Abstract
Genetic effects on mechanical properties have been demonstrated in rodents, but not confirmed in primates. Our aim was to quantify the proportion of variation in vertebral trabecular bone mechanical properties that is due to the effects of genes. L3 vertebrae were collected from 110 females and 46 male baboons (6–32 years old) from a single extended pedigree. Cranio-caudally oriented trabecular bone cores were scanned with microCT then tested in monotonic compression to determine apparent ultimate stress, modulus, and toughness. Age and sex effects and heritability (h2) were assessed using maximum likelihood-based variance components methods. Additive effects of genes on residual trait variance were significant for ultimate stress (h2=0.58), toughness (h2=0.64), and BV/TV (h2=0.55). When BV/TV was accounted for, the residual variance in ultimate stress accounted for by the additive effects of genes was no longer significant. Toughness, however, showed evidence of a non-BV/TV-related genetic effect. Overall, maximum stress and modulus show strong genetic effects that are nearly entirely due to bone volume. Toughness shows strong genetic effects related to bone volume and shows additional genetic effects (accounting for 10% of the total trait variance) that are independent of bone volume. These results support continued use of bone volume as a focal trait to identify genes related to skeletal fragility, but also show that other focal traits related to toughness and variation in the organic component of bone matrix will enhance our ability to find additional genes that are particularly relevant to fatigue-related fractures.
Introduction
Bone fragility results from low bone density and changes in bone quality, both of which show strong genetic effects. Until recently most research into the genetics of skeletal fragility focused on bone mineral density (BMD), but it is now well-accepted that BMD only partially explains fracture risk [1–3].
Mechanical properties provide a very direct and inclusive measure of bone’s resistance to fracture. Experimentally subjecting a bone or piece of bone to controlled loading can yield important information about the force required to break the bone, the stiffness of the bone, and the total amount of energy absorbed by the bone before failure.
A genetic effect on mechanical properties has been demonstrated in rodents [4–7]. Using progeny derived from inbred Fisher 344 and Lewis rats, Alam et al. detected significant quantitative trait loci (QTLs) for variation in midshaft femur ultimate force, energy to break, and stiffness [8]; fifth lumbar vertebra ultimate force [8]; and femoral neck ultimate force and energy to break [9]. Studies in another rat model (progeny of Copenhagen 233 and Dark Agouti rats) also revealed QTLs for femoral neck biomechanical properties[10] and femoral midshaft and fifth lumbar vertebra ultimate force [11]. In a study of femurs of HcB/8 and HcB/23 recombinant congenic mice, Saless [12] identified QTLs for total displacement, post yield deflection, and stiffness. Koller et al. [13] identified QTLs for load to failure, work to failure, and stiffness in a study of B6xC3H F2 mice. Together these studies provide strong evidence for the effect of genes on biomechanical properties.
The genetic effect on mechanical properties has not been confirmed in primates, including humans, and no studies in any species have documented the magnitude of the genetic effect (that proportion of the variance due to the additive effects of genes, or heritability (h2), on population level normal variation in mechanical properties. Substantial differences in fracture properties between primate and non-primate species [14] underscore the need for a genetically well-characterized non-human primate model to assess the genetics of bone mechanical properties.
The baboon shares physiological and developmental characteristics with humans that make it particularly well-suited to study the skeleton. This animal shares with humans a relatively long lifespan and bone loss with advancing age [15–18]. Baboon reproductive physiology and endocrinology are also quite similar to that of humans [19] in that the baboon menstrual cycle involves timing and phases similar to those of humans, they parallel humans with regard to hormonal changes accompanying pregnancy [20], and they undergo a natural menopause in the third decade of life [19–21]. Baboons offer another advantage over other popular animal models for vertebral mechanical properties (primarily rodents [22–25] and dogs [26–28]) that involves their tendency toward upright posture. Though they locomote quadrupedally, a substantial amount of their postural behavior is upright, resulting in transmission of biomechanical forces through the vertebrae that more closely approximate those of the human spinal column than is possible with a non-primate model.
The specific aim of this study is to detect and quantify the proportion of variation in vertebral trabecular bone mechanical properties in the baboon that is due to the additive effects of genes. Based on results from other bone phenotypes, we hypothesize that there will be a significant genetic effect on these mechanical properties.
Materials and Methods
Baboon sample
The sample consists of 156 baboons, Papio hamadryas, (110 females, 46 males) ranging in age from 6 to 32 years. During life all animals were housed out of doors in social group cages and maintained on commercial monkey chow to which they had ad libitum access. Animal care personnel and staff veterinarians provided daily maintenance and health care to all animals in accordance with the Guide for the Care and Use of Laboratory Animals [29]. All procedures related to their treatment during their lives at the Southwest Foundation for Biomedical Research (SFBR)/Southwest National Primate Research Center (SNPRC) were approved by the Institutional Animal Care and Use Committee in accordance with the established guidelines. All animals were euthanized for reasons unrelated to this project or died of natural causes. Clinical records for each animal were checked to be certain that animals with medical conditions known to affect bone metabolism (e.g. rheumatoid arthritis, diabetes, chronic renal disease) were not included in the sample. The third lumbar vertebra was collected from each animal at necropsy, wrapped in saline-soaked gauze, placed in air tight plastic bags, then frozen until preparation for testing. A detailed characterization of the baboon model is presented in Havill et. al. [30].
Preparation of Cores
Trabecular bone cores were obtained from the third lumbar vertebral body. Vertebral arches were removed with a Stryker saw (Stryker, Kalamazoo, MI). A trabecular bone core was obtained under constant irrigation in the cranial-caudal direction through the length of the vertebral body using a 9 mm diameter diamond-coated cylindrical core bit attached to a drill press. Each vertebral body was manually positioned such that the core location was in the center of the vertebral body in the medio-lateral and antero-posterior planes. Cranial and caudal endplates were removed from the core using an Isomet 1000 Precision Saw (Buehler, Lake Bluff, IL). Two trabecular bone samples, each 6 mm in height, were obtained for testing from each core, one sample representing the cranial portion of the core and one the caudal portion (Figure 1). Mean values for the data obtained from the two samples were used for all analyses. In two animals only the superior core was obtained due to difficulties in sample preparation.
Figure 1.
Location of 9 mm × 6 mm core samples from the vertebral body and an illustration of trabecular bone core structure obtained via µCT.
Trabecular Bone Volume
Trabecular bone cores were scanned using microCT (µCT 20) (SCANCO USA, Inc., Southeastern, PA) using an isotropic voxel size of 11 µm. A scout view was obtained to determine specimen height and to select locations for scanning. Five transverse slices, spaced 0.5 mm apart, were centered in the middle of the core. On each of the 2-dimensional images a region of interest was manually drawn. The region of interest excluded the outer rim of the core to avoid inclusion of debris resulting from the coring process. For each of the five slices the cross-sectional area (CSA, mm2), bone volume/tissue volume (BV/TV, %), trabecular thickness (Tb.Th, µm), and trabecular number (Tb.N, #/mm2) were determined. Values from all five slices were averaged for each core and this average was used in all subsequent calculations and analyses.
Mechanical Testing
Following microCT, cores were tested to failure in monotonic compression using a Model 858 Mini Bionix II servohydraulic testing machine (MTS Corp., Minneapolis, MN). Testing was conducted in displacement control at a rate of 0.5mm/min. Load and displacement data were collected at a rate of 10Hz and were converted to stress and strain data by dividing displacement data by initial specimen height, and by dividing force data by CSA. Because these tests were conducted on isolated bone cores, these data represent apparent-level mechanical properties. Ultimate stress was determined as the maximum stress value achieved during the test. Modulus was determined as the maximum slope in the linear portion of the stress-strain curve. Toughness was determined as the area under the stress-strain curve to the point of ultimate stress.
Statistical and Quantitative Genetic Analysis
Evaluation of the covariance between relative pairs in mechanical properties allows for quantification of the contribution of additive genetic effects (h2) on these focal traits (traits of interest). We used a variance decomposition approach, implemented in the computer package SOLAR (described in detail elsewhere [31]) to assess h2 and age and sex effects. This approach models the expected phenotypic covariance among relatives as: where , the additive genetic component, is the product of 2 times the kinship matrix (Φ) and the additive genetic variance (); and , the unique environmental component, is the product of the identity matrix (I) and the non-genetic variance component (). This approach is used to partition the phenotypic variance () into its additive genetic () and environmental () components and estimate the proportion of the phenotypic variance attributable to additive genetic effects (heritability) as , and that proportion attributable to non-genetic factors as e2 = 1− h2.
Phenotypes were modeled as: y = µ + β1x1 + β2x2 + … + βnxn + g + e; where µ is the population mean for the trait, xi are the covariate values, βi their mean effects coefficients, and g and e, respectively, are the genetic and environmental effects. The effects of age, sex, and age-by-sex were estimated simultaneously, allowing for estimation of the mean effects of any of these covariates found to significantly influence the mechanical properties phenotypes.
Significance of maximum likelihood estimates for heritability and other parameters was assessed by means of likelihood ratio tests [32]. The maximum likelihood for the general model in which all parameters are estimated was compared to that for a restricted model in which the value of the parameter to be tested is held constant at some value (usually zero). Twice the difference in the ln likelihoods of the two models is distributed asymptotically approximately as either a 1/2:1/2 mixture of χ2 and a point mass at zero for tests of parameters like h2 (for which a value of zero in a restricted model is at a parameter space boundary) or a χ2 variate for tests of covariates (for which zero is not a boundary value) [33]. Degrees of freedom equal the difference in the number of estimated parameters in the two models [33]. However, for tests of parameters like h2, whose values may be fixed at a boundary of their parameter space in the null model, the appropriate significance level is obtained by halving the p-value [34].
Age, sex and age-by-sex were selected for inclusion as covariates in the final model by means of a Bayesian model averaging procedure implemented in SOLAR. This procedure evaluates all possible covariates alone and in all possible combinations to identify the best set for inclusion based on a Bayesian Information Criterion for each covariate/combination and a posterior probability assigned to each covariate [35]. After these initial analyses, a subsequent set of analyses were conducted in which BV/TV was included as a covariate to determine the degree to which observed age, sex, and genetic effects were acting through effects on bone volume.
Results
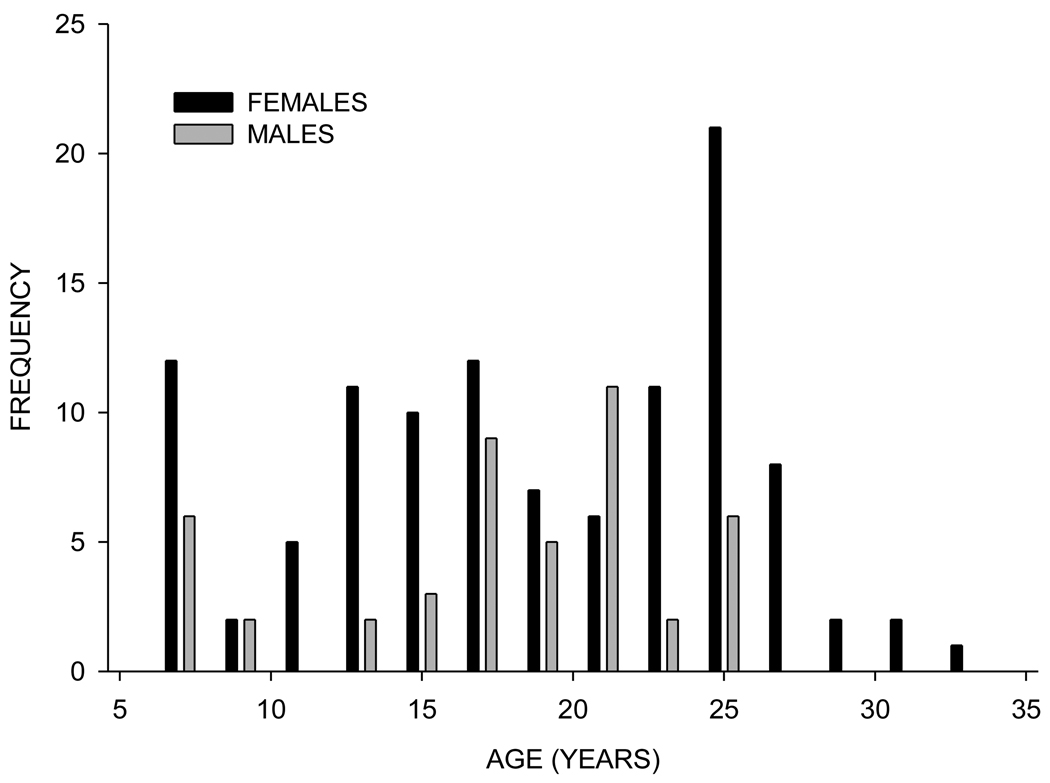
Table 1 shows the descriptive statistics for age and mechanical properties by sex. Qualitatively the females show higher mean values and wider ranges for all parameters. While the mean age is approximately 18 years for both sexes, Figure 2 clearly shows that the number of younger females (e.g. under the age of 15) is much higher than the number of males in this age range. It is also clear that only females are represented over the age of 26 years. The results of the maximum likelihood-based tests (which account for the relatedness among individuals in this sample) for significant effects of age, sex, and age*sex are presented in Table 2. A sex-specific age effect (age*sex) is significant for ultimate stress, toughness, and BV/TV. Ultimate stress also shows a significant sex effect that is not age-related. Age significantly affects modulus in a non-sex-specific manner. Overall, age and sex-related effects account for between six and 13% of the total trait variance in BV/TV and the mechanical properties.
Table 1.
Descriptive statistics for age, apparent mechanical properties, and bone architecture by sex.
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Variable | x̅ | sd | range | x̅ | sd | range |
| Age (years) | 18.63 | 6.71 | 6.06–32.45 | 17.51 | 5.51 | 6.06–25.51 |
| Maximum Stress (MPa) | 19.03 | 7.61 | 5.76–47.59 | 14.63 | 5.39 | 6.25–28.41 |
| Modulus (MPa) | 813.10 | 419.28 | 203.58–2176.87 | 627.58 | 416.24 | 172.93–1549.35 |
| Toughness (MPa) | 0.57 | 0.42 | 0.11–2.61 | 0.38 | 0.20 | 0.14–1.14 |
| BV/TV (%) | 29.93 | 8.16 | 12.96–53.80 | 25.71 | 5.76 | 15.53–39.18 |
| Tb.Th (µm) | 90.11 | 17.8 | 48.5–142.0 | 86.26 | 15.5 | 58.0–115.0 |
| Tb.N (#/mm) | 3.42 | 0.70 | 2.01–5.50 | 2.93 | 0.56 | 2.33–4.96 |
Figure 2.
Age distribution of sample by sex.
Table 2.
Results of quantitative genetic analysis of mechanical properties and bone volume.
| covariates | Total variation due to covariates |
Residual variation |
h2±SE (p-value) | Total variation due to genes |
|
|---|---|---|---|---|---|
| ultimate stress | sex, | 0.12 | 0.88 | 0.58 ± 0.20 (0.0005) | 0.51 |
| age*sex | |||||
| modulus | age | 0.06 | 0.94 | 0.29 ± 0.24 (0.0855) | 0.27 |
| toughness | age*sex | 0.08 | 0.92 | 0.64 ± 0.22 (0.0003) | 0.59 |
| BV/TV | age*sex | 0.13 | 0.87 | 0.55 ± 0.20 (0.0009) | 0.48 |
| adjusted for BV/TV | |||||
| ultimate stress | sex, | 0.86 | 0.14 | 0.15 ± 0.14 (0.1042) | 0.02 |
| age*sex, | |||||
| BV/TV | |||||
| modulus | age, | 0.44 | 0.56 | 0.02 ± 0.13 (0.4413) | 0.01 |
| BV/TV | |||||
| toughness | age*sex, | 0.65 | 0.35 | 0.29 ± 0.23 (0.0389) | 0.10 |
| BV/TV | |||||
Residual heritability estimates are presented in Table 2 for two sets of analyses (with and without BV/TV as a covariate). The first set of estimates show that additive effects of genes on residual trait variance (that part of the variance that remains after age and sex effects are accounted for) are significant for ultimate stress (h2=0.58), toughness (h2=0.64), and BV/TV (h2=0.55). The percent of the total trait variance accounted for by the genetic effect (calculated by multiplying the residual variance by the h2 estimate) is also provided in Table 2. These results show that genes account for 27% to 59% of the total trait variance in mechanical properties.
A much larger percentage of the total variation is accounted for when BV/TV is included as a covariate. Age, sex, and BV/TV effects together accout for 44 to 86% of the total trait variance (Table 2). When BV/TV is accounted for as a covariate, the residual variance in ultimate stress that is accounted for by genetics falls dramatically and is no longer significant. The h2 estimate for modulus also falls dramatically, though this estimate was not statistically significant in the first analysis (p=0.0855). Toughness shows the same trend with h2 falling from 0.64 to 0.29, but the latter estimate remains statistically significant. This indicates that variation in toughness is significantly affected by genes that are not accounted for by inclusion of BV/TV since the adjusted genetic effect accounts for 10% of the total trait variance.
Discussion
Our results clearly demonstrate that mechanical properties of trabecular bone of the spine are strongly heritable in this non-human primate model. In addition, the results show that these genetic effects are largely, but not entirely, due to genetic effects on trabecular bone volume. After accounting for relatively small but significant age and sex effects, 28% to 64% of the variation in mechanical properties is attributable to the effects of genes. Subsequent analyses in which the variation due to bone volume is accounted for, along with significant age and sex effects, non-significant heritability estimates for ultimate stress (14%) and modulus (1%) indicate that much of the genetic effect detected in the first analyses is due to genetic effects on trabecular bone volume. Conversely, when bone volume is accounted for, along with age and sex terms, a significant genetic effect on toughness (29%) remains. This indicates that variation in toughness is due, in part, to genes that affect bone volume, but also to a gene or set of genes independent of bone volume.
Age and sex effects together account for less than 12% of the variation in the bone mechanical properties. The magnitude of this effect is less than we expected, but is perhaps not surprising based on more careful consideration of the nature of the age and sex distribution of our sample. Our sample spans a large range of adult ages (6–33 years), including elderly males and females. The number of elderly animals for each sex, though, is very limited. Although female baboons undergo a natural menopause (average age of menopause in this colony is 26 years [36]) only a very small number of our female sample is older than this mean, so we do not have a large proportion of postmenopausal females as would be expected in a sample of adult humans. Most of our sample represents early and middle adulthood, a period before substantial age-related bone loss in either sex would be expected to occur. So, while we detect significant age effects, that are, for the most part, sex-specific, they do not explain a large percentage of the variation in the sample overall because this age effect is only apparent in a relatively small proportion of the population, the oldest animals. Age and sex effects would likely be of greater magnitude in a sample with a higher proportion of animals of advanced age, the stage of life during which bone loss would be expected to be most apparent. The potential sources of remaining variation are many and may include within-sex variation in body size, muscle mass, adiposity, hormone levels, and physical activity levels in addition to variation in bone-specific traits such as trabecular architechture, microstructure, compositional properties, microdamage, tissue matrix properties, tissue micro-porosity, collagen and water content, among others.
Baboons clearly offer advantages over other popular animal models (e.g. rodents or canines) for studies of the genetics of vertebral bone mechanical properties because of considerable time spent in upright posture, occasional bipedal walking, and relative genetic proximity to humans. Nevertheless, baboons, as other animal models, locomote predominantly quadrupedally and it is important to consider the potential implications of consequent differences in musculoskeletal biomechanics (including the role of trunk musculature, inter-vertebral discs, and other soft tissue effects) between this species and humans. In spite of these differences, a study of direct real-time in vivo forces in the lumbar spine of baboons revealed that maximum loads in the baboon lumbar spine were encountered when the animals were seated in a flexed position and that force data overall show similar trends to those reported for the human lumbar spine [37].
Considerable heterogeneity in vertebral body trabecular bone microstructure has been documented in lumbar vertebrae of humans [38, 39]. Although this intravertebral regional variation has not been characterized in the same detail in baboons, it is reasonable to expect that regional variation exists, so we took great care to assure that the vertebral cores from each animal represented the same relative anatomical location in the vertebral body. The one exception to this is that because we used a uniform core size for vertebral bodies of all sizes, the proportion of the center of the vertebral body represented by the core varies according to the size of the vertebra. This means that on average, cores from vertebrae of females will represent a higher proportion of the vertebral body than is the case for cores from the vertebrae of males. Since the central portion of the vertebral body appears to be the least dense portion of the body overall, this could explain the unexpected result of slightly higher bone volume and apparent mechanical properties in females. The decision to use a uniform core size for vertebrae of all sizes was influenced by the work of Linde et al. [40] that demonstrates that specimen geometry, in particular smaller cross-sectional area of the specimen (in this case, the core), can cause consistent differences in mechanical properties of specimens of differing sizes. Using a smaller core for females could have resulted in significantly different mechanical properties for female specimens that were actually due to the smaller core size rather than being due to sex. This issue would not affect our estimates of heritability because a) these estimates are based on covariance between relative pairs rather than being based on absolute values, and b) the effect of sex (including the significant higher mean effect of female sex) has been accounted for in the model in which the heritability is estimated. These data should not in isolation, however, be interpreted as suggesting that females have stronger vertebrae than males.
Our CT scanning protocol for these bones consisted of taking five equally spaced slices throughout the height of the specimen provides a representative overview of specimen architecture but does not allow us to investigate the role of trabecular bone architecture (connectivity and anisotropy) or any gradient in bone volume on apparent mechanical properties in these specimens. In addition, these data are not appropriate for heritability analyses of trabecular bone architecture. Quantification of Tb.Th and Tb.N are ideally made using a three dimensional volume for greater reliability. These limitations will be addressed in future studies in these same baboons in which entire specimens will be scanned in order to obtain three dimensional data.
It is important to note that the mechanical properties reported herein are apparent level properties, which are a function of multiple traits such as trabecular microstructure, bone matrix micro-porosity, bone matrix water content, microdamage, bone matrix mineral and collagen quality, and bone matrix material and mechanical properties. While these results clearly show that there are strong genetic effects in trabecular bone BV/TV and the apparent level mechanical behavior of trabecular bone (from this non-human primate model), particularly in bone toughness, the underlying traits contributing to the apparent level behavior remain unknown. Further, whether these underlying traits are under genetic control (highly likely) and to what extent co-variation in these traits contributes to overall bone structural integrity is an area of research that we are actively pursuing.
Our results confirm and build upon studies in rats and mice show that genetic background contributes to variation in bone mechanical properties. Li et al. (2005) report that changes in mechanical properties in response to ovariectomy differed significantly among strains of inbred mice (A/J, B6, and C3H strains). Alam et al. [8] identified quantitative trait loci for ultimate force, energy to break, and stiffness of the femur; and ultimate force and energy to break of the fifth lumbar vertebra in a study of Fischer 344 and Lewis rats.
Although rodent studies are a valuable first step in identifying genes that have direct effects on bone fracture resistance, studies of inbred rodents cannot provide information about the degree to which such genes are responsible for normal population-level variation in a non-inbred population. Our results show that genes contribute substantially to variation in mechanical properties in baboons. This is particularly relevant due to the genetic proximity of baboons to humans and consequent similarities in processes of bone maintenance and turnover.
Our results also provide valuable insight into the nature of the effects of genes on vertebral mechanical properties. The fact that the genetic effects on ultimate stress and modulus in compression are principally acting through bone volume, while significant non-bone volume genetic effects were evident for toughness, is consistent with our understanding of the primary determinants of bone mechanical properties. Bone strength (ultimate stress) and stiffness (modulus) are governed predominantly by the mineralized component of bone tissue [41]. Both the amount of bone (bone volume) and the degree of mineralization have significant effects on bone compression strength and stiffness [42, 43]. The organic matrix is only a minor contributor to strength and stiffness in compression [44], but it is the principal determinant of toughness [45, 46]. Genes that affect this or any other variation in the organic matrix could be responsible for the significant non-bone volume-related genetic effect on toughness.
Overall, we interpret these results to mean that maximum stress and modulus show strong genetic effects that are nearly entirely due to bone volume. Toughness, particularly relevant to fatigue-related fracture, also shows strong genetic effects related to bone volume fraction. Interestingly, though, toughness shows additional genetic effects (accounting for 10% of the total trait variance) that are not accounted for by bone volume. These results support continued use of bone volume as a focal trait to identify genes related to skeletal fragility. Importantly, these results also show that other focal traits, specifically those related to variation in the organic component of bone matrix, will enhance our ability to find additional genes that are particularly relevant to fatigue-related fractures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Dempster DW. The pathophysiology of bone loss. Clin Geriatr Med. 2003;19(2):259–270. doi: 10.1016/s0749-0690(02)00098-8. v-vi. [DOI] [PubMed] [Google Scholar]
- 2.Robbins JA, et al. Risk factors for hip fracture in women with high BMD: EPIDOS study. Osteoporos Int. 2004 doi: 10.1007/s00198-004-1661-y. [DOI] [PubMed] [Google Scholar]
- 3.Stone KL, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18(11):1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 4.Alam I, et al. Whole-Genome Scan for Linkage to Bone Strength and MIneral Density in Inbred Rats. American Journal of Bone and Mineral Research. 2005;19 Suppl 1:S69. doi: 10.1359/JBMR.050512. [DOI] [PubMed] [Google Scholar]
- 5.Alam I, et al. Genome-wide Linkage Analyses of Femoral Neck Density and Strength in Inbred Rats. American Journal of Bone and Mineral Research. 2005;19 Suppl 1:S130. [Google Scholar]
- 6.Jepsen KJ, et al. Hierarchical relationship between bone traits and mechanical properties in inbred mice. Mamm Genome. 2003;14(2):97–104. doi: 10.1007/s00335-002-3045-y. [DOI] [PubMed] [Google Scholar]
- 7.Li CY, et al. Genetic background influences cortical bone response to ovariectomy. J Bone Miner Res. 2005;20(12):2150–2158. doi: 10.1359/JBMR.050819. [DOI] [PubMed] [Google Scholar]
- 8.Alam I, et al. Whole-genome scan for linkage to bone strength and structure in inbred Fischer 344 and lewis rats. J Bone Miner Res. 2005;20(9):1589–1596. doi: 10.1359/JBMR.050512. [DOI] [PubMed] [Google Scholar]
- 9.Alam I, et al. Identification of a quantitative trait locus on rat chromosome 4 that is strongly linked to femoral neck structure and strength. Bone. 2006;39(1):93–99. doi: 10.1016/j.bone.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Alam I, et al. Identification of Sex-specific QTL for Femoral Neck Density and Strength in Inbred COP and DA rats. J Bone Miner Res. 2007;22 Suppl 1:S404. [Google Scholar]
- 11.Sun Q, et al. Genetic Loci for Bone Structure and Strength Identified in Inbred COP and DA Rats. J Bone Miner Res. 2007;22 Suppl 1:S408. [Google Scholar]
- 12.Saless N, et al. Biomechanics and Body Size Quantitative Trait Loci in HcB/8 x HcB/23 F2 Mice. J Bone Miner Res. 2007;22 Suppl 1:S481. [Google Scholar]
- 13.Koller DL, et al. Genetic effects for femoral biomechanics, structure, and density in C57BL/6J and C3H/HeJ inbred mouse strains. J Bone Miner Res. 2003;18(10):1758–1765. doi: 10.1359/jbmr.2003.18.10.1758. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Mabrey JD, Agrawal CM. An interspecies comparison of bone fracture properties. Biomed Mater Eng. 1998;8(1):1–9. [PubMed] [Google Scholar]
- 15.Cerroni AM, et al. Bone mineral density, osteopenia, and osteoporosis in the rhesus macaques of Cayo Santiago. Am J Phys Anthropol. 2000;113(3):389–410. doi: 10.1002/1096-8644(200011)113:3<389::AID-AJPA9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Aufdemorte TB, et al. A non-human primate model for the study of osteoporosis and oral bone loss. Bone. 1993;14(3):581–586. doi: 10.1016/8756-3282(93)90197-i. [DOI] [PubMed] [Google Scholar]
- 17.Kammerer CM, Sparks ML, Rogers J. Effects of age, sex, and heredity on measures of bone mass in baboons (Papio hamadryas) J Med Primatol. 1995;24(4):236–242. doi: 10.1111/j.1600-0684.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 18.DeRousseau CJ. Aging in the musculoskeletal system of rhesus monkeys: III. Bone loss. Am J Phys Anthropol. 1985;68(2):157–167. doi: 10.1002/ajpa.1330680203. [DOI] [PubMed] [Google Scholar]
- 19.Brommage R. Perspectives on using nonhuman primates to understand the etiology and treatment of postmenopausal osteoporosis. Journal of Musculoskeletal Neuron Interaction. 2001;1(4):307–325. [PubMed] [Google Scholar]
- 20.Hendrickx A, Dukelow W. Reproductive Biology. In: Henrickson R, editor. Nonhuman Primates in Biomedical Research: Biology and Management. San Diego, CA: Academic Press; 1995. pp. 147–191. [Google Scholar]
- 21.Chen LD, et al. Effect of naturally reduced ovarian function on plasma lipoprotein and 27-hydroxycholesterol levels in baboons (Papio sp.) Atherosclerosis. 1998;136(1):89–98. doi: 10.1016/s0021-9150(97)00190-1. [DOI] [PubMed] [Google Scholar]
- 22.Silva MJ, Brodt MD, Uthgenannt BA. Morphological and mechanical properties of caudal vertebrae in the SAMP6 mouse model of senile osteoporosis. Bone. 2004;35(2):425–431. doi: 10.1016/j.bone.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Brzoska MM, Moniuszko-Jakoniuk J. Low-level exposure to cadmium during the lifetime increases the risk of osteoporosis and fractures of the lumbar spine in the elderly: studies on a rat model of human environmental exposure. Toxicol Sci. 2004;82(2):468–477. doi: 10.1093/toxsci/kfh275. [DOI] [PubMed] [Google Scholar]
- 24.Ito M, et al. Contribution of trabecular and cortical components to the mechanical properties of bone and their regulating parameters. Bone. 2002;31(3):351–358. doi: 10.1016/s8756-3282(02)00830-x. [DOI] [PubMed] [Google Scholar]
- 25.Ammann P, et al. Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J Bone Miner Res. 2004;19(12):2012–2020. doi: 10.1359/JBMR.040906. [DOI] [PubMed] [Google Scholar]
- 26.Ding M, et al. Canine cancellous bone microarchitecture after one year of high-dose bisphosphonates. Calcif Tissue Int. 2003;72(6):737–744. doi: 10.1007/s00223-002-2066-6. [DOI] [PubMed] [Google Scholar]
- 27.Hirano T, et al. Does suppression of bone turnover impair mechanical properties by allowing microdamage accumulation? Bone. 2000;27(1):13–20. doi: 10.1016/s8756-3282(00)00284-2. [DOI] [PubMed] [Google Scholar]
- 28.Mashiba T, et al. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28(5):524–531. doi: 10.1016/s8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 29.Council" NR. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy of Sciences; 1996. [Google Scholar]
- 30.Havill LM, et al. Bone mineral density reference standards in adult baboons (Papio hamadryas) by sex and age. Bone. 2003;33(6):877–888. doi: 10.1016/s8756-3282(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 31.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards AWF. Likelihood. Baltimore: The Johns Hopkins University Press; 1992. p. 275. [Google Scholar]
- 33.Hopper JL, Mathews JD. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet. 1982;46 (4):373–383. doi: 10.1111/j.1469-1809.1982.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 34.Boehnke M, et al. Partitioning the variability of fasting plasma glucose levels in pedigrees. Genetic and environmental factors. Am J Epidemiol. 1987;125(4):679–689. doi: 10.1093/oxfordjournals.aje.a114581. [DOI] [PubMed] [Google Scholar]
- Blangero J, et al. Oligogenic Model Selection Using the Bayesian Information Criterion: Linkage Analysis of the P300 Cz Event-Related Brain Potential. Genet Epidemiol. 1999;17 Suppl 1:S67–S72. doi: 10.1002/gepi.1370170712. [DOI] [PubMed] [Google Scholar]
- 36.Martin LJ, et al. Lifespan in captive baboons is heritable. Mech Ageing Dev. 2002;123(11):1461–1467. doi: 10.1016/s0047-6374(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 37.Ledet EH, et al. Direct real-time measurement of in vivo forces in the lumbar spine. Spine J. 2005;5(1):85–94. doi: 10.1016/j.spinee.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Banse X, et al. Inhomogeneity of human vertebral cancellous bone: systematic density and structure patterns inside the vertebral body. Bone. 2001;28(5):563–571. doi: 10.1016/s8756-3282(01)00425-2. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, et al. Regional variations of vertebral trabecular bone microstructure with age and gender. Osteoporos Int. 2008;19(10):1473–1483. doi: 10.1007/s00198-008-0593-3. [DOI] [PubMed] [Google Scholar]
- 40.Linde F, Hvid I, Madsen F. The effect of specimen geometry on the mechanical behaviour of trabecular bone specimens. J Biomech. 1992;25(4):359–368. doi: 10.1016/0021-9290(92)90255-y. [DOI] [PubMed] [Google Scholar]
- 41.Turner CH. Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos Int. 2002;13(2):97–104. doi: 10.1007/s001980200000. [DOI] [PubMed] [Google Scholar]
- 42.Currey J. Incompatible mechanical properties in compact bone. J Theor Biol. 2004;231(4):569–580. doi: 10.1016/j.jtbi.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Currey JD. Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B Biol Sci. 1984;304(1121):509–518. doi: 10.1098/rstb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 44.Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45(2):108–116. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 45.Burr D. The contribution of the organic matrix to bone's material properties. Bone. 2002;31(1):8–11. doi: 10.1016/s8756-3282(02)00815-3. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, et al. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31(1):1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]