Abstract
Over a 9-year period, 58 patients who had previous portasystemic shunt procedures underwent orthotopic liver transplantation (OLTx) under a cyclosporine-steroid immunosuppressive regimen. The types of shunt used were distal splenorenal (18 patients), mesocaval (17 patients), end-to-side portacaval (11 patients), side-to-side portacaval (5 patients) and proximal splenorenal (7 patients). The mean interval between shunt and transplantation was 6 years. There was no statistical difference in survival between patients with previous shunts and the entire population of patients with primary liver transplantation performed during the same period of time. Age, sex, shunt patency, status of portal vein, and use of vein or artery graft did not affect survival. Child’s classification had a significant influence on graft survival, even though no difference was subsequently observed in patient survival. A progressively improved intraoperative strategy and the rwe of veno-venous bypass and University of Wisconsin preservation solution had a significant impact on blood loss, length of operation, length of stay in intensive care unit, and ultimately, on survival. Distal splenorenal and mesocaval shunts with no or minimal hilum dissection are safer shunts if subsequent transplantation is planned; in fact, their 9-year survival was 87%, whereas all other shunts were associated with a survival no better than 52% (p <0.006).
Operative portasystemic decompression has been documented to control variceal bleeding in cirrhotic patients [1-3]. Numerous prospective randomized trials have been reported that compare the various surgical procedures. Among the many options, the selective or distal splenorenal shunt has the advantage of resulting in a lower incidence of postshunt encephalopathy [4-7]. Sclerotherapy is another very successful option for the short-term management of variceal bleeding, but the overall rebleeding rate is higher in patients undergoing this procedure when compared with patients undergoing operative portasystemic decompression [8-11]. Neither shunting procedures nor sclerotherapy have had an impact on patient survival [1-3,5,9,10,12]. However, both can be highly effective temporizing measures.
Orthotopic liver transplantation (OLTx) is the only potentially curative treatment for end-stage liver disease [13] and is the ideal treatment to relieve portal hypertension caused by sinusoidal (intrahepatic) block. Patients with previous portasystemic shunt procedures have a resulting set of complicating technical and anatomic factors that make the performance of liver transplantation even more challenging; hemodynamic disturbances, portal vein abnormalities, and arterialized adhesions are examples of these complications. Nonetheless, successful liver transplantation in patients with previous portasystemic shunt has already been reported by us [14] and others [15].
In this paper, we present our experience over a 9-year period of cyclosporine immunosuppression in 58 consecutive patients with end-stage liver disease and some type of operative portasystemic shunt who subsequently underwent OLTx. The results demonstrate that the survival in this unique candidate group is similar to those obtained in our overall liver transplantation population.
PATIENTS AND METHODS
Between March 1980 and March 1989, a total of 1,445 primary OLTx procedures were performed at the University of Colorado in Denver, and beginning in January 1981, at the University of Pittsburgh. Fifty-eight of these patients had undergone previous operative portasystemic decompression for variceal bleeding. They have now been followed from 6 months to 10 years after transplantation.
There were 26 male and 32 female patients, with a mean age of 39.2 ± 13 years (range: 4 to 64 years). Six of the patients were less than 18 years old at the time of OLTx. Twenty-three patients were classified as Child’s class B and 35 as Child’s C at the time of the pre-OLTx workup. All patients were given immunosuppression with cyclosporine and prednisone [16] to which azathioprine and antilymphoid globulins were added when clinically required.
The following parameters were reviewed for this study: type of previous shunt, time interval from the creation of the portasystemic shunt to OLTx, shunt patency at the time of OLTx, size and characteristics of the portal vein, management of the actual shunt during the transplantation procedure, use of vein or artery homograft during surgery, duration of anesthesia, intraoperative blood loss, duration of ischemia of the graft, length of time in intensive care unit, and graft and patient survival after OLTx.
Because of the refinements in surgical technique and organ preservation that occurred over the years, the results were partitioned in three consecutive groups. At first, the use of veno-venous bypass was used to divide the patients into Group 1 (no bypass) and Group 2 (bypass used). In Group 3, the University of Wisconsin solution was used for graft preservation, replacing the Collins solution previously used for Groups 1 and 2 (Figure 1).
Figure 1.
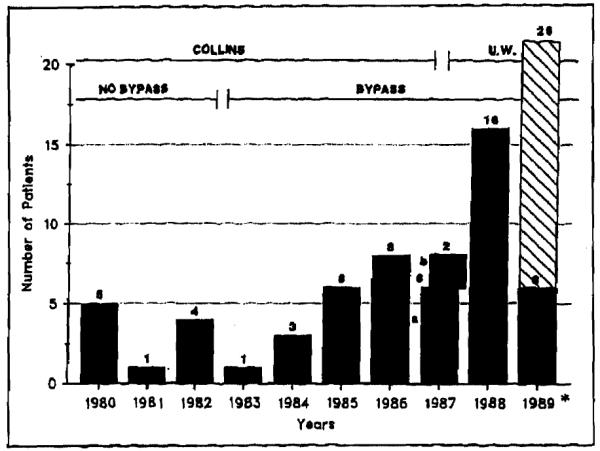
Different time periods in the surgical treatment of patients with previous portasystemic shunts. The number of patients who underwent transplantation per year is given. For 1987, a indicates the period from January to October when Collin’s preservation solution was used; b indicates the period from November to December when the University of Wisconsin (UW) preservation solution was used. Asterisk indicates the patients who underwent transplantation in the first 3 months of 1989; the predicted number for the year is shown.
The results, unless indicated, are expressed as mean ± standard deviation of the mean and analyzed using Fisher’s exact test. Estimated survivals were calculated and compared using the generalized Savage (Mantel-Cox) and Wilcoxon (Brenslow) life-table analysis, run on the BMDP-2L statistical software package (University of California).
RESULTS
The liver disease, type of previous shunt, and mean interval between the shunt procedure and OLTx are summarized in Tables I and II. Postnecrotic and primary biliary cirrhosis accounted for 45% of the cases. The types of shunts used were distal splenorenal (18 patients), mesocaval (17 patients), end-to-side portacaval (11 patients), proximal splenorenal (7 patients), and side-to-side portacaval (5 patients). The mean interval between the shunt and OLTx was approximately 6 years. This averaged 3.5 years in patients with side-to-side portacaval shunts and 7.9 years in patients with proximal splenorenal shunts. These differences were not statistically significant.
TABLE I.
Primary Diagnosis of 58 Liver Recipients with Previous Portasystemic Shunt
| Postnecrotic cirrhosis | 15 |
| Primary biliary cirrhosis | 11 |
| Alpha-1-antitrypsin deficiency | 6 |
| Wilson’s disease | 5 |
| Budd-Chiari syndrome | 5 |
| Congenital hepatic fibrosis | 4 |
| Sclerosing cholangitis | 3 |
| Alcoholic cirrhosis | 3 |
| Autoimmune chronic hepatitis | 3 |
| Hemophilia (plus hemosiderosis) | 1 |
| Neonatal hepatitis | 1 |
| Secondary biliary cirrhosis | 1 |
TABLE II.
Characteristics of the Study Population
| Type of Previous Shunt |
No. of Patients |
Age (median & range) |
Interval in Years from Shunt to OLTx (mean ± SD) |
|---|---|---|---|
| Portal venous—maintaining | |||
| Distal splenorenal | 18 | 41 (12–61) | 5.6 ± 0.9 |
| Portal venous—diverting | |||
| Mesocaval | 17 | 39 (7–56) | 5.1 ± 0.9 |
| Portacaval end-to-side | 11 | 42 (4–51) | 7.7 ± 2.5 |
| Portacaval stde-to-side | 5 | 32 (20–64) | 3.5 ± 0.7 |
| Proximal splenorenal | 7 | 21 (21–41) | 7.9 ± 1.9 |
There was no statistically significant difference in the actuarial 9-year survival between patients who underwent previous portasystemic shunt before transplantation and those who did not. In Figure 2, survival of portasystemic shunt patients is plotted against the survival of the entire population of 1,445 patients who received primary OLTx during the same period of time. Sixty-seven percent of the patients with portasystemic shunts were alive 5 years after OLTx compared with 65% of those without previous shunts.
Figure 2.
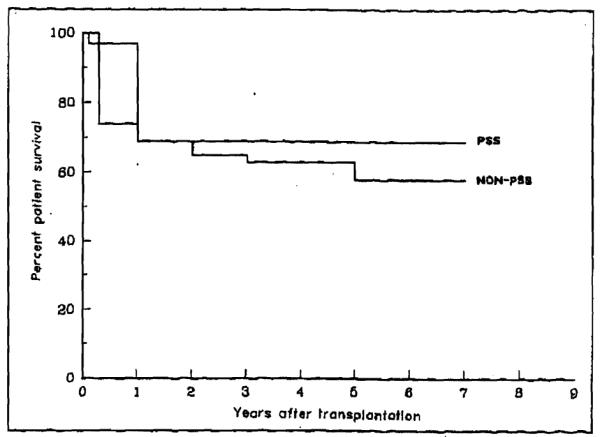
Survival of patients with portasystemic shunt (PSS) (58 patients) versus the entire population without PSS (1,445 patients) who underwent transplantation under cyclosporine immunosuppression from March 1980 to March 1989. No statistical difference is noted.
Patient age and sex did not influence the outcome. Shunt patency at the time of OLTx, an atrophic or sclerotic portal vein, or the use of vein or arterial graft did not have a statistical impact on either graft or patient survival. The pretransplantation Child’s status of the candidate had a definite influence on intraoperative blood loss, duration of the operation, length of ICU stay (Table III), and ultimately, on graft survival. The graft survival was significantly different between Child’s B and Child’s C patients (Figure 3). However, because of the timely resort to retransplantation in cases of graft failure, no significant difference was observed in patient survival (Figure 3), which was 74% for the Child’s B and 67% for the Child’s C groups.
TABLE III.
Blood Loss and Duration of Surgery, Ischemia, and ICU stay in Different Groups of Patients
| Pre-OLTx Status |
|||||
|---|---|---|---|---|---|
| Group 1 (n = 10) |
Group 2 (n = 24) |
Group 3 (n = 24) |
Child’s Class B (n = 23) |
Child’s Class C (n = 35) |
|
| Blood loss (U) | 80 ± 34 | 39 ± 33 | 19 ± 25 | 30 ± 34 | 37 ± 32 |
| OR time (hrs) | 15 ± 4 | 12 ± 4 | 12 ± 4 | 13 ± 3 | 14 ± 5 |
| Ischemia (hrs) | 7 ± 2 | 7 ± 2 | 13 ± 4 | 9 ± 4 | 11 ± 5 |
| ICU stay (days) | 96 ± 113 | 16 ± 25 | 8 ± 11 | 11 ± 21 | 43 ± 170 |
ICU = Intensive care unit; OR = operating room.
Figure 3.
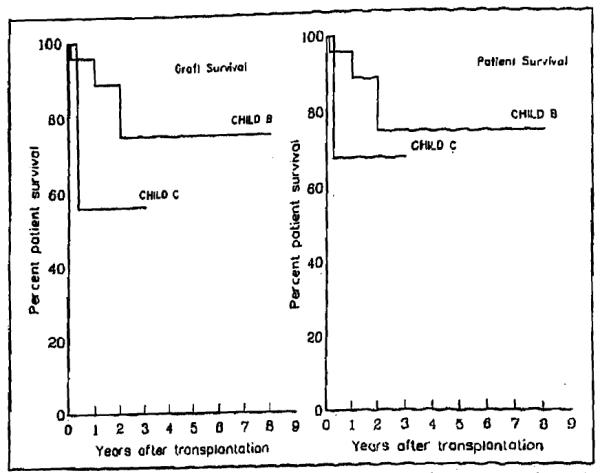
The graft survival was significantly different (by Mantel-Cox and Breslow) between patients in Child’s class B and those in child’s class C. Because of retransplantation in the case of graft failure, no difference was observed in patient survival.
The first 10 patients in the series (seen from 1980 to 1983) did not have the advantage of the intraoperative veno-venous bypass system [17]. In a second group of 24 patients (seen from 1983 to October 1987), the native hepatectomy was performed under bypass conditions (Figure 4 and Table III). In those patients, at least one cavacaval bypass was always used, and the portal limb of the system was added whenever allowed by the intraoperative conditions of the portal vein and the previous shunt. In a third group of 24 patients (seen from October 1987 to March 1989), the bypass was also employed, and the University of Wisconsin solution was used for graft preservation [18], replacing the Collins solution previously employed in Groups 1 and 2.
Figure 4.
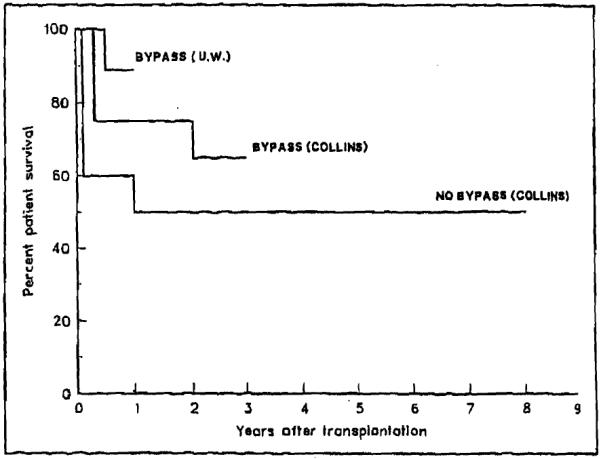
Differences in survival between different groups of patients (p <0.01 by Breslow, <0.008 by Mantel-Cox).
The use of veno-venous bypass was probably responsible for reducing the intraoperative blood loss from 60 units in Group 1 to 39 units in Group 2 and may have contributed to a shorter operation time (from 15 hours to 12 hours, respectively). There was a reduction of the average intensive care unit stay from 96 days for the patients without bypass to 16 days when veno-venous bypass was used.
With the introduction of the Wisconsin preservation solution, there was a significantly longer duration of cold ischemia of the graft, but the postreperfusion liver function was improved [18]. In Group 3 patients (bypass and Wisconsin solution), the duration of ischemia of the graft increased to 13 hours from a previous average time of 7 hours when the solution was not used (Groups 1 and 2). The longer and more effective preservation allowed a further planning of the operation, with better control of the bleeding and elimination of haste in reperfusing the organ. These facts are reflected in a further decrease in blood loss (to 19 units) and intensive care unit stay (to 8 days) in patients who received the solution (Table III). The actuarial survival also improved significantly (Figure 4).
The type of previous shunt had an impact on survival (Figure 5). Previous shunt procedures with no liver hilum dissection were safer. Patients with mesocaval and distal splenorenal shunts had a 5- and 9-year survival of 95% and 87%, respectively, whereas no previous shunt was associated with a survival better than 52% at the same time intervals. Those differences were highly significant (p <0.01 by Breslow, <0.006 by Mantel-Cox).
Figure 5.
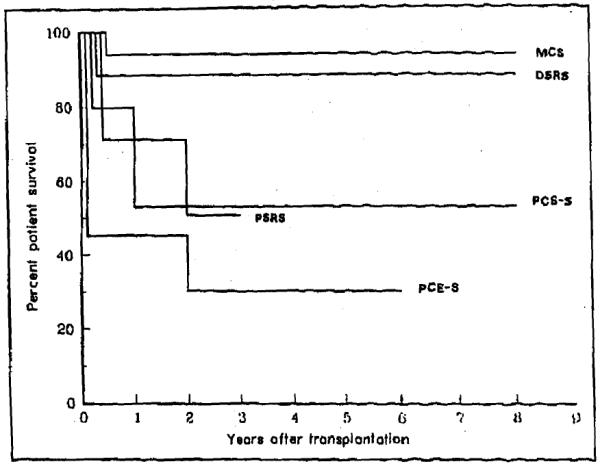
Influence of different types of previous shunt on survival after transplantation. Both mesocaval and distal splenorenal shunt procedures had a significant positive impact on survival when compared with other shunts (p <0.01 Breslow; <0.006 Mantel Cox). DSRS = distal splenorenal; MCS = mesocaval; PCE-S = portacaval end-to-side; PCS-S = portacaval side-to-side; PSRS = proximal splenorenal.
Thirty-one of the 58 patients had definite portal vein abnormalities determined by angiography or at the time of surgery. A thrombosed, small-caliber, or partially occluded portal vein did not preclude transplantation and did not influence survival. The portal limb of the veno-venous bypass was omitted in most of these situations, while a partial (femoral-axillary) bypass was always employed in Groups 2 and 3. Complete bypass was used in most cases in which there was a thrombosed or nonfunctional previous portasystemic shunt.
Abnormalities of the portal vein just distal to the confluence of the superior mesenteric and splenic veins required the use of a short segment of iliac vein graft for anastomosis to the donor portal vein. Ten patients with portal vein graft had no difference in survival compared with the rest of the present series. The technique of placing a vein homograft on a clotted or atrophic portal vein has been previously described [19]. More recently, an additional method of a vein jump-graft from the superior mesenteric vein has been developed [20].
Some shunt-specific technical modifications of the transplantation procedure were commonly applied. Arterialization of the graft was carried out first whenever restoration of portal flow was difficdt for any reason.
Distal splenorenal shunt
A double venous bypass was used in 9 of our 18 patients. Of the nine patients in whom bypass was not employed, three underwent transplantation prior to the bypass era, and in the other six, only partial femoral bypass was used because of either a thin-walled portal vein (one patient), thrombosed previous shunt (one patient), or thrombosed portal vein (four patients). A vein graft was necessary to reconstruct the thrombosed portal vein, and the arterial anastomosis was performed first in 10 of 18 patients because of a concern for adequate portal flow after unclamp. The closure of a previous patent splenorenal shunt is recommended at the end of the transplantation procedure after reperfusion of the new graft and can be performed simply with a splenectomy. Removal of the spleen avoids any dangerous dissection around the splenic vein or the old splenorenal anastomosis.
Mesocaval shunt
A patent shunt obviated the need for the portal limb of the bypass, but the shunt was often isolated and encircled at the onset of the operation in order to be prepared for immediate ligation after portal revascularization. When prosthetic materials had been used for the previous mesocaval anastomosis, a double firing of a TA35 intestinal stapler was usually sufficient to obstruct the old shunt. Complete bypass was performed in six of our patients when the shunt was thrombosed.
Portacaval shunt (end-to-side, side-to-side)
The previous surgery on the liver hilum renders the dissection for OLTx quite difficult in these patients compared with the previous two kinds of shunt. Thirteen of the 16 patients underwent transplantation prior to the bypass era. We recommend partial (femoro-axillary) bypass, leaving the shunt open as long as possible, since it serves the same purpose as the portal bypass. The lower caval anastomosis was usually performed while leaving the shunt undisturbed. Difficulties in dissection and the desire to preserve the shunt as long as possible frequently resulted in primary arterial revascularization (13 of 16 patients).
Proximal splenorenal shunt
As with the meso-caval shunt, a patent shunt obviated the need for portal bypass. A partial bypass was performed in five of the patients, and the shunt was ligated immediately after portal vein unclamping. In two of those cases, with a concomitant thrombosed portal vein, the arterial anastomosis was performed prior to the portal vein anastomosis. In one case with a thrombosed shunt, total bypass was performed even though the portal vein wall was abnormally thin.
COMMENTS
Our cumulative experience suggests that while either previous shunts, portal vein abnormalities, or the need for portal vein reconstruction significantly increase the complexity of the procedure as determined by the operative time and blood loss, these conditions do not prohibit successful hepatic transplantation.
A number of factors can contribute to good results. Careful patient evaluation and selection may be important. There was a very low incidence of alcoholic cirrhosis (three patients), while metabolic liver disease, primary biliary cirrhosis, and congenital hepatic fibrosis formed a higher relative proportion of the patients who underwent portasystemic shunt. Survival after portasystemic shunt is much lower in patients with alcoholic cirrhosis (45% 10-year survival) than in those patients in whom portal hypertension results from some other form of underlying liver disease (67% 10-year survival) [21-23]. The small number of alcoholic patients in our series may have been a favorable condition. The importance of patient selection was shown by the fact that preoperative transplantion status expressed by Child’s class was a significant determinant of graft survival (Figure 3).
Careful preoperative evaluation of the portal vein and shunt anatomy in liver transplantation candidates can be carried out using arteriography, magnetic resonance imaging, and Doppler ultrasonography to evaluate portal flow and shunt patency. These studies help in the development of an appropriate surgical strategy at the time of transplantation of the liver. Surgical technique is certainly a dominant factor in the treatment of such patients, who were operated on by our most experienced surgeons. There was no significant impact on prognosis exerted per se by intraoperative difficulties such as thrombosis of the portal vein or use of vein graft.
Distal splenorenal and mesocaval shunts presented a better situation for OLTx than any other portasystemic shunt in terms of both technical ease and graft or patient survival. There was easier access to the hepatic hilum of patients with those types of shunts at the time of transplantation, and if the portal vein was patent, there was no contraindication to the use of veno-venous bypass. Post-operative portal vein thrombosis occurred in three patients in the entire series of portasystemic shunt patients. It was previously reported that prior operations on the splanchnic circulation predisposed to peri-transplantation portal vein complications [24].
The role for OLTx in the treatment of portal hypertension should no longer be considered controversial. Liver transplantation was able to achieve 79% 1-year and 71% 5-year survival in patients who had end-stage liver disease in addition to a history of bleeding esophageal varices or who were actively bleeding at the time of transplantation [25]. These results were obtained regardless of the cause of cirrhosis (including alcoholism) and were better than those obtained with shunt operations [1,4-7,12,21-23], especially in patients with advanced hepatic failure (Child’s class C). However, shunt operations still have a role in the treatment of portal hypertension and should be considered complementary to transplantation in selected cases. The Warren shunt is the best of these procedures.
More and more reliance has been placed on sclerotherapy, with which survival similar to that of selective shunts can be achieved but with an higher incidence of rebleeding [8-11,26] and a significant incidence of esophageal perforation or strictures [27,28]. Nonetheless, sclerotherapy has been established in the control of acute variceal hemorrhage and in guaranteeing better candidates for liver transplantation [27]. Thus, a distal splenorenal anastomosis may be the preferred way to relieve portal hypertension only in patients who have Child’s A status.
Acknowledgments
Supported in part by Research Grants from the Veterans Administration, Washington, DC, and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Warren WD, Millikan WJ, Henderson JM, et al. Ten years portal hypertensive surgery at Emory: results and new perspectives. Ann Surg. 1982;95:530–42. doi: 10.1097/00000658-198205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson JM, Warren WD. Portal hypertension. Curr Probl Surg. 1988;25:151–223. doi: 10.1016/0011-3840(88)90013-5. [DOI] [PubMed] [Google Scholar]
- 3.Terblanche J, Burroughs AK, Hobbs KEF. Controversies in the management of bleeding esophageal varices. N Engl J Med. 1989;320:1393–8. 1469–75. doi: 10.1056/NEJM198905253202107. [DOI] [PubMed] [Google Scholar]
- 4.Millikan WJ, Warren WD, Henderson JM, et al. The Emory prospective randomized trial: selective versus non-selective shunt to control variceal bleeding. Ann Surg. 1985;201:712–22. doi: 10.1097/00000658-198506000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer B, Taylor BR, Mackenzie DR, Gilas T, Stone RM, Blendis L. Further report of a prospective randomized trial comparing distal splenorenal shunt with end to side portacaval shunt: an analysis of encephalopathy, survival and quality of life. Gastroenterology. 1985;88:424–9. doi: 10.1016/0016-5085(85)90502-5. [DOI] [PubMed] [Google Scholar]
- 6.Henderson JM. Variceal bleeding: which shunt [Editorial]? Gastroenterology. 1986;93:1021–3. doi: 10.1016/0016-5085(86)90710-9. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas A, Busuttil RWA. Comparative analysis of the mesocaval H graft versus the distal splenorenal shunt. Curr Surg. 1982;39:151–7. [PubMed] [Google Scholar]
- 8.Warren WD, Henderson JM, Millikan WJ, et al. Distal splenorenal shunt versus endoscopic sclerotherapy for long term management of variceal bleeding: preliminary report of a prospective, randomized trial. Ann Surg. 1986;203:454–62. doi: 10.1097/00000658-198605000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rikkers LF, Burnett DA, Volentine GD, Buchi KN, Cormier RA. Shunt surgery versus endoscopic sclerotherapy for long term treatment of variceal bleeding. Ann Surg. 1987;206:261–71. doi: 10.1097/00000658-198709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Copenhagen Esophageal Varices and Sclerotherapy Project Sclerotherapy after first variceal hemorrhage in cirrhosis. A randomized multicenter trial. N Engl J Med. 1984;311:1594–1600. doi: 10.1056/NEJM198412203112502. [DOI] [PubMed] [Google Scholar]
- 11.Eckhauser FE, Pomerantz RA, Knol JA, Strodel WE, Williams DM, Turcotte JG. Early variceal rebleeding after successful distal splenorenal shunt. Arch Surg. 1986;121:547–52. doi: 10.1001/archsurg.1986.01400050065008. [DOI] [PubMed] [Google Scholar]
- 12.Terblanche J. The surgeon’s role in the management of portal hypertension. Ann Surg. 1989;209:381–95. doi: 10.1097/00000658-198904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Demetris AJ, Van Thiel DH. Medical progress: liver transplantation. N Engl J Med. 1989;321:1014–22. 1092–9. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esquivel CO, Klintmalm G, Iwatsuki S, et al. Liver transplantation in patients with patent splenorenal shunts. Surgery. 1987;207:430–2. [PMC free article] [PubMed] [Google Scholar]
- 15.Brems JJ, Hiatt JR, Klein AS, et al. Effect of a prior portasystemic shunt on subsequent liver transplantation. Ann Surg. 1989;209:51–6. doi: 10.1097/00000658-198901000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD, Esquivel CO. Immunosuppression and other non-surgical factors in the improved results of liver transplantation. Semin Liver Dis. 1985;5:325–8. doi: 10.1055/s-2008-1040630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–34. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–4. [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Iwatsuki S, Shaw BW., Jr . Techniques of liver transplantation. In: Blumgart LH, editor. Surgery of the liver and biliary tract. Churchill Livingstone; Edinburgh: 1988. pp. 1537–52. [Google Scholar]
- 20.Tzakis A, Todo S, Stieber A, Starzl TE. Venous jump grafts for liver transplantation in patients with portal vein thrombosis. Transplantation. 1989;48:530–1. doi: 10.1097/00007890-198909000-00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson JM, Millikan WJ, Jr, Wright-Bacon L, Kutner MH, Warren WD. Hemodynamic differences between alcoholic and nonalcoholic cirrhotics following distal splenorenal shunt: effect of survival? Ann Surg. 1983;198:325–34. doi: 10.1097/00000658-198309000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeppa R, Hensley GT, Levi J, et al. Tbe comparative survivals of alcoholics versus nonalcoholics after distal splenorenal shunt. Ann Surg. 1978;187:510–4. doi: 10.1097/00000658-197805000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harley HAJ, Morgan T, Redeker AG, et al. Results of randomized trial of end to side portacaval shunt and distal splenorenal shunt in alcoholic liver disease and variceal bleeding. Gastroenterology. 1986;91:802–9. doi: 10.1016/0016-5085(86)90679-7. [DOI] [PubMed] [Google Scholar]
- 24.Lerut J, Tzakis AG, Bron K, et al. Complication of venous reconstruction in human orthotopic liver transplantation. Ann Surg. 1987;205:404–14. doi: 10.1097/00000658-198704000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwatsuki S, Starzl TE, Todo S, et al. Liver transplantation in the treatment of bleeding esophageal varices. Surgery. 1988;104:697–706. [PMC free article] [PubMed] [Google Scholar]
- 26.Cello JP, Grendell JH, Crass RA, Weber TE, Trunkey DD. Endoscopic sclerotherapy versus portacaval shunt in patients with severe cirrhosis and acute variceal hemorrhage. N Engl J Med. 1988;316:11–5. doi: 10.1056/NEJM198701013160103. [DOI] [PubMed] [Google Scholar]
- 27.Crass RA, Keeffe EB, Pinson CW. Management of variceal hemorrhage in the potential liver transplant candidate. Am J Surg. 1989;157:476–8. doi: 10.1016/0002-9610(89)90638-7. [DOI] [PubMed] [Google Scholar]
- 28.Conn HO, Atterbury CE. Cirrhosis. In: Shiff L, Shiff ER, editors. Diseases of the liver. 6th ed. JB Lippincott; Philadelphia: 1981. pp. 725–864. [Google Scholar]


