscarinic receptor have not been characterized in one cell type. This comparison is important because differences in the kinetics or extent of internalization, recycling or downregulation would be an indication that distinct mechanisms regulate the activity of muscarinic receptors in a subtype-specific manner. In this investigation, we compared the carbachol-dependent internalization and downregulation of M1-M5 receptors in CHO cells. We also compared the recycling of M1-M5 receptors after a brief treatment with carbachol. In general, M2 receptors internalized faster and more extensively than the other subtypes. M3, M4, and M5 receptors recycled more extensively than M1 and M2 receptors. Lastly, the extent of receptor downregulation elicited to 24 h carbachol treatment was greater for the M1 receptor than for the M2, M3, M4 and M5 receptors.
2. Materials and Methods
2.1 Cell culture
CHO cells stably expressing each subtype of muscarinic receptor (M1-M5) were obtained from Dr. Tom I. Bonner at the National Institutes of Mental Health. The preparation of these cell lines was described by Buckley and coworkers (Buckley et al., 1989). Cell lines were stored in the vapor phase of liquid nitrogen and revived in growth medium (F-12K, supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin). Cells were maintained in growth medium containing geneticin (500 μg/ml) in a humidified incubator set at 37°C in an atmosphere of 5% CO2/95% air and were subcultured every 2 to 3 days. Cells were passed a minimum of three times prior to use in experiments and were not passed more than twenty times. For experiments, cells (1.65 × 105 cells/well) were plated in 24-well plates in growth medium (500 μl) and incubated in a humidified incubator set at 37°C atmosphere of 5% CO2/95% air. After 24 h, medium in each well was exchanged for fresh growth medium (500 μl) and the cells were incubated in a humidified incubator for an additional 24 h (48 h total) before conducting experiments.
2.2 Receptor internalization assay
CHO cells stably expressing a subtype of muscarinic receptor were plated in 24-well plates as described above in section 2.1 “Cell culture”. Cells were washed with F-12K (3 × 500 μl; warmed to 37°C) to remove serum and then incubated with the muscarinic receptor agonist carbachol (1 mM) in F-12K (500 μl) for various periods of time for up to 4 h (6 wells for each time point) in a humidified incubator set at 37°C in an atmosphere of 5% CO2/95% air. This time period was chosen because the carbachol-induced internalization of each subtype of muscarinic receptor plateaued or nearly plateaued after 4 h of treatment. To remove carbachol and to prevent further receptor internalization, cells were washed extensively on ice with ice-cold PBS (3 × 500 μl). Intact, whole cell binding assays were then performed using a single concentration (1.6 nM) of the membrane impermeable muscarinic receptor selective radioligand [3H]N-methylscopolamine ([3H]NMS; PerkinElmer, Boston, MA) as described below in section 2.5 “Intact, whole cell receptor binding assays”. Specific [3H]NMS binding is used to determine the amount of receptor expressed on the plasma membrane of CHO cells.
2.3 Receptor recycling assay
CHO cells stably expressing a subtype of muscarinic receptor were plated in 24-well plates (2.5 plates) as described above in section 2.1 “Cell culture”. On the day of the experiment, plates were divided into two equal groups (1.25 plates per group) and cells were washed with F-12K (3 × 500 μl; warmed to 37°C) to remove serum. One group of cells was then incubated with F-12K (500 μl) for 1 h in a humidified incubator set at 37°C in an atmosphere of 5% CO2/95% air. This group of cells was used to determine the amount of receptor delivered to the plasma membrane in a constitutive manner. At the same time, the other group of cells was incubated with carbachol (1 mM) in F-12K (500 μl) for 1 h in a humidified incubator set at 37°C. This incubation time was chosen because it was the minimal time necessary to obtain maximal or nearly maximal receptor internalization for all five subtypes of receptor. This group of cells was used to measure the amount of constitutive receptor plasma membrane delivery plus recycled receptor. After this 1 h incubation, both groups of cells were washed on ice with F-12K (3 × 500 μl). This wash was conducted on ice to limit the amount of receptor trafficking. Both groups of cells were incubated with cyclized benzilylcholine mustard (BCM, 50 nM) for 5 min in a humidified incubator set at 37°C. BCM (10 μM) (obtained from Dr. Fred Ehlert, UC Irvine) was cyclized in PBS during a 30 min incubation at 37°C. The half-time for BCM cyclization is 2.5 min at 37°C, thus a 30 min incubation is adequate to ensure complete cyclization of BCM to its membrane-impermeable aziridinium form (Gill and Rang, 1966). Cells were washed on ice with F-12K (3 × 500 μl) to remove BCM and then incubated for various periods of time for up to 90 min (6 wells for each time point in untreated and carbachol treated plates) in a humidified incubator set at 37°C. Cells were then washed with ice-cold PBS (3 × 500 μl) and the amount of receptor expressed on the plasma membrane was determined using intact, whole cell [3H]NMS binding assays as described below in section 2.5 “Intact, whole cell receptor binding assays”.
As a control, 12 wells of a 24-well plate were plated at the same time as experimental plates. Each well of the control plate was washed with F-12K (3 × 500 μl) to remove serum and then incubated in the absence (6 wells) or presence of carbachol (1 mM; 6 wells) for 1 h in an incubator set at 37°C in an atmosphere of 5% CO2/95% air. Each well was washed with ice-cold PBS (3 × 500 μl) and then used in intact, whole cell [3H]NMS binding assays. The specific [3H]NMS binding of untreated wells was used to normalize specific binding of experimental plates. The specific [3H]NMS binding of carbachol treated wells was used to determine how much internalization occurred during the 1 h treatment with carbachol.
2.4 Receptor downregulation assay
CHO cells stably expressing each subtype of muscarinic receptor were plated in a 24-well plate as described above under section 2.1 “Cell culture”. Plates were divided into two equal groups (12 wells per group) and cells were washed with F-12K (3 × 500 μl) to remove serum. One group of wells was incubated with F-12K (500 μl) for 24 h in a humidified incubator set at 37°C and in an atmosphere of 5% CO2/95% air. This group of wells was used as a control. The other group of wells was incubated with carbachol (1 mM) in F-12K (500 μl) for 24 h to induce receptor downregulation. We chose this incubation time because Shockley and coworkers (Shockley et al., 1997) showed that muscarinic receptor downregulation is a slow process that requires approximately 24 h for a substantial loss of receptor to be observed. Cells from both groups were washed extensively on ice with ice-cold PBS (3 × 500 μl). Intact, whole cell binding assays were then performed using a single concentration of [3H]NMS (6 wells in untreated and carbachol treated plates) to determine the amount of plasma membrane expressed receptor. To determine the total amount of muscarinic receptor in CHO cells, intact, whole cell binding assays using a single concentration of [3H]3-quinuclidinyl benzylate (6 wells in untreated and carbachol treated plates) ([3H]QNB; PerkinElmer, Boston, MA) were also performed as described below in section 2.5 “Intact, whole cell receptor binding assays.”
2.5 Intact, whole cell receptor binding assays
Intact, whole cell binding assays were performed using either a single concentration of [3H]NMS (1.6 nM) or [3H]QNB (1.2 nM). [3H]NMS was used to determine the amount of plasma membrane expressed receptor and [3H]QNB was used to determine the total receptor expressed. 1.6 nM [3H]NMS should occupy approximately 86%, 72%, 84%, 94% and 60% of human muscarinic M1, M2, M3, M4 and M5 receptors, respectively (Ehlert et al., 1996). 1.2 nM [3H]QNB should occupy approximately 97%, 98%, 93%, 97% and 96% of human muscarinic M1, M2, M3, M4 and M5 receptors, respectively (Bolden et al., 1992). In each assay (i.e., internalization, downregulation and recycling) a control was used to determine specific [3H]NMS or [3H]QNB binding in untreated CHO cells expressing a particular receptor subtype. All other binding data from each assay was divided by the control binding and then reported as percent of control. Subtype-specific differences in affinity for [3H]NMS and [3H]QNB were accounted for by this transformation.
Washed cells were incubated with either [3H]NMS or [3H]QNB in the absence (three wells for each time point; total binding) and presence (three wells for each time point; nonspecific binding) of atropine (10 μM) in 500 μl binding buffer (25 mM HEPES, 113 mM NaCl, 6 mM dextrose, 3 mM CaCl2, 3 mM KCl, 2 mM MgSO4, 1 mM NaH2PO4, pH 7.4) for either one h ([3H]NMS) or 18 h ([3H]QNB) at 4°C. Unbound [3H]NMS or [3H]QNB was then removed by rapidly and gently washing cells with ice-cold PBS (2 × 1 ml). Bound [3H]NMS or [3H]QNB was recovered as described previously (Griffin et al., 2003). Recovered radioactivity was pipetted into scintillation vials and 5 ml of scintillation cocktail (Scintiverse BD cocktail, Fisher Scientific, Fairlawn, NJ) was added prior to counting using a Beckman LS 6500 scintillation counter.
The average amount of protein expressed in CHO cells was determined for each radioligand binding assay performed. Briefly, three wells of a 24-well plate were plated at the same time as experimental plates as described above in section 2.1 “Cell culture”. Cells were treated the same as the cells in each experiment performed and then cells were washed two times with 500 μl mannitol wash buffer (0.29 M mannitol, 0.01 M Tris, 0.5 mM Ca(NO3)2, pH 7.4). The protein concentration was determined for each well using the bicinchoninic acid (BCA) protocol as described previously (Goldschmidt and Kimelberg, 1989).
2.6 Filtration receptor binding assays
To verify BCM irreversibly binds muscarinic receptors, CHO M5 cells were plated (5 × 106 cells/plate) in six 10-cm dishes in growth medium (15 ml, see section 2.1 “Cell culture”). On the following day, cells were washed three times with F12K (15 ml) to remove serum. Five of the six plates were incubated with cyclized BCM (50 nM) in F12K (15 ml) for 5 min at 37°C. The remaining plate was incubated in F12K for 5 min at 37°C. Cells on each plate were rapidly washed (3 times, 15 ml) with ice cold PBS to remove unbound BCM, then incubated 5 min at room temperature with 5 ml hypotonic buffer (1 mM Tris-HCl, 1mM EGTA, 1mM MgCl2, 120 mM sucrose, pH 7.6) (Chang et al., 1981). Cells were scraped from plates and transferred into individual tubes (15 ml conical), then homogenized using a Tissue-TearorTM on setting five (15 sec). The untreated homogenate was retained on ice. The BCM treated homogenates were incubated in an humidified incubator set at 37°C for 0, 15, 30, 60 or 90 min, then retained on ice. Aliquots of homogenates (100 μl) were then incubated in 12 × 75 mm polypropylene tubes with [3H]NMS (1.6 nM) in binding buffer (see section 2.5 “Intact, whole cell receptor binding assays”, 400 μl) in the absence (3 tubes, total binding) and presence (3 tubes, nonspecific binding) of atropine (10 μM) for 1 h at 4°C with constant shaking. Bound [3H]NMS was trapped on Whatman glass-fiber filters (GF/B fired, Clifton, NJ) using a cell harvester (Brandel, Gaithersburg, MD). The filters were washed three times with approximately three ml of ice-cold saline (0.9%) each wash. The assay was repeated twice.
To verify that [3H]QNB permeates intracellular compartments containing muscarinic receptor in intact, whole CHO cells, CHO M1 cells were plated (5 × 106 cells/plate) in two 10-cm dishes in growth medium (15 ml, see section 2.1 “Cell culture”). On the following day, cells were washed three times with sterile F12K (15 ml) to remove serum. Plates were incubated 24 h in a humidified incubator set at 37°C in an atmosphere of 5% CO2/95% air in the absence (one plate) and presence (one plate) of carbachol (1 mM). Cells on each plate were washed (3 times, 15 ml) with ice cold PBS to remove carbachol, then incubated 5 min at room temperature with hypotonic buffer (5 ml). Cells were scraped from plates and transferred into individual tubes (15 ml conical), then homogenized using a Tissue-TearorTM on setting five (15 sec). The homogenates were pelleted (100,000 × g, 1 h) and resuspended in 1 ml binding buffer (see section 2.5 “Intact, whole cell receptor binding assays”) containing protease inhibitor (Complete Mini EDTA-free protease inhibitor cocktail tablet, Roche). Aliquots of homogenates (100 μl) were then incubated in 12 × 75 mm polypropylene tubes with [3H]QNB (1.6 nM) in binding buffer (400 μl) in the absence (3 tubes, total binding) and presence (3 tubes, nonspecific binding) of atropine (10 μM) for 6 h at 4°C with constant shaking. Bound [3H]QNB was trapped on Whatman glass-fiber filters (GF/B fired, Clifton, NJ) using a cell harvester (Brandel, Gaithersburg, MD). The filters were washed three times with approximately three ml of ice-cold saline (0.9%) each wash. The assay was repeated twice.
2.7 Data analysis
Significance values (p values) were calculated using either Student's t-test, one-way ANOVA with Tukey post-hoc test (GraphPad Prism, ver. 5.01; San Diego, CA), or two-way ANOVA with Bonferroni post-hoc test and are reported where appropriate (GraphPad Prism, ver. 5.01). Data with a p value < 0.05 was considered significant. Estimates of the rate constants for muscarinic M1-M5 receptor internalization were made by fitting data using a single-phase exponential decay equation (GraphPad Prism, ver. 5.01). Estimates of the rate constants for the plasma membrane delivery of M1-M5 receptors in untreated and carbachol treated cells were made by fitting data using a single-phase exponential association equation (GraphPad Prism, Ver 5.01).
3. Results
3.1 Comparison of the kinetics and extent of muscarinic M1-M5 receptor internalization
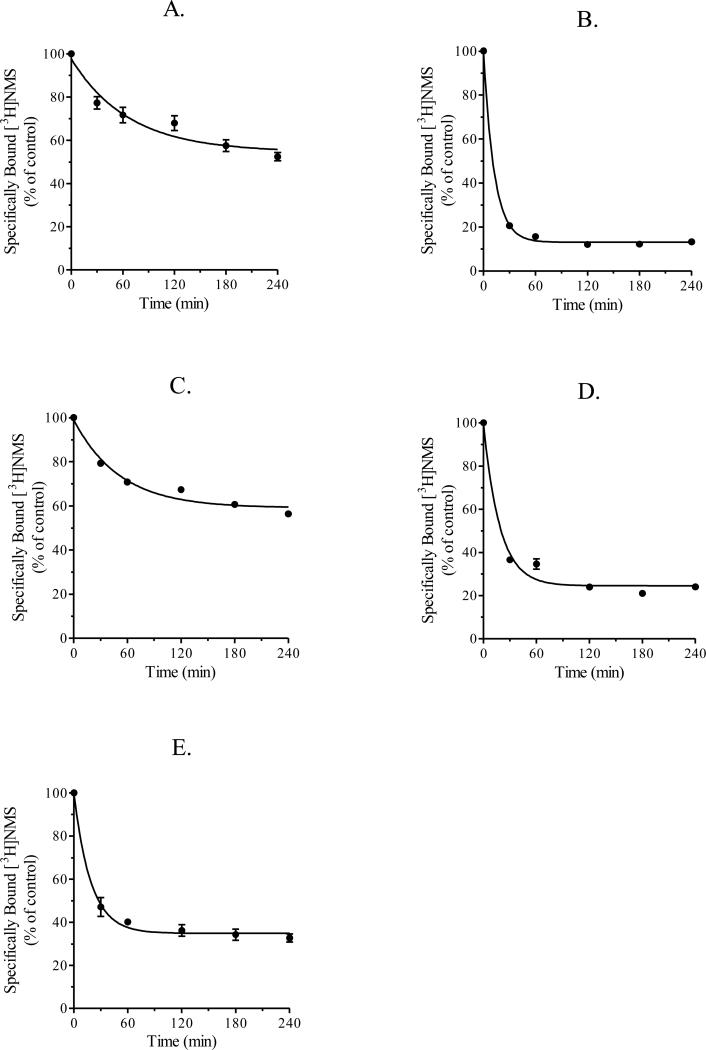
We investigated the kinetics of agonist-induced internalization of human muscarinic M1-M5 receptors stably expressed in CHO cells. CHO cells expressing muscarinic M1-M5 receptors were incubated with the muscarinic receptor agonist carbachol (1 mM) for various times up to four h and then receptor binding at the cell surface was measured using a single concentration of [3H]NMS (1.6 nM). As shown in Figure 1, these data were consistent with a first-order decay process.
Figure 1.
Internalization of muscarinic M1-M5 receptors. CHO cells stably expressing muscarinic M1 (A), M2 (B), M3 (C), M4 (D) or M5 (E) receptors were incubated with carbachol (1 mM) for various periods of time for up to 240 min at 37°C. Cells were then used in intact, whole cell [3H]NMS binding assays as described in section 2.5 “Intact, whole cell receptor binding assays”. Each data point represents the mean ± S.E.M. of three experiments conducted in triplicate.
The estimates of the rate constant for carbachol-induced internalization of each subtype over the time interval of 0 to 4 h are listed in Table 1. The M2 receptor internalized faster than M1, M3, M4 and M5 receptors. The half-time (t½) for M2 receptor internalization was 8.6 min and specific [3H]NMS binding decreased 87% during the 4 h treatment with carbachol (Figure 1B and Table 1). The half-times for the carbachol-induced internalization of M1 and M3 receptors were similar (t½ = 46.3 min and 35.5 min, respectively), but were significantly longer than that for M2 receptors (Figure 1A and 1C, Table 1). Specific [3H]NMS binding decreased 45% and 41% in CHO cells expressing M1 and M3 receptors, respectively, during carbachol treatment (Figure 1A and 1C, Table 1). The half-times for M4 and M5 receptor internalization were also similar (t½ = 12.8 min and 13.1 min, respectively) and significantly longer than that for M2 receptors (Figures 1D and 1E, Table 1). During 4 h carbachol treatment, specific [3H]NMS binding decreased 75% in CHO cells expressing M4 receptors and 65% in CHO cells expressing M5 receptors (Figures 1D and 1E, Table 1).
Table 1.
Comparison of specific [3H]NMS binding and rate constants for M1-M5 receptor internalizationa.
| Muscarinic Receptor Subtypeb | Specific [3H]NMS Binding (fmol/mg protein) |
K (min-1)d,e | Plateau (% of control)i,j | |
|---|---|---|---|---|
| Control | Carbachol treatedc | |||
| M1 (3) | 1395.2 ± 42.5 | 731.5 ± 34.1 | 0.015 ± 0.004f | 54.6 ± 3.4k |
| M2 (3) | 177.6 ± 12.8 | 23.8 ± 3.0 | 0.080 ± 0.005g | 13.0 ± 0.53l |
| M3 (3) | 549.4 ± 42.7 | 309.3 ± 21.2 | 0.020 ± 0.002h | 59.1 ± 1.4m |
| M4 (3) | 439.2 ± 16.3 | 105.5 ± 5.5 | 0.054 ± 0.006 | 24.5 ± 1.6n |
| M5 (3) | 262.6 ± 31.6 | 84.6 ± 5.0 | 0.053 ± 0.007 | 34.8 ± 1.4 |
Data from Figure 1.
Number of experiments is shown in parenthesis.
Binding observed after 4 h of carbachol treatment.
The rate constant for internalization was determined by fitting data shown in Figure 1 to a single-phase decay equation (see section 2.7, “Data analysis”).
The rate constants for carbachol-induced internalization differed significantly across the receptor subtypes (F (4, 10) = 43.7, p < 0.0001) as determined by one-way ANOVA.
Tukey post-hoc comparisons indicate that the rate constant (K) of M1 receptors (95% CI [0.007, 0.02]) is significantly different (p < 0.01) from M2 (95% CI [0.07, 0.09]), M4 (95% CI [0.04, 0.07]), and M5 (95% CI [0.04, 0.07]) receptors.
Tukey post-hoc comparisons indicate that the rate constant (K) of M2 receptors (95% CI [0.07, 0.09]) is significantly different (p < 0.01) from M3 (95% CI [0.01, 0.03]), M4 (95% CI [0.04, 0.07]), and M5 (95% CI [0.04, 0.07]) receptors.
Tukey post-hoc comparisons indicate that the rate constant (K) of M3 receptors (95% CI [0.01, 0.03]) is significantly different (p < 0.01) from M4 (95% CI [0.04, 0.07]) and M5 (95% CI [0.04, 0.07]) receptors.
The plateau for internalization was determined by fitting data shown in Figure 1 to a single-phase decay equation (see section 2.7, “Data analysis”).
The plateaus for carbachol-induced internalization differed significantly across the receptor subtypes (F (4, 10) = 253.2, p < 0.0001) as determined by one-way ANOVA.
Tukey post-hoc comparisons indicate that the plateau of M1 receptors (95% CI [47, 62]) is significantly different from M2 (95% CI [12, 14], p < 0.001), M3 (95% CI [56, 62], p < 0.05), M4 (95% CI [22, 27], p < 0.001), and M5 (95% CI [32, 38], p < 0.001) receptors.
Tukey post-hoc comparisons indicate that the plateau of M2 receptors (95% CI [12, 14]) is significantly different (p < 0.001) from M3 (95% CI [56, 62]), M4 (95% CI [22, 27]), and M5 (95% CI [32, 38]) receptors.
Tukey post-hoc comparisons indicate that the plateau of M3 receptors (95% CI [56, 62]) is significantly different (p < 0.001) from M4 (95% CI [22, 27]), and M5 (95% CI [32, 38]) receptors.
Tukey post-hoc comparisons indicate that the plateau of M4 receptors (95% CI [22, 27]) is significantly different (p < 0.001) from M5 (95% CI [32, 38]) receptors.
3.2 Comparison of M1-M5 receptor downregulation
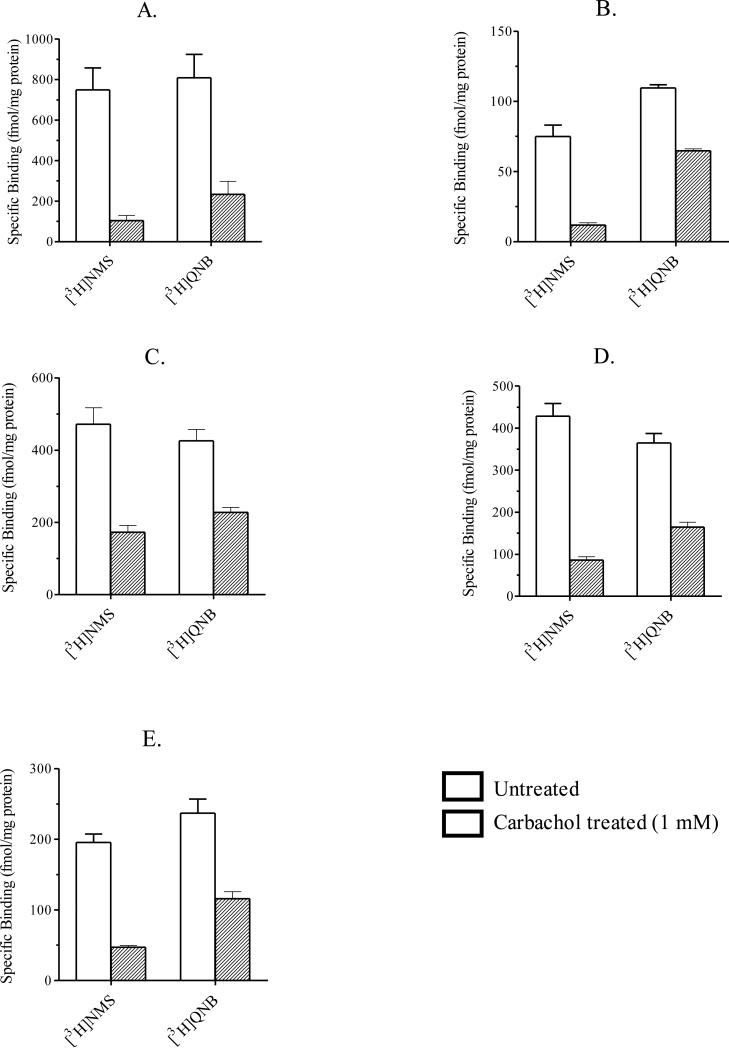
[3H]QNB is a membrane permeable muscarinic receptor selective antagonist that is used to determine the total amount of muscarinic receptor expressed in cells. In our investigation, we used [3H]QNB to assess the change in total M1-M5 receptor expressed in intact, whole CHO cells after 24 h carbachol treatment (i.e., receptor downregulation) (see section 2.4, “Receptor downregulation assay”). To determine whether [3H]QNB can permeate intracellular compartments containing muscarinic receptors, we incubated CHO M1 cells in the absence and presence of carbachol (1 mM) for 24 h. Untreated and carbachol treated cells were ruptured using a hypotonic buffer and the resulting membranes were washed and pelleted (see section 2.6, “Filtration receptor binding assays”). Untreated and carbachol treated membranes were then used in [3H]QNB binding assays as described in section 2.6, “Filtration receptor binding assays”. We found that 24 h carbachol treatment caused a 78% reduction in specific [3H]QNB binding in carbachol treated membranes compared to untreated membranes. This reduction was comparable to that observed in intact, whole cell [3H]QNB binding assays conducted on CHO cells expressing M1 receptors after 24 h carbachol treatment (1 mM) (see Figure 2A and Table 2). These data suggest that [3H]QNB permeates intracellular compartments in intact, whole CHO cells. Consequently, we used intact, whole cell binding assays to measure M1-M5 receptor downregulation elicited to 24 h carbachol treatment.
Figure 2.
Downregulation of muscarinic M1-M5 receptors. CHO cells stably expressing muscarinic M1 (A), M2 (B), M3 (C), M4 (D) or M5 (E) receptors were incubated in the absence (open bars) and presence (closed bars) of carbachol (1 mM) for 24 h at 37°C. Cells were washed and then used in intact, whole cell binding assays using either [3H]NMS or [3H]QNB as described in section 2.5 “Intact, whole cell receptor binding assays”. Each bar represents the mean ± S.E.M. of three experiments conducted in triplicate.
Table 2.
Comparison of the specific [3H]NMS and [3H]QNB binding for M1-M5 receptors in CHO cells incubated in the absence and presence of carbachol (1 mM) 24 hoursa.
| Receptor Subtype/Conditionb | Specific [3H]NMS Binding (fmol/mg protein) | Specific [3H]NMS Binding Remaining after Treatment (%)c | Specific [3H]QNB Binding (fmol/mg protein) | Specific [3H]QNB Binding Remaining after Treatment (%)g |
|---|---|---|---|---|
| M1 (3) | ||||
| Untreated | 748.5 ± 109.2 | 808.1 ± 116.7 | ||
| 24 h | 103.2 ± 26.6 | 13.4 ± 1.6d | 233.0 ± 64.6 | 27.8 ± 3.6h |
| M2 (3) | ||||
| Untreated | 74.9 ± 8.3 | 109.8 ± 2.3 | ||
| 24 h | 11.8 ± 1.6 | 15.7 ± 0.6e | 64.9 ± 1.4 | 59.1 ± 0.1 |
| M3 (3) | ||||
| Untreated | 471.0 ± 45.9 | 425.6 ± 31.1 | ||
| 24 h | 172.2 ± 18.8 | 36.6 ± 1.5f | 227.2 ± 13.4 | 53.5 ± 2.0 |
| M4 (3) | ||||
| Untreated | 428.2 ± 30.3 | 364.4 ± 22.6 | ||
| 24 h | 86.0 ± 8.1 | 20.1 ± 0.9 | 164.6 ± 11.5 | 45.1 ± 0.8 |
| M5 (3) | ||||
| Untreated | 195.4 ± 12.1 | 237.1 ± 20.0 | ||
| 24 h | 47.0 ± 2.5 | 24.3 ± 2.2 | 115.9 ± 10.0 | 50.0 ± 7.6 |
Data from Figure 2.
Number of experiments are shown in parenthesis.
The specific [3H]NMS binding remaining after 24 h carbachol treatment (1 mM) differed significantly across the receptor subtypes (F (4, 10) = 39.9, p < 0.0001) as determined by one-way ANOVA.
Tukey post-hoc comparisons indicate that the [3H]NMS binding remaining in CHO M1 cells (95% CI [7.5, 20]) is significantly different from M3 (95% CI [30, 43], p < 0.001) and M5 (95% CI [15, 34], p < 0.01) receptor CHO cells.
Tukey post-hoc comparisons indicate that the [3H]NMS binding remaining in CHO M2 cells (95% CI [13, 18]) is significantly different from M3 (95% CI [30, 43], p < 0.001) and M5 (95% CI [15, 34], p < 0.05) receptor CHO cells.
Tukey post-hoc comparisons indicate that the [3H]NMS binding remaining in CHO M3 cells (95% CI [30, 43]) is significantly different from M4 (95% CI [16, 24], p < 0.001) and M5 (95% CI [15, 34], p < 0.001) receptor CHO cells.
The specific [3H]QNB binding remaining after 24 h carbachol treatment (1 mM) differed significantly across the receptor subtypes (F (4, 10) = 9.4, p < 0.01) as determined by one-way ANOVA.
Tukey post-hoc comparisons indicate that the [3H]QNB binding remaining in CHO M1 cells (95% CI [12, 43]) is significantly different from M2 (95% CI [58, 59], p < 0.01), M3 (95% CI [42, 62], p < 0.01) and M5 (95% CI [17, 82], p < 0.05) receptor CHO cells.
In downregulation assays, CHO cells expressing M1-M5 receptors were incubated with carbachol (1 mM) for 24 h. Residual receptors were measured using a single concentration of [3H]QNB (see section 2.5, “Intact, whole cell receptor binding assays”). Following carbachol-treatment, specific [3H]QNB binding in CHO cells expressing muscarinic M1, M2, M3, M4 and M5 receptors decreased by 72.2 ± 3.6%, 40.9 ± 0.1%, 46.5 ± 2.0%, 54.9 ± 0.8% and 50.0 ± 7.6%, respectively (Figure 2 and Table 2).
We also determined specific [3H]NMS binding in intact, whole CHO cells expressing M1-M5 receptors after 24 h of carbachol treatment (1 mM). In CHO cells expressing M1, M2, M3, M4 and M5 receptors, specific [3H]NMS binding decreased 86.6 ± 1.6%, 84.3 ± 0.6%, 63.4 ± 1.5%, 79.9 ± 0.9% and 75.7 ± 2.2%, respectively (Figure 2 and Table 2). When comparing these [3H]NMS binding data with the data in Figure 1, it can be seen that the loss of M1, M3 and M5 receptors consists of two components. A rapid component occurs during a short-term treatment with carbachol, and is nearly complete in approximately 4.5 half times (1 - 3.5 h) (Figure 1A, 1C, and 1E). Longer treatment with carbachol (24 h), however, caused a further loss of M1, M3 and M5 receptors (Figure 2A, 2C, and 2E). This slower component was not apparent with M2 and M4 receptors (see Figures 1B and 1D, 2B and 2D).
3.3 Recycling of muscarinic receptors
The assay for receptor recycling in CHO cells consisted of three sequential phases: 1) incubation of cells with carbachol (1 mM, 1 hr) followed by washing, 2) treatment with BCM followed by washing, and 3) measurement of the binding of [3H]NMS (1.6 nM) at various times up to 90 min. An increase in phase 3 binding in response to phase 1 carbachol treatment was defined as recycling. Control cells received no carbachol during phase 1.
BCM is an irreversible muscarinic receptor selective antagonist that alkylates muscarinic M1-M5 receptors. To ensure selective alkylation of plasma membrane expressed muscarinic receptor, we cyclized BCM at 37°C for 30 min, which results in a 99.99% conversion of the parent mustard to a membrane impermeable quaternary aziridinium ion (Gill and Rang, 1966). We then incubated CHO cells stably expressing M3 receptors with increasing concentrations of cyclized BCM (10 nM, 50 nM, and 100 nM) for either 5 min or 15 min (data not shown) at 37°C. From these data, we determined that a 5 min treatment with 50 nM BCM was adequate to alkylate greater than 95% of plasma membrane expressed M3 receptor (data not shown). Since a majority of M3 receptors were akylated using these conditions, we incubated CHO cells at 37°C for 5 min with BCM (50 nM) in phase 2 of recycling experiments. Additionally, this short treatment with BCM should minimize the amount of receptor trafficking to the plasma membrane during the alkylation phase (phase 2) of recycling experiments.
We performed intact, whole cell [3H]NMS binding assays (see section 2.5 “Intact, whole cell receptor binding assays”) on untreated and BCM treated CHO cells expressing M1- M5 receptors. When compared to untreated cells, specific [3H]NMS binding in BCM treated cells (5 min, 50 nM) expressing M1, M2, M3, M4 and M5 receptors decreased 90.4 ± 1.8%, 93.9 ± 1.0%, 97.0 ± 0.3%, 94.3 ± 0.7% and 85.4 ± 2.7%, respectively. We also performed filtration binding assays (see section 2.6 “Filtration receptor binding assays”) on homogenates of untreated and BCM treated CHO cells expressing M5 receptors. Intact, whole CHO M5 cells were incubated at 37°C for 5 min in the absence (control) and presence of cyclized BCM (50 nM). Cells were washed and homogenized and the untreated homogenate was retained on ice. The BCM treated homogenates were incubated for 0, 15, 30, 60 and 90 min at 37°C. 5 min BCM treatment caused a 82% reduction in specific [3H]NMS binding and a similar reduction was observed after 15 (83%), 30 (82%), 60 (84%) and 90 (85%) min incubations at 37°C. These data indicate that BCM irreversibly alkylates the plasma membrane expressed M5 receptor under conditions similar to those used in recycling assays. Presumably, BCM irreversibly alkylates M1-M4 receptors in recycling assays as well.
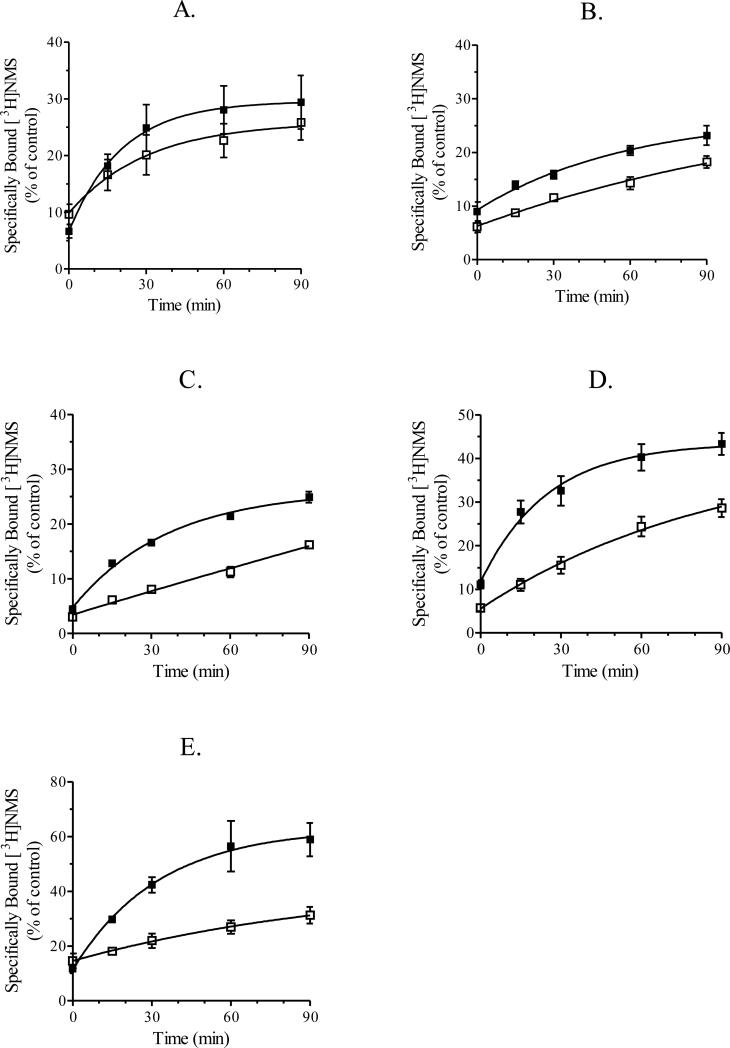
In CHO cells expressing the muscarinic M2 receptor, 1 h carbachol treatment (1 mM) caused a 79% reduction in specific [3H]NMS binding (Table 3). After BCM alkylation (50 nM, 5 min), the initial rate of recovery of [3H]NMS binding in CHO cells expressing the M2 receptor was comparable in untreated and carbachol-treated cells, indicating no significant recycling (Figure 3B and Table 3).
Table 3.
Comparison of the recovery of specific [3H]NMS binding for M1-M5 receptors in untreated and carbachol (1 mM) treated cellsa.
| Receptor Subtype/Conditionb | Amount Internalizedc (fmol/mg protein) | Initial Rated (% of control min-1) | Recovery of Specific [3H]NMS Bindinge (% of control) |
|---|---|---|---|
| M1 (5) | |||
| Untreated | - | 0.55 ± 0.15 | 16.2 ± 1.6 |
| 1 h | 262.8 ± 42.7 | 1.17 ± 0.13f | 22.8 ± 3.7f |
| M2 (3) | |||
| Untreated | - | 0.28 ± 0.03 | 12.1 ± 0.6 |
| 1 h | 204.9 ± 3.0 | 0.41 ± 0.03 | 14.2 ± 0.1 |
| M3 (3) | |||
| Untreated | - | 0.16 ± 0.01 | 13.2 ± 0.2 |
| 1 h | 186.4 ± 27.4 | 0.61 ± 0.07f | 20.5 ± 0.9f |
| M4 (3) | |||
| Untreated | - | 0.42 ± 0.07 | 22.9 ± 1.7 |
| 1 h | 364.2 ± 23.0 | 1.30 ± 0.21f | 32.2 ± 1.6f |
| M5 (3) | |||
| Untreated | - | 0.27 ± 0.10 | 16.6 ± 5.3 |
| 1 h | 96.1 ± 2.1 | 1.67 ± 0.11f | 47.1 ± 7.3f |
Data from Figure 3.
Number of experiments are shown in parenthesis and the duration of carbachol treatment (1 mM) is indicated.
The amount of receptor internalized during carbachol treatment was calculated by subtracting specific [3H]NMS binding for carbachol treated control cells from that obtained for untreated control cells (see section 2.3, “Receptor recycling assay”).
The initial rate was determined for untreated and carbachol treated cells by fitting data shown in Figure 3 to a single-phase association equation (see section 2.7, “Data analysis”).
Recovery of specific [3H]NMS binding was calculated by subtracting specific binding at 0 min after BCM treatment from that at 90 min after BCM treatment.
Significantly different from untreated cells (p < 0.05) as determined using a paired Student's t test (two-tailed).
Figure 3.
Recycling of muscarinic M1-M5 receptors. CHO cells stably expressing muscarinic M1 (A), M2 (B), M3 (C), M4 (D) or M5 (E) receptors were incubated with (■) or without (□) carbachol for 1 h. Cells were then washed and treated with cyclized BCM (50 nM) for 5 min as described in section 2.3 “Receptor recycling assay”. Cells were then incubated for various periods of time for up to 90 minutes at 37°C and then used in intact, whole cell [3H]NMS binding assays as described in section 2.5 “Intact, whole cell receptor binding assays”. Each data point represents the mean ± S.E.M. of three to five experiments conducted in triplicate.
Unlike the M2 receptor, M1, M3, M4, and M5 receptors recycled after carbachol-induced internalization, albeit at different rates and to different extents. When expressed relative to control, the initial rate of recovery of [3H]NMS binding was 2.5 ± 0.4-, 3.8 ± 0.5-, 3.1 ± 0.01- and 8.9 ± 3.9-fold greater in carbachol treated cells expressing M1, M3, M4 and M5 receptors, respectively. The corresponding increases in [3H]NMS binding at 90 min were 1.4 ± 0.1-, 1.6 ± 0.1-,1.4 ± 0.03-fold, and 2.8 ± 1.0-fold. These data are summarized in Table 3 and Figure 3A, C-E.
4. Discussion
Each subtype of human muscarinic receptor was stably expressed in CHO cells using a viral promoter (i.e., CMV). Consequently, the wild-type transcriptional control of the receptor subtypes was lost. However, agonist-dependent internalization, recycling and downregulation of muscarinic receptors was observed and several proteins that play a role in these agonist-dependent processes (e.g., G protein-coupled receptor kinases, β-arrestin, clathrin, dynamin, etc.) are expressed in CHO cells (Gaborik et al., 2001; Menard et al., 1997; Santini et al., 2000). Since these proteins are expressed in CHO cells, they are a useful model for investigating the agonist-dependent regulation of G protein-coupled receptors.
In this investigation, we observed subtype-specific differences in the kinetics and extent of internalization, recycling and downregulation of M1-M5 receptors elicited to carbachol. These differences were unrelated to whether the subtype signaled through Gq (M1, M3 and M5) or Gi (M2 and M4), suggesting that there are subtype-specific differences in the mechanisms mediating the agonist-dependent regulation of muscarinic receptors. Since many different cell types express similar G protein-coupled receptor kinases and arrestins (proteins playing a central role in the agonist-dependent regulation of G protein-coupled receptors) (Attramadal et al., 1992; Komori et al., 1998; Parruti et al., 1993; Shenoy and Lefkowitz, 2003), subtype-specific differences in agonist-dependent internalization, recycling and downregulation of muscarinic receptors likely exists in other cell types. The magnitude and the rate of these processes may differ, however, because of cell-type specific differences in the mechanism.
Consistent with this postulate, the extent of muscarinic M1, M3 and M4 receptor internalization after 1 h of carbachol treatment (1 mM) in HEK293 tsA201 cells (Lee et al., 1998) was comparable to that observed in our study. However, the extent of muscarinic M2 receptor internalization in HEK293 tsA201 cells after 1 h of carbachol treatment (1 mM) (23% of control) was different from what we observed in CHO cells (84% of control) (Pals-Rylaarsdam et al., 1997).
Under similar assay conditions in COS-7 cells (i.e., 1 mM carbachol treatment), the half-times for carbachol-induced muscarinic M1 and M5 receptor internalization (t½ = 43 and 12 min, respectively; (Tsuga et al., 1998b) were comparable to our study. In contrast, the half-time for M3 receptor internalization in COS-7 cells (t½ = 11 min) was 31% of what we observed (Tsuga et al., 1998b). More muscarinic M1, M3 and M5 receptors internalized in CHO cells (32, 33 and 63% of control, respectively) than in COS-7 cells (10, 20 and 25% of control, respectively) after 2 h of carbachol treatment (1 mM) (Tsuga et al., 1998b). Tsuga and coworkers, (1998b) also determined that 10 μM and 100 μM carbachol treatment elicited maximal M2 and M4 receptor internalization in COS-7 cells. Under these assay conditions, the half-times for M2 and M4 receptor internalization (t½ = 37 and 49 min, respectively) were different than those observed in our study (t½ = 8.6 and 12.8 min, respectively) (Tsuga et al., 1998b). Additionally, muscarinic M2 and M4 receptors internalized to a lesser extent in COS-7 cells (47 and 45% of control, respectively) than in CHO cells (88 and 77% of control, respectively) after two hours of carbachol treatment (Tsuga et al., 1998b).
Large proportions of the M2 and M4 receptor populations (87 and 75%, respectively) internalized with half-times of 8.6 and 12.8 min, respectively, during short-term treatment with carbachol, and no significant additional receptor internalization was observed at 24 h (84 and 80%, respectively). Short-term carbachol treatment also caused M1, M3 and M5 receptors to internalize consistent with a first-order process (t½ = 46.3, 35.5, and 13.1 min, respectively), but the component of each receptor population that internalized (45, 41, and 65%, respectively) was much less that that observed at 24 h (87, 63 and 76%, respectively). We found that the internalization of M1, M3 and M5 receptors was inconsistent with a single first-order process, but could be described by a two-component model, although we did not characterize the slow component in detail. Thus, there appears to be both fast and slow components to the carbachol-induced internalization of M1, M3 and M5 receptors.
BCM was used for the alkylation phase of recycling experiments because it has a short half-time for alkylation (t½ = 33 sec). We found that a 5-min incubation with BCM (50 nM) alkylated 85% or more of muscarinic M1-M5 receptors (see section 3.3 “Recycling of muscarinic receptors”). It is likely that only a few muscarinic receptors traffic to the plasma membrane of CHO cells during this 5 min incubation with BCM. Thus, in recycling experiments we alkylated muscarinic receptors with BCM at 37°C instead of a temperature that inhibits receptor trafficking (e.g., 15°C). In previous investigations using PrBCM mustard (t½ for alkylation = 2 min), it was necessary to incubate cells at 15°C or 20°C to inhibit receptor trafficking during the 20 to 30 min incubation with PrBCM (Koenig and Edwardson, 1994a; b; 1996). Using our approach, we greatly reduced the time necessary to alkylate a significant fraction of plasma membrane expressed muscarinic receptor and the complexity of the experiment by incubating cells at 37°C with BCM instead of 15°C to 20°C.
In general, two processes contribute to the recovery of [3H]NMS binding in intact, whole CHO cells after BCM treatment; constitutive delivery of newly synthesized receptors from the biosynthetic-secretory pathway and delivery of receptors from an endosomal pool (i.e. receptor recycling). Presumably, carbachol-treatment increases the number of receptors in the endosomal pool and, once in the endosomal pool, receptors may either recycle or be degraded (downregulation). In CHO cells expressing M1, M3, M4 and M5 receptors, the initial rate for the recovery of [3H]NMS binding in carbachol treated cells (1 mM, 1 h) was 2- to 9-fold greater than the initial rate for the recovery in untreated cells (see Table 3). This difference in the initial rates indicates that M1, M3, M4 and M5 receptors recycle from the endosomal pool and that delivery from this pool occurs at a faster rate than delivery from the biosynthetic-secretory pathway. Thus, the recovery of [3H]NMS binding consists of two components, a rapid and slow component. The rapid component is apparent for subtypes that recycle (i.e., M1, M3, M4 and M5 receptors) and appears to be a first-order process that is nearly complete at approximately 90 min after BCM treatment (see Figure 3A, C-E). The slow component, which represents receptor delivery from the biosynthetic-secretory pathway, should cause [3H]NMS binding to increase to the level observed prior to BCM treatment, if given enough time.
The slow component (i.e., receptor delivery from the biosynthetic-secretory pathway) appears to predominate in untreated cells expressing M3, M4 and M5 receptors (Figure 3C-E). Carbachol treatment (1 mM, 1 h) caused an increase in the number of M3, M4 and M5 receptors in the endosomal pool, and thus, caused an increase in the contribution of the rapid component (Figure 3C-E and Table 3). In CHO cells expressing M1 receptors, the rapid component appears to contribute to the recovery of receptor binding in untreated cells (Figure 3A). This suggests that M1 receptors may recycle even in the absence of agonist. Carbachol treatment (1 mM, 1 h) also caused an increase in the number of M1 receptors in the endosomal pool and an increase in the contribution of the rapid component (Figure 3A and Table 3). Carbachol treatment (1 mM, 1 h) did not affect the rate of recovery or the amount of [3H]NMS binding for the M2 receptor (Figure 3B and Table 3). Based on the internalization data presented in Figure 1B, and the recovery of [3H]NMS binding in recycling assays, it appears that the majority of M2 receptors that internalize during carbachol treatment do not recycle. Presumably, these subtype-specific differences arise as a consequence of subtype-specific structural differences that restrict the numbers of receptors recycling from endosomes to the plasma membrane.
The downregulation of M2, M3, M4 and M5 receptors observed after 24-h carbachol treatment (1 mM) was comparable (see Figure 2 and Table 2). However, the downregulation of M1 receptors was significantly greater than that observed for the other subtypes (see Figure 2 and Table 2). The amount of M1, M2 and M3 receptor downregulation observed in our study was comparable to that previously described in CHO cells (Shockley et al., 1999; Tsuga et al., 1998a). Prior to our study, the agonist-induced downregulation of the M5 receptor had not been determined and that of the M4 receptor had not been characterized in CHO cells.
In conclusion, carbachol treatment caused muscarinic receptor internalization, recycling and downregulation in CHO cells. However, there were subtype-specific differences in the rate or extent or both of carbachol-induced internalization, downregulation and recycling. Based on these findings, it would be interesting to determine whether there are subtype-specific differences in the G protein-coupled receptor kinases and arrestins that interact with the receptors during agonist treatment. The type of G protein-coupled receptor kinase (e.g., GRK 2, 3, 5 and 6) and arrestin (e.g., arrestin 2 and 3) recruited to a particular muscarinic receptor subtype may be responsible for the subtype-specific differences observed in this study.
Acknowledgements
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant 1R15-NS057742].
We would like to thank Crystal A. Shults for her excellent technical assistance and Drs. Fred Ehlert and Craig W. Stevens for reading and critiquing our manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to customersour customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attramadal H, Arriza JL, Aoki C, Dawson T, Codina J, Kwatra MM, Snyder SH, Caron MG, Lefkowitz RJ. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. Journal of Biological Chemistry. 1992;267:17882–17890. [PubMed] [Google Scholar]
- Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. Journal of Pharmacology & Experimental Therapeutics. 1992;260:576–580. [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Buckley CM, Brann MR. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Molecular Pharmacology. 1989;35:469–476. [PubMed] [Google Scholar]
- Chang TY, Limanek JS, Chang CC. A simple and efficient procedure for the rapid homogenization of cultured animal cells grown in monolayer. Analytical Biochemistry. 1981;116:298–302. doi: 10.1016/0003-2697(81)90360-2. [DOI] [PubMed] [Google Scholar]
- DeLapp NW, McKinzie JH, Sawyer BD, Vandergriff A, Falcone J, McClure D, Felder CC. Determination of [35S]guanosine-5'-O-(3-thio)triphosphate binding mediated by cholinergic muscarinic receptors in membranes from Chinese hamster ovary cells and rat striatum using an anti-G protein scintillation proximity assay. Journal of Pharmacology & Experimental Therapeutics. 1999;289:946–955. [PubMed] [Google Scholar]
- Ehlert FJ, Oliff HS, Griffin MT. The quaternary transformation products of N-(3-chloropropyl)-4-piperidinyl diphenylacetate and N-(2-chloroethyl)-4-piperidinyl diphenylacetate (4-DAMP mustard) have differential affinity for subtypes of the muscarinic receptor. Journal of Pharmacology & Experimental Therapeutics. 1996;276:405–410. [PubMed] [Google Scholar]
- Gaborik Z, Szaszak M, Szidonya L, Balla B, Paku S, Catt KJ, Clark AJ, Hunyady L. Beta-arrestin- and dynamin-dependent endocytosis of the AT1 angiotensin receptor. Molecular Pharmacology. 2001;59:239–247. doi: 10.1124/mol.59.2.239. [DOI] [PubMed] [Google Scholar]
- Gill EW, Rang HP. An alkylating derivative of benzilylcholine with specific and long-lasting parasympatholytic activity. Molecular Pharmacology. 1966;2:284–297. [PubMed] [Google Scholar]
- Goldschmidt RC, Kimelberg HK. Protein analysis of mammalian cells in monolayer culture using the bicinchoninic assay. Analytical Biochemistry. 1989;177:41–45. doi: 10.1016/0003-2697(89)90010-9. [DOI] [PubMed] [Google Scholar]
- Goodman OBJ, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Griffin MT, Hsu JC, Shehnaz D, Ehlert FJ. Comparison of the pharmacological antagonism of M2 and M3 muscarinic receptors expressed in isolation and in combination. Biochemical Pharmacology. 2003;65:1227–1241. doi: 10.1016/s0006-2952(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Hammer R. Muscarinic receptors in the stomach. Scandinavian Journal of Gastroenterology. 1980;66(Supplement):5–11. [PubMed] [Google Scholar]
- Hulme EC, Birdsall NJ, Buckley NJ. Muscarinic receptor subtypes. Annual Review of Pharmacology & Toxicology. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Kashihara K, Varga EV, Waite SL, Roeske WR, Yamamura HI. Cloning of the rat M3, M4 and M5 muscarinic acetylcholine receptor genes by the polymerase chain reaction (PCR) and the pharmacological characterization of the expressed genes. Life Sciences. 1992;51:955–971. doi: 10.1016/0024-3205(92)90403-c. [DOI] [PubMed] [Google Scholar]
- Koenig JA, Edwardson JM. Kinetic analysis of the trafficking of muscarinic acetylcholine receptors between the plasma membrane and intracellular compartments. Journal of Biological Chemistry. 1994a;269:17174–17182. [PubMed] [Google Scholar]
- Koenig JA, Edwardson JM. Routes of delivery of muscarinic acetylcholine receptors to the plasma membrane in NG108-15 cells. British Journal of Pharmacology. 1994b;111:1023–1028. doi: 10.1111/j.1476-5381.1994.tb14846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JA, Edwardson JM. Intracellular trafficking of the muscarinic acetylcholine receptor: importance of subtype and cell type. Molecular Pharmacology. 1996;49:351–359. [PubMed] [Google Scholar]
- Komori N, Cain SD, Roch JM, Miller KE, Matsumoto H. Differential expression of alternative splice variants of beta-arrestin-1 and -2 in rat central nervous system and peripheral tissues. European Journal of Neuroscience. 1998;10:2607–2616. [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. Journal of Biological Chemistry. 2000;275:23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- Lee KB, Pals-Rylaarsdam R, Benovic J, Hosey MM. Arrestin-independent internalization of the m1, m3, and m4 subtypes of muscarinic cholinergic receptors. Journal of Biological Chemistry. 1998;273:12967–12972. doi: 10.1074/jbc.273.21.12967. [DOI] [PubMed] [Google Scholar]
- Menard L, Ferguson SS, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS. Synergistic regulation of beta2-adrenergic receptor sequestration: intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Molecular Pharmacology. 1997;51:800–808. [PubMed] [Google Scholar]
- Offermanns S, Wieland T, Homann D, Sandmann J, Bombien E, Spicher K, Schultz G, Jakobs KH. Transfected muscarinic acetylcholine receptors selectively couple to Gi-type G proteins and Gq/11. Molecular Pharmacology. 1994;45:890–898. [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Gurevich VV, Lee KB, Ptasienski JA, Benovic JL, Hosey MM. Internalization of the m2 muscarinic acetylcholine receptor. Arrestin-independent and -dependent pathways. Journal of Biological Chemistry. 1997;272:23682–23689. doi: 10.1074/jbc.272.38.23682. [DOI] [PubMed] [Google Scholar]
- Parker EM, Kameyama K, Higashijima T, Ross EM. Reconstitutively active G protein-coupled receptors purified from baculovirus-infected insect cells. Journal of Biological Chemistry. 1991;266:519–527. [PubMed] [Google Scholar]
- Parruti G, Peracchia F, Sallese M, Ambrosini G, Masini M, Rotilio D, De Blasi A. Molecular analysis of human beta-arrestin-1: cloning, tissue distribution, and regulation of expression. Identification of two isoforms generated by alternative splicing. Journal of Biological Chemistry. 1993;268:9753–9761. [PubMed] [Google Scholar]
- Santini F, Penn RB, Gagnon AW, JL. B, JH. K. Selective recruitment of arrestin-3 to clathrin coated pits upon stimulation of G protein-coupled receptors. Journal of Cell Science. 2000;113:2463–2470. doi: 10.1242/jcs.113.13.2463. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochemical Journal. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley MS, Burford NT, Sadee W, Lameh J. Residues specifically involved in down-regulation but not internalization of the m1 muscarinic acetylcholine receptor. Journal of Neurochemistry. 1997;68:601–609. doi: 10.1046/j.1471-4159.1997.68020601.x. [DOI] [PubMed] [Google Scholar]
- Shockley MS, Tolbert LM, Tobin AB, Nahorski SR, Sadee W, Lameh J. Differential regulation of muscarinic M1 and M3 receptors by a putative phosphorylation domain. European Journal of Pharmacology. 1999;377:137–146. doi: 10.1016/s0014-2999(99)00303-9. [DOI] [PubMed] [Google Scholar]
- Tsuga H, Kameyama K, Haga T, Honma T, Lameh J, Sadee W. Internalization and down-regulation of human muscarinic acetylcholine receptor m2 subtypes. Role of third intracellular m2 loop and G protein-coupled receptor kinase 2. Journal of Biological Chemistry. 1998a;273:5323–5330. doi: 10.1074/jbc.273.9.5323. [DOI] [PubMed] [Google Scholar]
- Tsuga H, Okuno E, Kameyama K, Haga T. Sequestration of human muscarinic acetylcholine receptor hm1-hm5 subtypes: effect of G protein-coupled receptor kinases GRK2, GRK4, GRK5 and GRK6. Journal of Pharmacology & Experimental Therapeutics. 1998b;284:1218–1226. [PubMed] [Google Scholar]