Abstract
Herein we report studies with a novel combination vaccine that, when administered to mice, conferred protection against highly virulent strains of Francisella tularensis by stimulating both arms of the immune system. Our earlier studies with Ft.LVS::wbtA, an O-polysaccharide (OPS)–negative mutant derived from the available live vaccine strain of F. tularensis (Ft.LVS), elucidated the role of antibodies to the OPS—a key virulence determinant—in protection against virulent type A organisms. However, when expressed on the organism, the OPS enhances virulence. In contrast, in purified form, the OPS is completely benign. We hypothesized that a novel combination vaccine containing both a component that induces humoral immunity and a component that induces cellular immunity to this intracellular microbe would have an enhanced protective capacity over either component alone and would be much safer than the LVS vaccine. Thus we developed a combination vaccine containing both OPS (supplied in an OPS–tetanus toxoid glycoconjugate) to induce a humoral antibody response and strain Ft.LVS::wbtA (which is markedly attenuated by its lack of OPS) to induce a cell-mediated protective response. This vaccine protected mice against otherwise-lethal intranasal and intradermal challenge with wild-type F. tularensis strains Schu S4 (type A) and FSC 108 (type B). These results represent a significant advance in our understanding of immunity to F. tularensis and provide important insight into the development of a safer vaccine effective against infections caused by clinical type A and B strains of F. tularensis.
Keywords: Francisella tularensis, Combination vaccination, protective immunity
1. Introduction
Given the potential of Francisella tularensis as an agent of biological warfare and bioterrorism, the development of a fully protective and safe vaccine for the prevention of tularemia has become the main focus of several research laboratories. Unfortunately, the precise mechanisms of host protective immunity to this category A pathogen are still poorly defined. Because F. tularensis is an intracellular pathogen, some investigators have thought it likely that immunity to this organism would prove to rely mainly on cell-mediated mechanisms [1]. However, studies from several laboratories have demonstrated that humoral immunity also plays a role [2–7]. An empirically derived, still-unlicensed vaccine strain of F. tularensis (live vaccine strain, or LVS) was created more than 50 years ago by exhaustive in vitro passage of a type B clinical isolate and is now available as an investigational new drug for at-risk individuals [8]. Unfortunately, routine use of F. tularensis LVS (Ft.LVS) as a vaccine is complicated by several issues, including adverse side effects, incomplete immunity, and the unknown derivation and undefined immunogenic properties of the strain [8–10]. These issues have prompted investigation of a new generation of live attenuated and subunit vaccines [11–14].
The nontoxic nature of the lipopolysaccharide (LPS) of Ft.LVS and the documented transfer of some degree of immunity by specific antibodies to the LPS make this molecule an attractive candidate for inclusion in a subunit vaccine [4]. Our recent studies suggest an important role for O-polysaccharide (OPS) -specific antibodies in conferring protection against type A strains [11]. In immunization studies, an OPS–bovine serum albumin glycoconjugate (OPS-BSA) completely protected mice against intradermal challenge with Ft.LVS and partially protected the animals against aerosol challenge [5]. In addition, this vaccine provided partial protection against intradermal challenge with an F. tularensis type A strain. However, the glycoconjugate failed to protect mice against aerosol challenge with the type A strain [5]. These studies indicate that the development of a fully protective and safe vaccine against tularemia may depend on a combination containing both the surface polysaccharide and immunodominant F. tularensis proteins that are recognized by T cells and that elicit effective local and systemic immune responses. It is important to note however, that when presented on the organism, the OPS contributes substantially to the virulence of the microbe. In this study, we used a novel combination vaccine containing both OPS (provided by an OPS–tetanus toxoid [OPS-TT] glycoconjugate) and immunodominant protein antigens of Ft.LVS (provided by Ft.LVS::wbtA—a live, severely attenuated OPS-negative mutant). We hypothesized (1) that the use of Ft.LVS::wbtA in a combination vaccine would allow the cellular immune system to respond to F. tularensis protein antigens and (2) that the use of an OPS-TT glycoconjugate would generate a robust specific antibody response to OPS. Indeed, the combination vaccine displayed protective efficacy comparable to that obtained with LVS, along with markedly reduced toxicity.
2. Materials and Methods
2.1. Bacterial strains and growth conditions
F. tularensis subspecies holarctica strain LVS was provided by Karen Elkins (U.S. Food and Drug Administration, Rockville, MD). Ft.LVS was grown at 37°C in modified Mueller-Hinton broth (Difco) supplemented with glucose (0.1%), ferric pyrophosphate (0.025%), and Isovitalex (2%) or on cysteine heart agar (Difco) supplemented with 1% hemoglobin solution (VWR, http://www.vwrsp.com). F. tularensis subspecies tularensis type A wild-type strain Schu S4 (FSC237) was obtained from the Francisella Strain Collection (FSC) of the Swedish Defense Research Agency (Umea). F. tularensis type B wild-type strain FSC108 was provided by Dr. John Cherwonogrodsky (Defense Research and Development Canada, Alberta, Canada). The two wild-type strains were grown in Mueller-Hinton broth, harvested, and frozen at −70°C in 1-mL aliquots (1010 cfu/mL) in the presence of 10% (w/v) sucrose, as described previously.
2.2. Adoptive transfer studies
The CD3+ T cell population was enriched from whole splenocytes harvested 2 weeks after intradermal immunization of BALB/c mice (n = 8) with ~108 cfu of Ft.LVS::wbtA or 104 cfu of the parent LVS strain. For enrichment, the Pan T cell isolation kit was used according to the manufacturer’s instructions (Miltenyi Biotech, Auburn, CA). Adoptive transfer studies were performed as previously described. In brief, groups of 8 mice received an intraperitoneal injection of 0.1 mL of cell suspension containing 1 × 107 CD3+ T cells. The mice were challenged 2 h after cell transfer with ~8 times the intraperitoneal LD50 of Ft.LVS. Survival was monitored and mean time to death determined over a 28-day observation period.
2.3. Ft.LVS OPS purification
For production of OPS, Ft.LVS from a fresh cysteine heart agar plate was inoculated into five 250-mL vented-cap erlenmeyer flasks, each containing 100 mL of tryptic soy broth with 0.1% cysteine, 0.025% ferric pyrophosphate, and 0.1% Antifoam 204 (T soy with additives). After incubation overnight at 37°C and aeration at 200 rpm, the cultures were used to inoculate five 3-L vented-cap erlenmeyer flasks containing 2 L of T-soy with additives, which were then incubated as described above for 72 h. Cells were harvested by centrifugation and frozen at −80°C until used for OPS purification.
OPS was purified by the addition of a hot solution of fresh 50% phenol to thawed Ft.LVS cells (at a ratio of 1 g of cells to 10 mL of phenol solution), with subsequent mixing for 2 h at 68°C with use of sterile glass beads and an overhead mixer. After further mixing overnight at 4°C, cell debris and phenol were removed by centrifugation (6K × g for 20 min at 4°C) in Teflon FEP centrifuge bottles (Nalgene). After the top aqueous phase was removed, additional phenol was removed by dilution with one volume of water and two volumes of ether. The solution was mixed vigorously for 10 min in a separatory funnel and allowed to separate overnight at room temperature. The bottom aqueous phase was decanted, and residual ether was removed with a rotary evaporator. The sample was lyophilized to reduce its volume before dialysis against water and enzyme treatment. Nucleic acid and protein were degraded by sequential treatment with DNase, RNase, and pronase (Seward Ltd., Worthington, UK). The solution was then clarified by low-speed centrifugation (5K × g overnight at 4°C). LPS was sedimented and washed three times with water by ultracentrifugation (60K × g overnight at 4°C). Lipid A was hydrolyzed by treatment with acetic acid (6%, v/v) at 100°C for 2 h. The solution was cooled to room temperature before centrifugation (15K × g for 10 min at 4°C) to sediment the cleared lipid. OPS was further purified by gel filtration. NMR and wavelength scanning at 206, 260, and 280 nm were performed to establish purity.
2.4. Preparation of the OPS-TT glycoconjugate
OPS was conjugated to TT as previously described [5]. In brief, OPS was suspended in water and activated with cyanogen bromide at pH 10.5 and then with adipic dihydrazide at pH 8.5 and 4°C, with subsequent dialysis and lyophilization. For conjugation, activated OPS and monomeric TT (in a ratio of 1.5:1) were suspended in saline and allowed to react with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide HCl at pH 5.5 in an ice bath. The conjugate was then passed over a Superdex 200 column, and void-volume fractions were pooled and dialyzed against saline with thimerosal. The conjugate ratio of OPS to TT was 1:2.5.
2.5. Immunization and Ft.LVS challenge studies
Male BALB/cByJ mice (6–8 weeks old; Jackson Laboratory, Bar Harbor, ME) were caged in a microisolator in a pathogen-free environment in the animal facility at Harvard Medical School. For these experiments, there were seven mice per group. Mice received two doses (2 weeks apart) of mutant strain Ft.LVS::wbtA (~107 cfu, 50 µL per mouse, given intranasally) and OPS-TT (8 µg of OPS, 500 µL per mouse, given subcutaneously with an 18-gauge needle). The glycoconjugate was emulsified with either complete Freund’s adjuvant (first dose) or incomplete Freund’s adjuvant (second dose) in a 1:1 ratio. Mice immunized with an equivalent dose of Ft.LVS::wbtA alone, OPS-TT alone, or adjuvant alone served as controls. After a 4-week rest period, all mice were challenged intranasally on day 42 with 3.7 × 105 Ft.LVS organisms (~370 times the intranasal LD50). Survival was monitored for 28 days after challenge, at which point the survivors were humanely sacrificed.
2.6. Immunization and wild-type challenge studies
Specific pathogen–free female BALB/c mice were purchased from Charles River Laboratories (St. Constant, Quebec, Canada) and were used in experiments starting at 8–10 weeks of age. Mice were maintained and used in accordance with the recommendations of the Guide to the Care and Use of Experimental Animals from the Canadian Council on Animal Care. For immunization with the combination, mice were inoculated twice (with doses 2 weeks apart), as described above, with 50 µL of an Ft.LVS::wbtA suspension (1.5 × 107 cfu per mouse, given intranasally) and 0.1 mL of an emulsified suspension of OPS-TT (8 µg of OPS) and incomplete Freund’s adjuvant at a ratio of 1:1 (given subcutaneously). Challenge with virulent type A strain FSC237 (Schu S4) or type B strain FSC 108 took place in a federally certified and Select Agent–approved small-animal level 3 biocontainment facility. Mice that survived the challenge for 21 days were killed; their tissues were removed, homogenized, and plated onto cysteine heart agar supplemented with 1% hemoglobin solution (CHAH). Actual bacterial cfu counts in the inocula and the challenge dose were determined by plating of 10-fold serial dilutions onto CHAH.
2.7. Dissemination studies
To determine LVS bacterial burden, tissues from mice were first hand-mashed and then homogenized with a StomacherR 80-paddle action blender (Seward). Serial dilutions were prepared with sterile 1X PBS supplemented with 2% fetal bovine serum. A 10-µL volume of each dilution was streaked onto CHAH plates, and the plates were incubated at 37°C in the presence of 5% CO2 until colonies became visible. To determine bacterial burdens of FSC 108 and Schu S4, organs were minced with scissors and homogenized in aerosol-proof homogenizers. Serial dilutions of homogenates were plated as described above.
2.8. Histopathology
Spleen and liver tissues were harvested from each group and fixed in Bouin’s fixative solution for histopathologic assessment. Fixed tissues were embedded in paraffin, and sections were subjected to hematoxylin and eosin staining. All histopathology slides were evaluated and scored by a veterinary pathologist in a blinded fashion.
2.9. Statistical analysis
Kaplan-Meier survival analysis and the log-rank test to determine P values for survival curves were performed with GraphPad Prism Version 4.0 (GraphPad Software, San Diego, CA).
3. Results
3.1. Induction of both cellular and humoral immunity is required for an effective tularemia vaccine
Previous studies from our laboratory have demonstrated that Ft.LVS::wbtA, an OPS-negative mutant of Ft.LVS, is highly attenuated (~107 times less virulent in terms of mouse lethality). Despite this significant attenuation, the Ft.LVS::wbtA mutant remains immunogenic and confers protective immunity to mice against challenge with an ordinarily lethal intradermal dose of Ft.LVS or a fully virulent clinical type B isolate of F. tularensis. Because Ft.LVS::wbtA lacks OPS expression, we hypothesized that the protective efficacy of this mutant might be due to the induction of an effective cellular immune response by bacterial protein antigens. To examine the role of immune cells (i.e., cell-mediated immunity), we performed adoptive transfer experiments with a splenic CD3+ T cell–enriched population from immunized mice. In brief, 2 weeks after primary immunization of mice with mutant strain Ft.LVS::wbtA or parent strain Ft.LVS, 1 × 107 splenic CD3+ T cells were transferred to naïve mice by the intraperitoneal route (see Materials and Methods). Two hours after the initial cell transfer, recipient mice were challenged by the intraperitoneal route with 8 times the intraperitoneal LD50 of Ft.LVS, and survival was monitored. As expected, all mice that had received Ft.LVS-primed CD3+ T cells survived homologous challenge (Fig. 1A, *, P = 0.0001). The level of protection was also significant among mice that received Ft.LVS::wbtA-primed CD3+ T cells: 5 of 8 mice in this group survived otherwise-lethal challenge with the parent LVS strain (Fig. 1A, **, P = 0.0001). In contrast, all mice that received naïve CD3+ T cells succumbed to infection 5–7 days after a similar challenge dose.
Fig. 1.
Adoptive transfer studies: (A) Splenic CD3+ T cells were enriched after a 2-week prime with either Ft.LVS or Ft.LVS::wbtA. CD3+ T cells enriched from splenocytes of age- and sex-matched naïve mice served as controls. Approximately 1 × 107 CD3+ T cells of each population [Ft.LVS-primed (■), Ft.LVS::wbtA-primed (▲), and naïve (●)] were adoptively transferred via the intraperitoneal route to naïve male BALB/c mice. All groups were challenged 2 h after adoptive transfer with an ordinarily lethal dose of Ft.LVS (8 times the intraperitoneal LD50). Mice were monitored for 28 days after challenge. The survival curves for groups receiving Ft.LVS-primed and Ft.LVS::wbtA-primed CD3+ T cells, respectively, differed significantly from that for mice given naïve CD3+ T cells (*,**; P = 0.0001); (B) Passive serum transfer studies. Mice (8 per group) received 100 µL of polyclonal rabbit serum [Ft.LVS polyclonal serum (■), Ft.LVS::wbtA polyclonal serum (▲), or normal rabbit polyclonal serum (●)] via the intracardiac route and were challenged after 24 h with Ft.LVS (30 times the intradermal LD50). The survival curve for the Ft.LVS polyclonal serum group differed significantly from those for the other two groups (***, P < 0.0001).
Our previous immunization/challenge studies indicate that active immunization with Ft.LVS—but not with Ft.LVS::wbtA—protects mice against a low but otherwise lethal intradermal challenge dose of a fully virulent type A strain [11]. The major difference between the two immunizing strains lies in the complete lack of surface OPS expression in the wbtA mutant strain; thus antibodies to OPS may play an important role in delaying the progression of disease due to virulent type A F. tularensis. In passive serum transfer studies, all mice receiving Ft.LVS::wbtA immune serum succumbed to infection, as did control mice that received normal rabbit serum. However, all mice receiving Ft.LVS polyclonal immune serum survived this otherwise-lethal Ft.LVS challenge (Fig. 1B, ***, P < 0.0001). These studies, which are consistent with our previous passive serum transfer studies, suggest that antibodies (most likely those to OPS) are important in conferring protection, as inferred by comparison of the protective capacities of anti-Ft.LVS polyclonal immune serum and Ft.LVS::wbtA immune serum. Taken together, these adoptive cell transfer and passive serum transfer studies strengthen the evidence indicating that both cellular and humoral immunity are important for an effective tularemia vaccine.
3.2. Development of the OPS-TT glycoconjugate
Despite the greater protection observed after Ft.LVS immunization than after Ft.LVS::wbtA immunization, Ft.LVS-based vaccine has met with limited acceptance both because of the lack of knowledge about the mechanism of its attenuation and because of the toxicity associated with its use. In an attempt to incorporate all the immunogenic components of Ft.LVS vaccine while eliminating potential adverse effects, we developed a novel approach using an OPS-TT glycoconjugate in combination with attenuated mutant strain Ft.LVS::wbtA. High-molecular-weight OPS from Ft.LVS was purified and conjugated to TT as previously described [5]. Silver staining and immunoblot analysis using α-TT and an Ft.LVS OPS-specific monoclonal antibody (mAb2033) confirmed the presence of the glycoconjugate (data not shown). Our initial studies of vaccine potential indicated that mice immunized with the glycoconjugate subcutaneously were fully protected against an otherwise-lethal intradermal Ft.LVS challenge (15 times the intradermal LD50). In contrast, only a delay in time to death was recorded in another group of glycoconjugate-immunized mice challenged via the intranasal route with 20 times the intranasal LD50 (data not shown). Quantitative studies of the bacterial burden in reticuloendothelial tissues yielded data consistent with the results of survival studies (data not shown).
3.3 The combination vaccine provides enhanced protection against lethal intranasal challenge with Ft.LVS
Mice received two doses (2 weeks apart) of both Ft.LVS::wbtA (via the intranasal route) and the OPS-TT glycoconjugate (via the subcutaneous route). Four weeks after the second immunization, animals were challenged via the intranasal route with a lethal dose of Ft.LVS (370 times the intranasal LD50) and survival monitored (Fig. 2A). Similar to those in the adjuvant control group, mice immunized with either the glycoconjugate or the Ft.LVS::wbtA strain alone showed obvious signs of infection and succumbed to intranasal Ft.LVS challenge (Fig. 2B). In contrast, mice given the combination vaccine remained asymptomatic and survived this high-dose intranasal challenge (Fig. 2B).
Fig. 2.
Survival after an ordinarily lethal intranasal challenge with Ft.LVS in a representative study: (A) Immunization schedule. BALB/c mice received two immunizing doses (2 weeks apart) and were challenged intranasally after a 4-week rest period with Ft.LVS (370 times the intranasal LD50). Animals were monitored daily for 28 days after challenge; (B) Survival of mice (7 per group) after immunization with the TT-OPS glycoconjugate alone (■), with Ft.LVS::wbtA (▼), with the combination vaccine (●), or with adjuvant alone (▲); (C and D) Bacterial burden in spleen, lung, and liver tissues on days 5 and 8, respectively, after an ordinarily lethal intranasal challenge with Ft.LVS in mice previously immunized with adjuvant alone (■), TT-OPS alone (●), Ft.LVS::wbtA alone (▲), or the combination vaccine (□); (E) Bacterial burden on day 28 after an otherwise-lethal intranasal challenge with Ft.LVS in mice receiving the combination vaccine.
The efficacy of the combination vaccine was further assessed by determination of the bacterial burden in reticuloendothelial tissues (lung, liver, and spleen) at days 5, 8, and 28 after intranasal challenge. By day 5 (Fig. 2C), mice given the combination vaccine harbored ~3–4 logs fewer bacteria in the spleen and liver than those in the adjuvant control group. Although significant bacterial loads were found in the lung tissue of combination-vaccine recipients, counts were nevertheless ~1 log lower than those in the adjuvant group. On day 8 (Fig. 2D), the bacterial loads in all tissues of mice receiving the combination vaccine were significantly lower than those of the other vaccinated groups. The organ-specific bacterial burden in the liver and spleen of combination-vaccine immunized mice was below the level of detection at day 28 after challenge (Fig. 2E). The bacterial load was reduced by ~4 logs in lung tissue from this group at day 28.
3.4. Mice given the combination vaccine exhibit less severe pathology after Ft.LVS challenge
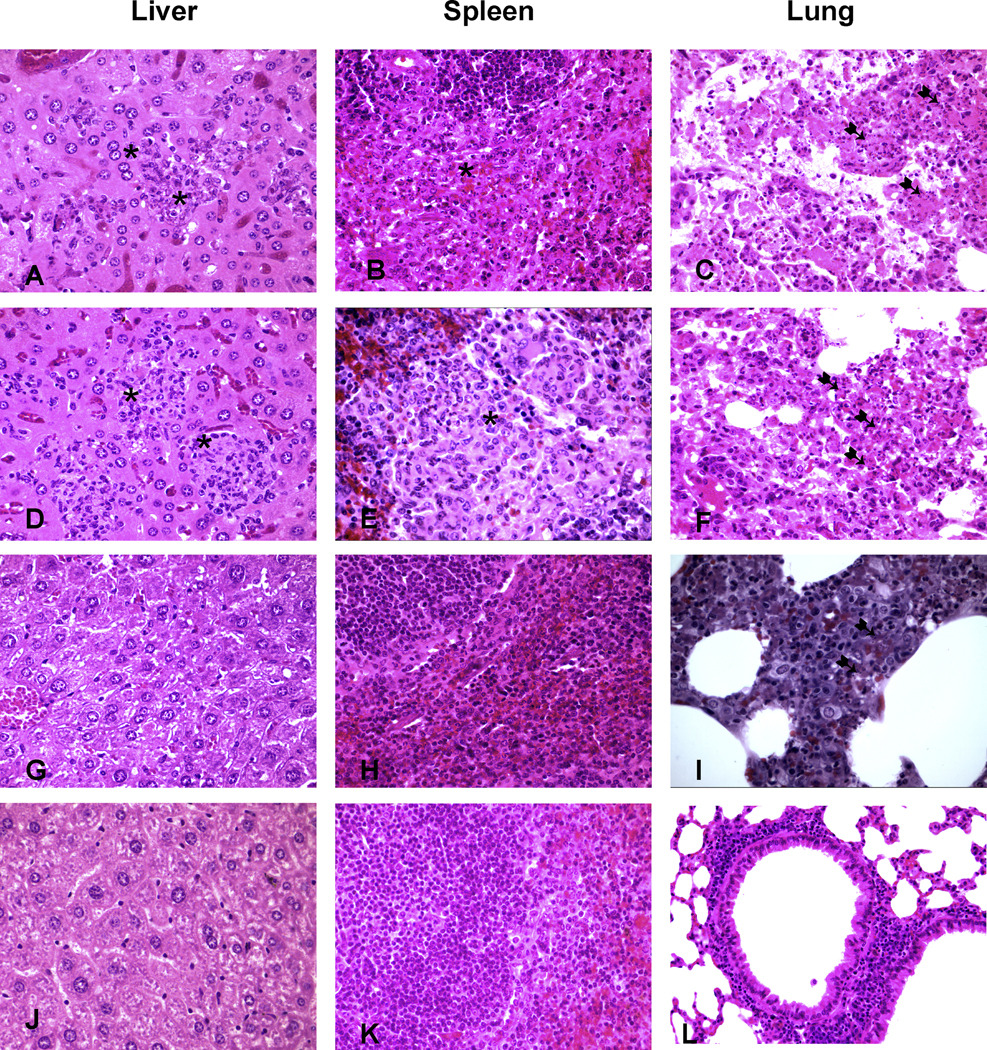
Histopathologic studies of liver tissue from mice vaccinated with the glycoconjugate or with mutant strain Ft.LVS::wbtA (Figs. 3A and 3D, respectively) showed obvious signs of inflammation and severe necrosis, with several foci of neutrophilic infiltration on day 7 after intranasal challenge. In stark contrast, liver tissue from mice given the combination vaccine contained only minor sporadic infiltrations, which were most likely due to the low numbers of bacteria in these tissues (Fig. 3G). Histopathologic examination of splenic tissue from glycoconjugate- or wbtA-vaccinated mice showed massive infiltration by neutrophils and macrophages (pyogranuloma) on day 7 after challenge (Figs. 3B and 3E), while normal architecture with only mild infiltration by polymorphonuclear leukocytes was evident in spleen tissue from mice immunized with the combination vaccine (Fig. 3H). In contrast to the findings on spleen and liver histology, histopathologic analysis of lung tissue from mice given the combination vaccine (Fig. 3I) revealed obvious signs of inflammation (bronchopneumonic lesions) and necrotic cellular debris similar to that found in lung tissue from mice vaccinated with the glycoconjugate (Fig. 3C) or the wbtA strain (Fig. 3F). On day 28, however, histopathologic examination of liver, spleen, and lung tissues from combination-vaccine recipients demonstrated only minor sporadic infiltration, with a histology closely resembling that of normal tissue (Figs. 3J, 3K, and 3L).
Fig. 3.
Histopathologic features: Tissues from mice vaccinated with TT-OPS alone (day 7, A–C), Ft.LVS::wbtA alone (day 7, D–F), or the combination vaccine (day 7, G–I; day 28, J–L) (hematoxylin and eosin). Asterisks indicate sites of massive infiltration in tissues; arrows indicate areas of severe necrosis in lung tissue.
The combination vaccine confers protection against intranasal challenge with wild-type F. tularensis
The efficacy of protective immunity generated by the combination vaccine was evaluated after an otherwise-lethal intranasal challenge with wild-type organisms of subspecies type A (strain Schu S4) or type B (strain FSC 108) (Fig. 4A). As shown in Fig. 4B, the time to death after a low but lethal type A challenge was significantly longer among mice given the combination vaccine (median survival time: 15 days) than among adjuvant control mice (median survival time: 7 days). Complete protection was documented when combination-vaccinated mice were challenged intranasally with ~10 cfu of virulent type B strain FSC 108 (Fig. 4C). Moreover, a high level of protection was noted when combination-vaccine recipients were challenged intranasally with ~100 cfu of virulent type B organisms 6 weeks after second immunization dose (Fig. 4D). Determination of the bacterial load in reticuloendothelial tissues after type A challenge indicated a 2-log bacterial reduction in spleen and liver tissues from combination-vaccinated mice (Table 1) and the presence of a similar bacterial burden in lung tissues from both groups. The bacterial burden documented in blood samples from the vaccinated group was 3 logs lower than that in blood from control mice after type A intranasal challenge (Table 1). Consistent with the complete protection observed after a low-dose intranasal challenge with type B organisms, all reticuloendothelial tissues from the vaccinated group exhibited a cfu reduction of ~4–5 logs from counts in the adjuvant control group (Table 1). Remarkably, a 106-fold reduction in bacterial burden was documented when blood samples from the vaccinated group were examined after type B challenge (Table 1).
Fig. 4.
Survival of mice (5 per group) after lethal intranasal challenge with a wild-type strain of F. tularensis: (A) Immunization schedule. BALB/c mice received two immunizing doses (2 weeks apart) and were challenged intranasally after a 4-week (panel B and C) or 6-week (panel D) rest period with an ordinarily lethal dose of a wild-type strain; (B) Survival after intranasal challenge with ~10 cfu of virulent type A strain Schu S4, (FSC 237); (C) Survival after intranasal challenge with ~10 cfu of virulent type B strain (FSC 108); (D) Survival after intranasal challenge with ~100 cfu of virulent type B strain (FSC 108).
Table 1.
Bacterial burdens in mock immunized and immunized mice challenged intranasally with type A or type B F. tularensis.
| Mice | Challenge strain |
Day of infection |
Log10 CFU ±SD / organ (proportion infected) n=5 |
|||
|---|---|---|---|---|---|---|
| liver | spleen | lungs | Blood /mL |
|||
| Mock immunized | SCHU S4 | 5 | 6.87±1.46a | 7.55±1.53a | 6.26±1.54 | 5.56±1.0a (4/5) |
| Combination vaccine | 4.88±1.18 | 5.10±1.64 | 6.30±0.89 | 2.48±0.67 (2/5) | ||
| Mock immunized | FSC 108 | 8 | 8.76±2.02a | 8.51±1.90a | 8.25±1.68a | 8.00±2.05a (4/5) |
| Combination vaccine | 2.92±0.86 | 3.97±0.62 (3/5) | 3.57±1.07 | 2.15±0.22 (2/5) | ||
Mice were challenged IN with 6 or 7 CFU of type A strain, SCHU S4 or type B strain, FSC 108 respectively, four weeks post-vaccination. The former mice were killed on day 5, and the latter mice on day 8 post-challenge.
Significantly higher burden (P<0.05) than in immunized mice by two tailed t test.
3.5. The combination vaccine confers protection against high-dose intradermal challenge with wild-type F. tularensis
Six weeks after the second combination vaccine immunization dose, mice were challenged intradermally with a high dose (1000 cfu) of wild-type F. tularensis type A (strain Schu S4). A high level of protection was observed, with 60% survival (Fig. 5A). Complete protection of immunized recipients was documented after intradermal challenge with the same dose of F. tularensis type B (strain FSC 108) (Fig. 5B). Although combination-immunized mice showed no overt signs of infection, bacteriologic studies of survivors on day 28 demonstrated ~2.256 ± 0.568 cfu per organ of the type B strain and ~3.174 ± 0.540 cfu per organ of the type A strain in splenic tissue.
Fig. 5.
Survival of BALB/c mice (5 per group) after high dose lethal intradermal challenge with wild-type strain of F. tularensis following 6-week post immunization: (A) Survival after challenge with ~1000 times the LD50 of F. tularensis wild-type type A strain Schu S4 (FSC 237); (B) Survival after challenge with ~1000 times the LD50 of F. tularensis wild-type type B strain (FSC 108).
4. Discussion
The pivotal role of T cell–mediated events in the control of tularemia has been well established [6, 15–20]. However, studies from several laboratories have shown that humoral immunity also plays a critical role, especially in the early phase of type B F. tularensis infection [21, 22]. Using B cell–deficient mice, Stenmark et al demonstrated the important role of humoral antibodies in protection against a type B strain of F. tularensis [23]. Drabick et al found that pooled immune sera from human LVS recipients protected mice fully against challenge with 10,000 times the LD50 of the LVS strain [24]. Fulop et al demonstrated the role of antibodies to LPS in protection against LVS in T cell–depleted mice [2, 4]. Our recent studies indicate that protective antibodies directed specifically toward the OPS of Ft.LVS LPS are an important component of humoral immunity [11]. On the basis of these findings, it seems reasonable to conclude that an efficacious tularemia vaccine should effectively elicit both humoral components (OPS-specific antibodies) and cell-mediated components (protein antigen[s]). Ft.LVS fulfills these criteria but has the potential to cause adverse side effects. The identification of key virulence factors and candidate protein antigens has been challenging because of a lack of sequenced genomes and of well-defined genetic manipulation techniques [8]. Recently, however, these impediments have been overcome, and significant progress has been made toward both genetic manipulation of F. tularensis and identification of candidate antigens [25]. Although several putative candidate protein antigens that are immunogenic and promote T cell proliferation have been identified, none have been shown to induce protective immunity against type A challenge in the mouse model [2, 26–29].
The currently available, empirically derived, still-unlicensed vaccine strain of F. tularensis is effective, but its use is complicated by significant undesirable side effects. To elucidate the role of immunodominant protein antigens and to address the need for a safe tularemia vaccine, we have used a novel combination containing the OPS-deficient mutant strain Ft.LVS::wbtA as a source of protein antigens (to induce cellular immunity) and an OPS-TT glycoconjugate as an inducer of a robust OPS antibody response (to elicit humoral immunity). Use of the Ft.LVS::wbtA strain is an attractive option for two reasons. First, this strain is markedly attenuated compared with LVS [11, 30]. Second, the lack of an immunodominant surface polysaccharide in the wbtA strain may permit better exposure of other antigens that may be critical for protective immunity. Furthermore, use of OPS from Ft.LVS is expected to facilitate cross protection against type A strain, since the chemical structure of OPS from F.tularensis subspecies tularensis (Schu S4) and F. tularensis subspecies holaractica (LVS) has been demonstrated to be identical [31, 32].
Pneumonic tularemia—the most potent form of the disease—is thought to result from inhalation of highly infectious type A F. tularensis via the aerosol route [1, 8, 29, 33]. After aerosol exposure, the bacterium proliferates exponentially in the lung. Once the organ load in the lung reaches a certain threshold, infection disseminates and becomes systemic; proliferation of organisms in spleen and liver tissues ultimately leads to organ failure and death. An ideal tularemia vaccine thus should contain both the proliferation of the pathogen at the portal of entry (the respiratory tract) and its dissemination to reticuloendothelial tissues. Compelling evidence from a growing body of literature has indicated the importance of aerosol and/or intranasal immunization for optimal priming of mucosal immunity [34]. In addition, the control of F. tularensis–mediated respiratory infections (especially those initiated by type A organisms) is enhanced after intranasal immunization[10, 35, 36]. Aerosol but not intradermal immunization with LVS protects mice against subsequent aerosol challenge with a highly virulent type A strain by an αβ T cell and interferon gamma dependent mechanism. It is important to note, however, that mice are much more susceptible to exposure to F. tularensis LVS via the intranasal route (LD50 =1000 cfu) than via the intradermal route (LD50 = 106 cfu) [35, 37]. Thus intranasal immunization with Ft.LVS often results in infection and sometimes causes death. In humans, aerosol vaccination with effective doses of LVS can also result in overt infection [10]. To ensure the safety of the immunization regimen, we have used Ft.LVS::wbtA, a highly attenuated OPS mutant of Ft.LVS. Our previous studies had shown that this mutant, although significantly attenuated, retains its ability to confer protective immunity to mice against challenge with an otherwise-lethal dose of either Ft.LVS or a fully virulent clinical isolate of F. tularensis type B [11]. The importance of LPS/OPS antibodies in protective immunity is well documented [4, 11]. To compensate for the lack of OPS in the wbtA vaccine strain, we purified the core OPS from Ft.LVS and chemically conjugated it to a tetanus toxoid carrier protein, thereby generating a T cell–dependent OPS-TT glycoconjugate as previously described [5]. We envisaged that use of the OPS mutant strain in combination with the OPS-TT glycoconjugate would yield a safe immunization regimen that retains all the immunogenic components of Ft.LVS. We administered the wbtA vaccine strain by the intranasal route and the OPS-TT glycoconjugate by the subcutaneous route.
This combination vaccine afforded complete protection against high-dose Ft.LVS intranasal challenge (~400 times the intranasal LD50). The combination prevented the proliferation of Ft.LVS; immunization was followed by the resolution of Ft.LVS-initiated respiratory tularemia. Histopathologic study of liver and spleen tissues from mice receiving the combination vaccine revealed only minor sporadic infiltrations that most likely were due to the low numbers of bacteria in these tissues. Although a significant level of infiltration and a higher bacterial count were observed in lung tissue from these mice during the initial stages of infection, the combination vaccine appeared to control the bacterial burden. By day 28 after challenge, the pneumonic lesions had resolved and the lung-tissue architecture was nearly normal, with only mild infiltrates. This approach was not associated with specific side effects like those that typically follow immunization with Ft.LVS (especially via the intranasal route) in the murine tularemia model.
Likewise, high-level protection was conferred when combination-immunized mice were challenged intranasally with both a low dose (~10 cfu) and a high dose (~100 cfu) of wild-type organisms of type B strain FSC 108. Moreover, time to death was significantly longer when similarly immunized mice were challenged intranasally with a low dose (~10 cfu) of type A strain Schu S4. The combination vaccine effectively protected mice against intradermal challenge with a high dose (~1000 cfu) of wild-type organisms of type A (Schu S4) or type B (FSC 108). The ability of this combination vaccine to control proliferation and dissemination more effectively for wild-type organisms of type B than for wild-type organisms of type A was further evidenced by the significantly greater reduction in the number of cfu in the blood and in organ cultures of immunized mice after type B challenge.
Although immunization with the combination vaccine conferred only partial protection against intranasal challenge with wild-type strain Schu S4 (type A), the overall level of protection was significantly greater than in previous glycoconjugate immunization studies [5]. Additionally, the overall protection observed here following a combination vaccine was far superior to the previously observed protection following single immunization with Ft.LVS::wbtA vaccine strain [11]. In those investigations, only partial protection was noted when mice were subjected to low-dose aerosol challenge with wild-type organisms of type B or to intradermal challenge with Schu S4, and no protection was observed against aerosol challenge with a highly virulent type A strain [5].
The exact reason for the inability of the combination regimen to completely control respiratory tularemia initiated by type A F. tularensis is not clear. It is important to note that comparative genomic analyses have indicated the existence of genetic differences between the F.tularensis subspecies [38]. This and other studies have indicated that more than 20 additional genes exists which are unique to subspecies tularensis and are missing from subspecies holaractica [13, 38]. The Ft.LVS::wbtA vaccine strain used in this study is a holaractica (type B) derivative; one likely explanation for the inability of the current combination vaccine to completely protect against a type A intranasal challenge is that the wbtA vaccine strain may lack the critical type A–specific antigen(s) that are instrumental in controlling type A–induced respiratory tularemia [38]. This possibility is supported by the findings of Wu et al., who have shown that mice primed with LVS raise a stronger type A–specific protective immune response upon receipt of a boost with type A—but not with the LVS strain—before type A challenge [35]. Further studies using a similar OPS-negative live attenuated strain with a type A background are warranted to address these issues. Nevertheless, the results of this study represent a significant advance toward the provision of a safer vaccine to combat tularemia due to wild-type strains of F. tularensis type A and type B.
Acknowledgments
The authors thank Dr. R. T Bronson for expert contributions and Hua Shen for studies employing virulent isolates of F. tularensis. This work was supported by the New England Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (AI057157).and grant AI48474 to Dr. Wayne Conlan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11(3):440–451. [PubMed] [Google Scholar]
- 2.Fulop M, Manchee R, Titball R. Role of lipopolysaccharide and a major outer membrane protein from Francisella tularensis in the induction of immunity against tularemia. Vaccine. 1995;13(13):1220–1225. doi: 10.1016/0264-410x(95)00062-6. [DOI] [PubMed] [Google Scholar]
- 3.Fulop M, Manchee R, Titball R. Role of two outer membrane antigens in the induction of protective immunity against Francisella tularensis strains of different virulence. FEMS Immunol Med Microbiol. 1996;13(3):245–247. doi: 10.1111/j.1574-695X.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 4.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19(31):4465–4472. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 5.Conlan JW, Shen H, Webb A, Perry MB. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine. 2002;20(29–30):3465–3471. doi: 10.1016/s0264-410x(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 6.Conlan JW, Sjostedt A, North RJ. CD4+ and CD8+ T-cell-dependent and -independent host defense mechanisms can operate to control and resolve primary and secondary Francisella tularensis LVS infection in mice. Infect Immun. 1994;62(12):5603–5607. doi: 10.1128/iai.62.12.5603-5607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlan JW, Vinogradov E, Monteiro MA, Perry MB. Mice intradermally-inoculated with the intact lipopolysaccharide, but not the lipid A or O-chain, from Francisella tularensis LVS rapidly acquire varying degrees of enhanced resistance against systemic or aerogenic challenge with virulent strains of the pathogen. Microb Pathog. 2003;34(1):39–45. doi: 10.1016/s0882-4010(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 8.Oyston PC, Quarry JE. Tularemia vaccine: past, present and future. Antonie Van Leeuwenhoek. 2005;87(4):277–281. doi: 10.1007/s10482-004-6251-7. [DOI] [PubMed] [Google Scholar]
- 9.Conlan JW. Vaccines against Francisella tularensis--past, present and future. Expert Rev Vaccines. 2004;3(3):307–314. doi: 10.1586/14760584.3.3.307. [DOI] [PubMed] [Google Scholar]
- 10.Eigelsbach HT, Tulis JJ, Overholt EL, Griffith WR. Aerogenic immunization of the monkey and guinea pig with live tularemia vaccine. Proc Soc Exp Biol Med. 1961;108:732–734. doi: 10.3181/00379727-108-27049. [DOI] [PubMed] [Google Scholar]
- 11.Sebastian S, Dillon ST, Lynch JG, Blalock LT, Balon E, Lee KT, et al. A defined O-antigen polysaccharide mutant of Francisella tularensis live vaccine strain has attenuated virulence while retaining its protective capacity. Infect Immun. 2007;75(5):2591–2602. doi: 10.1128/IAI.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Ryder C, Mandal M, Ahmed F, Azadi P, Snyder DS, et al. Attenuation and protective efficacy of an O-antigen-deficient mutant of Francisella tularensis LVS. Microbiology. 2007;153(Pt 9):3141–3153. doi: 10.1099/mic.0.2007/006460-0. [DOI] [PubMed] [Google Scholar]
- 13.Twine S, Bystrom M, Chen W, Forsman M, Golovliov I, Johansson A, et al. A mutant of Francisella tularensis strain SCHU S4 lacking the ability to express a 58-kilodalton protein is attenuated for virulence and is an effective live vaccine. Infect Immun. 2005;73(12):8345–8352. doi: 10.1128/IAI.73.12.8345-8352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjostedt A. Virulence determinants and protective antigens of Francisella tularensis. Curr Opin Microbiol. 2003;6(1):66–71. doi: 10.1016/s1369-5274(03)00002-x. [DOI] [PubMed] [Google Scholar]
- 15.Elkins KL, Rhinehart-Jones TR, Culkin SJ, Yee D, Winegar RK. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64(8):3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yee D, Rhinehart-Jones TR, Elkins KL. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1996;157(11):5042–5048. [PubMed] [Google Scholar]
- 17.Ericsson M, Kroca M, Johansson T, Sjostedt A, Tarnvik A. Long-lasting recall response of CD4+ and CD8+ alphabeta T cells, but not gammadelta T cells, to heat shock proteins of francisella tularensis. Scand J Infect Dis. 2001;33(2):145–152. doi: 10.1080/003655401750065562. [DOI] [PubMed] [Google Scholar]
- 18.Ericsson M, Sandstrom G, Sjostedt A, Tarnvik A. Persistence of cell-mediated immunity and decline of humoral immunity to the intracellular bacterium Francisella tularensis 25 years after natural infection. J Infect Dis. 1994;170(1):110–114. doi: 10.1093/infdis/170.1.110. [DOI] [PubMed] [Google Scholar]
- 19.Poquet Y, Kroca M, Halary F, Stenmark S, Peyrat MA, Bonneville M, et al. Expansion of Vgamma9 Vdelta2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66(5):2107–2114. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowley SC, Hamilton E, Frelinger JA, Su J, Forman J, Elkins KL. CD4-CD8- T cells control intracellular bacterial infections both in vitro and in vivo. J Exp Med. 2005;202(2):309–319. doi: 10.1084/jem.20050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elkins KL, Rhinehart-Jones T, Nacy CA, Winegar RK, Fortier AH. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect Immun. 1993;61(3):823–829. doi: 10.1128/iai.61.3.823-829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun. 2000;68(4):1988–1996. doi: 10.1128/iai.68.4.1988-1996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenmark S, Lindgren H, Tarnvik A, Sjostedt A. Specific antibodies contribute to the host protection against strains of Francisella tularensis subspecies holarctica. Microb Pathog. 2003;35(2):73–80. doi: 10.1016/s0882-4010(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 24.Drabick JJ, Narayanan RB, Williams JC, Leduc JW, Nacy CA. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994;308(2):83–87. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Lauriano CM, Barker JR, Nano FE, Arulanandam BP, Klose KE. Allelic exchange in Francisella tularensis using PCR products. FEMS Microbiol Lett. 2003;229(2):195–202. doi: 10.1016/S0378-1097(03)00820-6. [DOI] [PubMed] [Google Scholar]
- 26.Sandstrom G, Tarnvik A, Wolf-Watz H. Immunospecific T-lymphocyte stimulation by membrane proteins from Francisella tularensis. J Clin Microbiol. 1987;25(4):641–644. doi: 10.1128/jcm.25.4.641-644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjostedt A, Sandstrom G, Tarnvik A. Humoral and cell-mediated immunity in mice to a 17-kilodalton lipoprotein of Francisella tularensis expressed by Salmonella typhimurium. Infect Immun. 1992;60(7):2855–2862. doi: 10.1128/iai.60.7.2855-2862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjostedt A, Sandstrom G, Tarnvik A. Several membrane polypeptides of the live vaccine strain Francisella tularensis LVS stimulate T cells from naturally infected individuals. J Clin Microbiol. 1990;28(1):43–48. doi: 10.1128/jcm.28.1.43-48.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2(12):967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 30.Raynaud C, Meibom KL, Lety MA, Dubail I, Candela T, Frapy E, et al. Role of the wbt locus of Francisella tularensis in lipopolysaccharide O-antigen biogenesis and pathogenicity. Infect Immun. 2007;75(1):536–541. doi: 10.1128/IAI.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prior JL, Prior RG, Hitchen PG, Diaper H, Griffin KF, Morris HR, et al. Characterization of the O antigen gene cluster and structural analysis of the O antigen of Francisella tularensis subsp. tularensis. J Med Microbiol. 2003;52(Pt 10):845–851. doi: 10.1099/jmm.0.05184-0. [DOI] [PubMed] [Google Scholar]
- 32.Vinogradov EV, Shashkov AS, Knirel YA, Kochetkov NK, Tochtamysheva NV, Averin SF, et al. Structure of the O-antigen of Francisella tularensis strain 15. Carbohydr Res. 1991;214(2):289–297. doi: 10.1016/0008-6215(91)80036-m. [DOI] [PubMed] [Google Scholar]
- 33.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Tularemia as a biological weapon: medical and public health management. Jama. 2001;285(21):2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 34.Gallichan WS, Rosenthal KL. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184(5):1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect Immun. 2005;73(5):2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wayne Conlan J, Shen H, Kuolee R, Zhao X, Chen W. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma- dependent mechanism. Vaccine. 2005;23(19):2477–2485. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 37.Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59(9):2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broekhuijsen M, Larsson P, Johansson A, Bystrom M, Eriksson U, Larsson E, et al. Genome-wide DNA microarray analysis of Francisella tularensis strains demonstrates extensive genetic conservation within the species but identifies regions that are unique to the highly virulent F. tularensis subsp. tularensis. J Clin Microbiol. 2003;41(7):2924–2931. doi: 10.1128/JCM.41.7.2924-2931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]