Abstract
Background
Ischemia/reperfusion (IR) injury is an unavoidable consequence of tissue transplantation or replantation that often leads to inflammation and cell death. Excessive complement activation following IR induces endothelial cell injury, altering vascular and endothelial barrier function causing tissue dysfunction. To mitigate the IR response, various systemic anti-complement therapies have been tried. Recently, we developed a localized therapy that uses biotinylated fusogenic lipid vesicles (BioFLVs) to first incorporate biotin tethers onto cell membranes, which are then used to bind therapeutic fusion proteins containing streptavidin (SA) resulting in the decoration of cell membranes. The therapy is applied in two steps using solutions delivered intra-arterially.
Materials and methods
Alteration of formulation, concentration and duration of incubation of BioFLVs were conducted to demonstrate the ability of the system to modulate biotin tether incorporation in cultured cells. Using a rat hind limb model, the ability of BioFLVs to decorated endothelium of femoral vessels with FITC-labeled SA for 48 h of reperfusion was demonstrated. The feasibility of a BioFLV-based anti-complement therapy was tested in cultured cells using SA fused with vaccinia virus complement control protein (SA-VCP), a C3 convertase inhibitor. Human ovarian carcinoma (SKOV-3) cells were incubated with BioFLVs first and then with SA-VCP. To activate complement the cells were treated with a SKOV-3-specific antibody (trastuzumab) and incubated in human serum.
Results
Decoration of cells with SA-VCP effectively reduced complement deposition.
Conclusions
We conclude that BioFLV-mediated decoration of cell membranes with anti-complement proteins reduces complement activation and deposition in vitro and has the potential for application against inappropropriate complement activation in vivo.
Keywords: Ischemia-reperfusion injury, complement, transplantation, fusogenic lipid vesicles, vaccinia virus complement control protein
Introduction
The etiology of ischemia/reperfusion injury (IRI) is multi-factorial and occurs in a variety of clinical scenarios including replantation of traumatic limb amputations or transplantation of tissues/organs. During reperfusion, unregulated complement activation causes excessive complement deposition on the vascular endothelium which potentiates inflammation leading to severe tissue/organ damage.[1–3] Although IRI primarily elicits complement activation by the alternate pathway, in some organs the involvement of classical and lectin pathways also have been reported.[4;5] As a result, several proteins within the complement cascade have served as targets for complement inhibition.[6–8]
The complement system comprises of plasma and cell bound proteins that orchestrate the host innate immune response to pathogens by causing direct or indirect cell injury. Briefly, complement proteins are activated by three pathways, classical pathway by antigen-antibody interaction, the alternate pathway by spontaneous hydrolysis of C3 and the lectin pathway by binding of mannose-binding lectin to sugar moieties on bacterial cell surfaces.[9] Upon activation, all the pathways lead to the formation of C3 convertase which cleaves C3 to C3a and C3b. C3b is deposited on the cell surfaces and acts as a receptor for C5 with further cleavage to form C5a and C5b. C5b in turn binds sequentially to C6-9 forming the terminal membrane attack complex (MAC) which creates pores in cell membranes leading to cell lysis.[10]
Although previous complement inhibition therapies were effective, [11–13] variable half-life of inhibitors and potential complications such as vulnerability to infection and immune complex diseases posed significant challenges. Since complement-mediated damage in IRI disrupts the integrity of the endothelial barrier, a current strategy is to develop endothelial cell-protective targeted approaches. To this end, we describe a novel method to anchor vaccinia virus complement control protein (VCP), a potent anti-complement protein on cell membranes to reduce complement activation and deposition. This involves a two step process wherein the cell membranes were first functionalized with biotin tethers; these were then used to link with the streptavidin (SA) domain of the fusion protein SA-VCP. VCP binds to C3b and C4b in the presence of cofactor1 and blocks the formation of both alternate and classical C3 convertase complex, thus preventing further propagation of the cascade.[14;15]
The purpose of this study was to develop, characterize and evaluate whether a biotin-SA coupling strategy designed to link and display a potent anti-complement protein on cell membranes can reduce complement deposition in vitro. Here, we report that targeted delivery of biotin tethers using biotinylated FLVs (BioFLVs) and subsequent linkage with SA-VCP has the ability to reduce complement deposition initiated by classical pathway of complement activation.
Materials and Methods
Materials
Synthetic lipids 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC); 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate (monosodium salt) (POPA); and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(biotinyl) (sodium salt) (DGPB) were purchased from Avanti Polar Lipids (Alabaster, AL) and stored dissolved in liquid chloroform at −20°C. ImmunoPure® Streptavidin, conjugated with fluorescein (SA-FITC) was purchased from Pierce Biotechnology (Rockford, IL).
SA-VCP synthesis
Gene sequences of VCP and SA were obtained from NCBI’s Gene bank database. The SA-VCP plasmid was inserted in a pPIC9K expression vector (Invitrogen Corporation, Carlsbad, CA) and cloned into the genome of Pichia pastoris. SDS-PAGE gel analysis was used to identify SA-VCP. Protein was purified using an iminobiotin-AH-Sepharose 4B column and by gel filtration size exclusion column (Sephacryl S300).
Cell Culture
Immortalized human fibroblasts and ovarian carcinoma (SKOV-3) cells were seeded in 75cm2 BD Falcon flasks, maintained at 37°C, 5% CO2, 90% humidity in a Steri-Cult 200 tissue culture incubator (Thermo Scientific, Marietta, OH). Fibroblasts were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10% fetal bovine serum (FBS); 2mM L-glutamine and 200U/mL penicillin/streptomycin mixture, all from Sigma Inc. St. Louis, MO. SKOV-3 cells were grown in McCoy’s Complete Medium (Caisson Labs, North Logan, UT) with 10% FBS, 2mM L-glutamine and 100U of penicillin/streptomycin.
BioFLV synthesis
Synthetic lipids dissolved in liquid chloroform DOPC, POPA and DGPB at an approximate ratio of 5:1:1 were mixed and dried overnight. Following rehydration with PBS to a concentration of 1mg/mL, lipid solution was vortexed for 1 min and incubated at 37°C for 5 min for 6 cycles. The resulting solutions were sonicated using a Branson Sonifier® 450A (Branson Ultrasonics Corp. Danbury, CT) at 50% duty cycle for 1 min/mL to form smaller unilamellar fusogenic vesicles.
BioFLV formulation experiment
Fibroblasts were seeded in 96 well plates coated with 0.5% porcine gelatin solution (Sigma Inc. St Louis, MO) at a density of 8×104 cells/mL and incubated for 24 h at 37°C, 5% CO2, 90% humidity. The cells were washed with PBS and incubated for 20 min with BioFLVs formulated with the following concentrations of DGPB: 2.5, 5, 9.5, 21.1 and 36.1 ng/μL. Control groups received no lipid treatment. Cells were then incubated with 0.05mg/mL SA-FITC for 15 min and washed with PBS. Fluorescent intensity was measured at 494–518nm with the SpectraMax M2 (Molecular Devices, Sunnyvale, CA) running Softmax Pro software.
BioFLV concentration experiment
Fibroblasts were seeded as described above and incubated with BioFLVs for 20 min at the following total lipid concentrations: 1, 20, 100, 200 and 500 μg/mL. (From this point forward all BioFLV experiments were performed with the highest DGPB (36.1ng/μL) concentration tried). The cells were washed with PBS and then incubated for 15 min with 0.05mg/mL SA-FITC and fluorescent intensity was obtained as described above.
BioFLV incubation time experiment
Fibroblasts were prepared as described earlier, then incubated with BioFLVs at total lipid concentration of 200μg/mL, for 10, 20, 30 and 40 min. The cells were washed three times with PBS and were treated with 0.05 mg/mL SA-FITC for 15 min. Cells were washed three times and fluorescent intensity was measured.
In vivo Biotinylation of endothelium using BioFLVs
Male Fisher-344 rats (250–300 g) were used in this study. All handling of animals was performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Louisville and with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 86-23).
In vivo experiments were conducted in rat hind limbs (n=5); briefly, under tourniquet, the right femoral artery was cannulated, flushed, and perfused with 10mL of BioFLVs (200μg/mL) and incubated for 40 min. 3mL of SA-FITC (0.05mg/mL) was perfused next and incubated for 15 min. The limbs were flushed with 20mL of lactated Ringer’s after each step. Animals were euthanized at 48 h, bilateral femoral vessels were harvested and immediately pressure fixed (Safefix II™, Fisher Scientific, Kalamazoo MI) with the left side serving as control. Transverse sections of femoral vessels were photographed in layers (1μm in thickness) using confocal microscopy (Olympus Fluoview FV 1000, IX81, Olympus America Inc, Center Valley, PA) with laser excitation set to 488 nm. To quantify signal intensity at the intimal layer of vessels, ten random fields from each image were selected for grayscale analysis using NIH ImageJ software (http://rsb.info.nih.gov/ij/)
Complement deposition experiment
SKOV-3 cell membranes were functionalized with biotin tethers following the same protocol as for human fibroblasts. Briefly, SKOV-3 cells were seeded in 96 well microplates at 8×104 cells/mL at ~90% confluence. Four experimental groups (n=8/group) were used. Group 1 cells (spontaneous deposition) received no treatment. Group 2 cells (maximal deposition) were incubated on ice for 30 min with 50μg/mL of trastuzumab (Herceptin, Genentech, San Francisco, CA) to activate complement. Group 3 cells were incubated for 40 min with BioFLVs, then for 30 min with SA-VCP (0.4μM), and treated with trastuzumab (50μg/mL). Group 4 cells (background controls) were incubated with media containing FITC-labeled antibody only. To evaluate C3b deposition on SKOV-3 cells, all groups were incubated for 30 min with a FITC-conjugated F(ab′)2 fragment antibody to human C3 (cross-reacts with C3b) (MP-Biomedicals, Solomon, OH) at 1:100 dilution. After treatments, cells in groups 1–3 were incubated in diluted (1:2) human serum for 25 min at 37°C. Cells in all groups were washed 3 times with PBS between treatments and prior to fluorescence evaluation. Fluorescent intensity was measured with a microplate reader as described above.
Statistical analysis
All experiments were analyzed for statistical significance by one way ANOVA followed by Tukey post-hoc tests. Differences between control groups and experimental groups were evaluated using t-test for two samples assuming equal variances. P values less than 0.05 were considered significant. The data is reported as means ± SEM.
Results
BioFLV formulation experiment
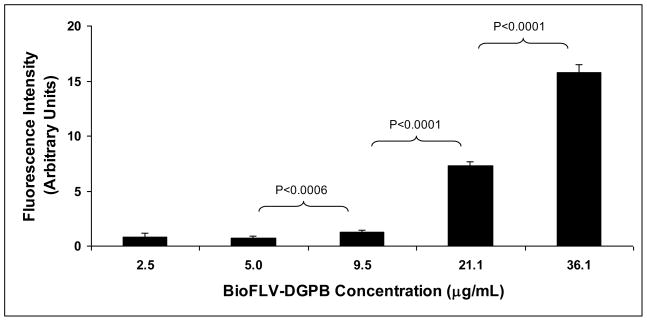
The purpose of this experiment was to establish the ideal lipid formulation of BioFLVs for efficient incorporation of biotin tethers into the target membranes. Our results demonstrate that the amount of biotin tethers displayed on fibroblast membranes is dose-dependent on the biotinylated lipid content (amount of DGPB) in BioFLVs (Fig. 1), and formulation with the final concentration of 36.1ng/μL DGPB achieved maximal biotinylation with no apparent loss of fusogenicity. DGPB at 21.1ng/μL was also effective but to a lesser degree. Both of these formulations achieved significantly higher membrane biotinylation levels (p<0.0001) as compared to other formulations.
Figure 1.
Display of biotin tethers on fibroblast cell membranes is altered by biotinylated fusogenic lipid vesicle (BioFLV) formulation. Cells were incubated for 20min with BioFLVs formulated with various levels of DGPB (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(biotinyl)) (n=8 wells per concentration). After washing cells, SA-FITC (0.05 mg/mL) was added to media, cells were incubated for 15 min and were washed. Fluorescent intensity measured relative levels of biotin tether incorporation on cell membranes. Reported values were corrected for background and reflect means ± SEM.
BioFLV concentration experiment
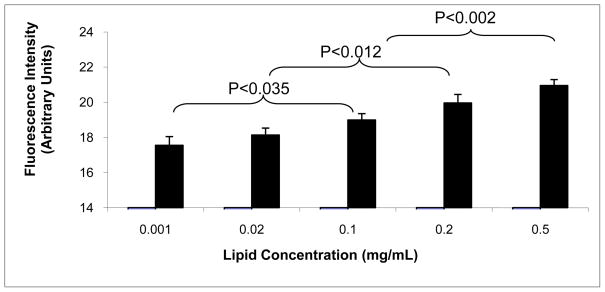
This experiment was conducted to determine the effect of BioFLV total lipid concentration in the incorporation of biotin tethers onto cell membranes at or below the maximal concentration of 0.5mg/mL. BioFLVs were administered at different dilutions in order to test total lipid concentrations ranging from 0.1–0.5mg/mL. All tested concentrations appeared to functionalize membranes in a dose-dependent manner (Fig. 2). Concentrations greater than 0.2mg/mL were statistically significant when compared to the lowest concentration (0.001mg/mL). However, there was no significant difference between the two lowest BioFLV and the two highest concentrations.
Figure 2.
Display of biotin tethers on fibroblast cell membranes is altered in a dose dependent manner by the concentration of biotinylated fusogenic lipid vesicles (BioFLVs) in media. Cells were incubated for 20 min with BioFLV concentrations ranging from 0.001 to 0.5mg/mL (n=8 wells per concentration). BioFLV formulation was the same for all concentrations. After washing cells, SA-FITC (0.05 mg/mL) was added to media, cells were incubated for 15 min and were washed. Fluorescent intensity measured relative levels of biotin tether incorporation on cell membranes. Reported values reflect means ± SEM.
BioFLV incubation time experiment
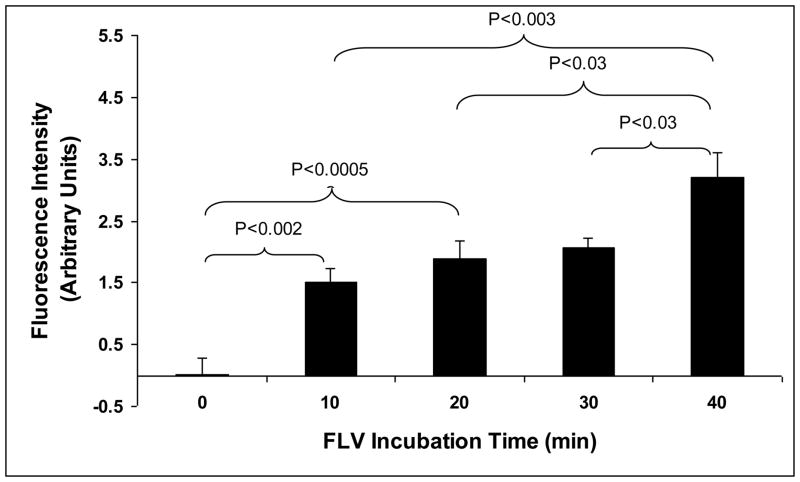
In surgical procedures that could benefit from localized anti-complement therapy to reduce IRI, a BioFLV incubation time of < 40 min seems reasonable. Therefore, the purpose of this experiment was to determine the rate at which BioFLVs biotinylated membranes within 40 min of incubation. The results demonstrated that as BioFLV incubation time increased, membrane biotinylation also increased in a dose-dependent manner (Fig. 3). Almost half of the maximal biotinylation attained at 40 min occurred during the first 10 min of incubation (~49%), and was statistically significant compared to 0 minute (p<0.002). The rate of biotinylation was slower during the last 30 min of incubation, as determined by rate of change of fluorescence intensity during the last 30 minutes of assessment (time point 20, 30, and 40 min)
Figure 3.
Display of biotin tethers on fibroblast cell membranes is altered by total incubation time of biotinylated fusogenic lipid vesicles (BioFLVs). Cells were incubated with BioFLVs of equal formulation and concentration for intervals of time between 10 and 40 min (n=8 wells per time interval). After washing cells, SA-FITC (0.05 mg/mL) was added to media, cells were incubated for 15 min and were washed. Fluorescent intensity was measured to quantify relative levels of biotin tether incorporation onto cell membranes. Reported values were corrected for background and represent means ± SEM.
In vivo Biotinylation of endothelium using BioFLVs
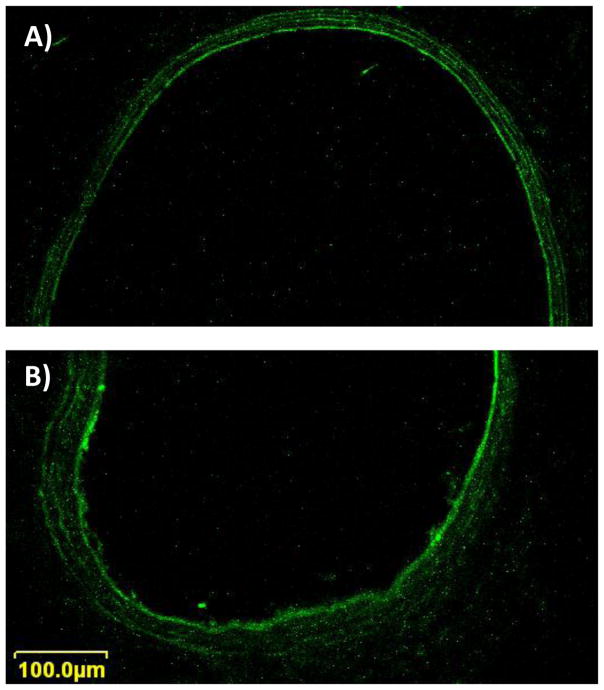
Since the success of any anti-complement therapy in IRI is dependent on the duration of its action in the first 24 h, in vivo experiments were conducted to determine whether biotin linked SA-FITC remained incorporated on the endothelial surface of vessels after 48 h of reperfusion. Our results confirm that biotin linked SA-FITC remained incorporated on the endothelial surface of vessels after 48 h of reperfusion; a significantly strong fluorescence signal was observed in the intima (Fig. 4) in the treated femoral arteries (70.0 ± 3.2 Arbitrary Units (AU)) compared to untreated contralateral control arteries (39.0 ± 4.9 AU; p<0.002). Likewise, treated femoral veins had a significantly strong fluorescence signal (58.8 ± 3.9 AU) compared to untreated controls (37.6 ± 5.2 AU; p<0.017). This suggested that proteins linked to biotin tethers on the intimal layer can be durably displayed up to 48 h and would be useful in therapies requiring short-term action.
Figure 4.
Confocal microscopy images of rat femoral artery cross sections (1μm thick) 48 h after intra-arterial biotinylated fusogenic lipid vesicle (BioFLV)/SA-FITC treatment to the right hindlimb. A) Shows a representative cross-section of the untreated left femoral artery, and B) shows a femoral artery from a treated hindlimb. Laser intensity setting was the same when images of treated and untreated sides were taken. Notice the higher signal intensity of the intima layer in the vessel from the treated hindlimb.
Complement deposition experiment
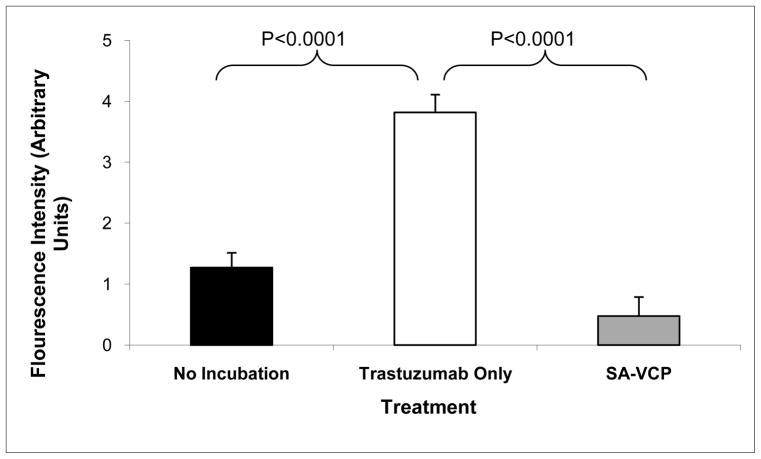
Assays were performed to determine whether membrane-localized SA-VCP is efficacious in reducing complement deposition upon activation of the classical pathway. Complement activation was achieved by incubating cells with the antibody, trastuzumab, which binds to HER-2/Neu receptors that are over-expressed in SKOV-3 cells and initiates classical complement activation by forming antigen-antibody complexes that bind to C1q. We found that cells displaying SA-VCP anchored to the membrane via biotin tethers exhibited significantly lower C3b deposition than the maximal activation controls (p<0.0001) (Fig. 5). No significant difference in C3b deposition was observed in untreated control cells exposed to human serum only compared to cells pre-treated with BioFLVs and SA-VCP exposed to human serum and trastuzumab. Since fluorescent intensity is directly correlated to the amount of complement deposition on SKOV-3 cells, it appears that SA-VCP displayed on the surface of cells retained bioactivity against classical complement activation and significantly reduced the amount of C3b deposited.
Figure 5.
SKOV-3 cells treated with biotinylated fusogenic lipid vesicles (BioFLVs) and SA-VCP reduced C3b deposition upon activation of complement. Human serum was the complement source which was added to the media. The black bar represents untreated cells showing spontaneous complement deposition. The white bar represents cells treated with the HER-2/Neu receptor antibody trastuzumab (50μg/mL) showing maximal deposition of complement. The grey bar represents cells treated with BioFLV/SA-VCP therapy and with trastuzumab (50μg/mL). Values were corrected for background and reflect means ± SEM.
Discussion
We present a novel approach of incorporating exogenous complement control proteins onto the surface of cell membranes that uses a biotin/SA coupling strategy, which has potential clinical application as an endothelium targeted therapy. The purpose of this study was 1. to demonstrate in vitro effective cell membrane biotinylation using BioFLVs without fusogens, 2. demonstrate in a rat hindlimb model that SA-FITC coupling with biotin tethers remain linked to the femoral vessel endothelium following 48 h of reperfusion, 3. demonstrate in vitro that membrane immobilized SA-VCP reduces complement deposition in a complement activation assay. In vivo findings in a rat hindlimb model demonstrated the ability of BioFLVs to target the limb’s whole vascular endothelium (arteries and veins) when administered via the femoral artery (Fig. 4). Endogenous auto-fluorescence was observed in the untreated control femoral vessels; however, fluorescence signal intensity appeared stronger in the treated femoral vessels, particularly in the intima (Fig 4A). The in vivo experiment also provided useful information regarding the durability of endothelial biotin/SA display subjected to the sheer forces of blood flow for 48 h. Anti-complement fusion proteins containing an SA domain can be expected to exert a protective effect against IR-induced injury in the first 48 h of reperfusion. The high affinity of biotin for SA (Kd ~ 10−15 M) makes this protein immobilization strategy extremely useful in this regard. SA-VCP was constructed by fusing SA to the carboxyl terminus of VCP to avoid interfering with the complement binding sites at the amino terminus.
In therapy, liposomes have been used primarily as drug delivery vehicles designed for long-acting sustained release (low fusogenicity) or engineered with fusogenic properties for intracellular delivery of encapsulated agents.[16–24] When FLVs are engineered to fuse with cells, as in the present study, incorporated FLV-derived lipids have been used to alter membrane surfaces. Two groups in the literature have used FLVs to modify cell surfaces. Fadok et al., performed in vitro studies in which T cells treated with FLVs formulated with phosphatidylserine induced co-cultured macrophages to engulf what appeared to be apoptotic T cells.[25] van Broekhoven et al., in an effort to develop a cancer vaccine used a zinc/polyhistidine (6) linking strategy in which FLVs formulated with a zinc chelator lipid incorporated zinc tethers on membranes to couple polyhistidine (6)-tagged co-stimulatory molecules on murine tumor cells.[26] In these experiments the adjuvant fusogen polyethylene glycol was employed to increase fusogenicity.[26] In contrast, our technique did not require fusogens since our BioFLVs were highly unstable due to their formulation and small radius of curvature.
Over the years other methods to modify cell membrane without FLVs have been developed.[27–30] Investigators have derivatized proteins with lipophilic moieties that directly embed into cell membranes. These moieties include glycosyl phosphatidylinositol,[30] palmitic acid,[27] and hydrophobic tails.[28] Previously, another study also described a biotin/SA linking strategy using sulfo-NHS-LC-biotin to biotinylate proteins on membranes linking SA-containing proteins.[29] Using this strategy, rodents immunized with splenocytes displaying SA-FasLigand effectively blocked alloreactive responses and prevented the rejection of pancreatic islets.
All the above approaches have various time requirements for drug application and duration of display. As these techniques gain clinical acceptance coupling strategies for protein display will be dictated by time constraints inherent to the procedure. For example, in tissue transplantation since ischemia time is a key factor that influences the outcome, rapid membrane modification would be highly desirable to improve outcome. In contrast, elective procedures involving ex vivo cell surface modification for cell-based therapies are less time sensitive and may be performed over several hours or days without compromising the outcome. Another issue is the duration of drug display which can be influenced by the bonds formed in the coupling strategy. In vaccine cell based therapy applications, long-term display of proteins would be desirable, thus, biotin/SA strategies would be ideal. Our BioFLV/SA-VCP therapy is being developed to protect the endothelial barrier from IR-induced complement damage in tissue transfer/transplantation. Based on the results, we believe that an effective surface modification can be accomplished within an hour, and the rat hind limb studies suggest that the biotin/SA strategy sufficiently maintains therapeutic levels of protein for the first 24 h of reperfusion, when most complement mediated damage occurs.
In the immediate post-operative period, IR-induced excessive complement activation has been implicated in delayed graft function of transplanted organs and also as a contributing factor for acute and chronic rejection.[31;32] Previous therapies to reduce IRI in organ/tissue transplantation have focused mainly on preservation solutions for protection during ischemia but not on complement-mediated injury during reperfusion. Since the formation of C3 convertase is a common step of the three complement pathways, we selected to fuse SA with VCP to form a potent C3 convertase inhibitor.
Earlier studies using soluble complement receptor 1 (sCR1) [33] and anti-C5 monoclonal antibody[34] have demonstrated effectiveness in reducing IRI. However, their systemic use places patients at risk for bacterial infection,[35] and autoimmune diseases.[36] Systemic therapy can severely restrict complement’s role in antigen interaction, and also suppress complement tagging for clearance of microorganism and immune complexes by the reticulo-endothelial system. More recently, endothelium targeted agents TP20 and APT070 have shown to be useful in transplantation and IRI animal models. TP20 contains sialylated and fucosylated tetrasaccharide sialyl Lewisx (sLex) moieties targeted to E-selectin and P-selectin adhesion molecules,[37;38] and APT070 a derivative of CR1 coupled to a myristoylated peptide allows it to anchor into membranes upon contact.[39;40] The same membrane-linking strategy applied to APT070 has been tried with less success on CD59 and CD55.[41] However, to date neither TP20 nor APT070 has made it to clinical trials.[42] Our BioFLV based therapy appears to effectively target the endothelium of an ischemic hindlimb in which the vasculature has been isolated. In future experiments, we want to demonstrate that immobilized SA-VCP will block complement activation by staying firmly anchored to the endothelium as demonstrated by the SA-FITC experiments.
Conclusion
In this study we have demonstrated that our novel approach of targeted anti-complement therapy is effective in reducing complement deposition in vitro. Our BioFLV delivery method designed to decorate the endothelium of isolated tissue or cells with immobilized SA-VCP appears superior to existing systemic anti-complement therapies because it not only simulates endogenous anti-complement defenses on endothelial cells but can also be delivered locally at much higher levels without causing serious systemic side effects. Furthermore, in IRI where the endogenous complement regulatory proteins on endothelial cells are overwhelmed, this therapy can be easily administered ex vivo during ischemia to serve as a valuable adjunct bolstering the local defense mechanism. The BioFLV system is flexible and can be formulated with various functional groups to bind and display various drugs simultaneously, making this strategy highly versatile for investigational and therapeutic purposes. We conclude that BioFLV-mediated decoration of cell membranes with anti-complement proteins reduces complement activation and deposition in vitro and has the potential for application against inappropropriate complement activation in vivo.
Acknowledgments
Grant sponsors: This work was funded in part by a grant from the National Institutes of Health (NIH) R41HL079855, and by EndoProtech, Inc.
List of Abbreviations
- AU
Arbitrary units
- BioFLVs
Biotinylated fusogenic lipid vesicles
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DGPB
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(biotinyl) (sodium salt)
- DMEM
Dulbecco’s Modified Eagle’s Medium
- POPA
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate (monosodium salt)
- FITC
Fluorescein isothiocyanate
- IR
Ischemia/reperfusion
- IRI
Ischemia/reperfusion injury
- MAC
Membrane attack complex
- PBS
Phosphate buffered saline
- SA
Streptavidin
- sCR1
Soluble complement receptor 1
- SKOV-3
Ovarian carcinoma cells
- sLex
Sialylated and fucosylated tetrasaccharide sialyl Lewisx
- VCP
Vaccinia virus complement control protein
Footnotes
Financial disclosure
The authors Drs. Gustavo Perez-Abadia and Claudio Maldonado declare that they have conflict of interest.
Drs. Ledia Goga, Sathnur B Pushpakumar, Paul Olson, Gary Anderson, John Barker, and Mr. Chirag Soni do not have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babu AN, Murakawa T, Thurman JM, Miller EJ, Henson PM, Zamora MR, Voelkel NF, Nicolls MR. Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis. J Clin Invest. 2007;117:3774–3785. doi: 10.1172/JCI32311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamada K, Miwa T, Liu J, Nangaku M, Song WC. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol. 2004;172:3869–3875. doi: 10.4049/jimmunol.172.6.3869. [DOI] [PubMed] [Google Scholar]
- 4.Heijnen BH, Straatsburg IH, Padilla ND, Van Mierlo GJ, Hack CE, Van Gulik TM. Inhibition of classical complement activation attenuates liver ischaemia and reperfusion injury in a rat model. Clin Exp Immunol. 2006;143:15–23. doi: 10.1111/j.1365-2249.2005.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol. 2008;181:8068–8076. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damman J, Schuurs TA, Ploeg RJ, Seelen MA. Complement and renal transplantation: from donor to recipient. Transplantation. 2008;85:923–927. doi: 10.1097/TP.0b013e3181683cf5. [DOI] [PubMed] [Google Scholar]
- 7.Lewis AG, Kohl G, Ma Q, Devarajan P, Kohl J. Pharmacological targeting of C5a receptors during organ preservation improves kidney graft survival. Clin Exp Immunol. 2008;153:117–126. doi: 10.1111/j.1365-2249.2008.03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pratt JR, Harmer AW, Levin J, Sacks SH. Influence of complement on the allospecific antibody response to a primary vascularized organ graft. Eur J Immunol. 1997;27:2848–2853. doi: 10.1002/eji.1830271116. [DOI] [PubMed] [Google Scholar]
- 9.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 10.Podack ER, Tschopp J. Membrane attack by complement. Mol Immunol. 1984;21:589–603. doi: 10.1016/0161-5890(84)90044-0. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson C, Song H, Lu B, Qiao F, Burns TA, Holers VM, Tsokos GC, Tomlinson S. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson C, Qiao F, Song H, Gilkeson GS, Tomlinson S. Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J Immunol. 2008;180:1231–1238. doi: 10.4049/jimmunol.180.2.1231. [DOI] [PubMed] [Google Scholar]
- 13.Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD., Jr Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol. 1992;149:1723–1728. [PubMed] [Google Scholar]
- 14.Jha P, Kotwal GJ. Vaccinia complement control protein: multi-functional protein and a potential wonder drug. J Biosci. 2003;28:265–271. doi: 10.1007/BF02970146. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie R, Kotwal GJ, Moss B, Hammer CH, Frank MM. Regulation of complement activity by vaccinia virus complement-control protein. J Infect Dis. 1992;166:1245–1250. doi: 10.1093/infdis/166.6.1245. [DOI] [PubMed] [Google Scholar]
- 16.Allen TM. Liposomes. Opportunities in drug delivery. Drugs. 1997;54 (Suppl 4):8–14. doi: 10.2165/00003495-199700544-00004. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee R. Liposomes: applications in medicine. J Biomater Appl. 2001;16:3–21. doi: 10.1106/RA7U-1V9C-RV7C-8QXL. [DOI] [PubMed] [Google Scholar]
- 18.Fraley R, Straubinger RM, Rule G, Springer EL, Papahadjopoulos D. Liposome-mediated delivery of deoxyribonucleic acid to cells: enhanced efficiency of delivery related to lipid composition and incubation conditions. Biochemistry. 1981;20:6978–6987. doi: 10.1021/bi00527a031. [DOI] [PubMed] [Google Scholar]
- 19.Garrett FE, Goel S, Yasul J, Koch RA. Liposomes fuse with sperm cells and induce activation by delivery of impermeant agents. Biochim Biophys Acta. 1999;1417:77–88. doi: 10.1016/s0005-2736(98)00258-2. [DOI] [PubMed] [Google Scholar]
- 20.Khatri K, Rawat A, Mahor S, Gupta PN, Vyas SP. Hepatitis B surface protein docked vesicular carrier for site specific delivery to liver. J Drug Target. 2005;13:359–366. doi: 10.1080/10611860500230294. [DOI] [PubMed] [Google Scholar]
- 21.Pagano RE, Weinstein JN. Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng. 1978;7:435–468. doi: 10.1146/annurev.bb.07.060178.002251. [DOI] [PubMed] [Google Scholar]
- 22.Schiffelers R, Storm G, Bakker-Woudenberg I. Liposome-encapsulated aminoglycosides in pre-clinical and clinical studies. J Antimicrob Chemother. 2001;48:333–344. doi: 10.1093/jac/48.3.333. [DOI] [PubMed] [Google Scholar]
- 23.Trigiante G, Huestis WH. Selective virus-mediated intracellular delivery of membrane-impermeant compounds by means of plasma membrane vesicles. Antiviral Res. 2000;45:211–221. doi: 10.1016/s0166-3542(00)00073-5. [DOI] [PubMed] [Google Scholar]
- 24.Zakaria eR, Ehringer WD, Tsakadze N, Li N, Garrison RN. Direct energy delivery improves tissue perfusion after resuscitated shock. Surgery. 2005;138:195–203. doi: 10.1016/j.surg.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 26.van Broekhoven CL, Parish CR, Vassiliou G, Altin JG. Engrafting costimulator molecules onto tumor cell surfaces with chelator lipids: a potentially convenient approach in cancer vaccine development. J Immunol. 2000;164:2433–2443. doi: 10.4049/jimmunol.164.5.2433. [DOI] [PubMed] [Google Scholar]
- 27.Chen A, Zheng G, Tykocinski ML. Hierarchical costimulator thresholds for distinct immune responses: application of a novel two-step Fc fusion protein transfer method. J Immunol. 2000;164:705–711. doi: 10.4049/jimmunol.164.2.705. [DOI] [PubMed] [Google Scholar]
- 28.Wahlsten JL, Mills CD, Ramakrishnan S. Antitumor response elicited by a superantigen-transmembrane sequence fusion protein anchored onto tumor cells. J Immunol. 1998;161:6761–6767. [PubMed] [Google Scholar]
- 29.Yolcu ES, Askenasy N, Singh NP, Cherradi SE, Shirwan H. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity. 2002;17:795–808. doi: 10.1016/s1074-7613(02)00482-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Schmidt WG, Hou Y, Williams AF, Jacobson K. Spontaneous incorporation of the glycosyl-phosphatidylinositol-linked protein Thy-1 into cell membranes. Proc Natl Acad Sci U S A. 1992;89:5231–5235. doi: 10.1073/pnas.89.12.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocha PN, Plumb TJ, Crowley SD, Coffman TM. Effector mechanisms in transplant rejection. Immunol Rev. 2003;196:51–64. doi: 10.1046/j.1600-065x.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 32.Sacks SH, Chowdhury P, Zhou W. Role of the complement system in rejection. Curr Opin Immunol. 2003;15:487–492. doi: 10.1016/s0952-7915(03)00100-6. [DOI] [PubMed] [Google Scholar]
- 33.Pratt JR, Hibbs MJ, Laver AJ, Smith RA, Sacks SH. Effects of complement inhibition with soluble complement receptor-1 on vascular injury and inflammation during renal allograft rejection in the rat. Am J Pathol. 1996;149:2055–2066. [PMC free article] [PubMed] [Google Scholar]
- 34.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 35.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan KE. Complement deficiency and autoimmunity. Curr Opin Pediatr. 1998;10:600–606. doi: 10.1097/00008480-199810060-00011. [DOI] [PubMed] [Google Scholar]
- 37.Linton SM, Williams AS, Dodd I, Smith R, Williams BD, Morgan BP. Therapeutic efficacy of a novel membrane-targeted complement regulator in antigen-induced arthritis in the rat. Arthritis Rheum. 2000;43:2590–2597. doi: 10.1002/1529-0131(200011)43:11<2590::AID-ANR29>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.Zacharowski K, Otto M, Hafner G, Marsh HC, Jr, Thiemermann C. Reduction of myocardial infarct size with sCR1sLe(x), an alternatively glycosylated form of human soluble complement receptor type 1 (sCR1), possessing sialyl Lewis x. Br J Pharmacol. 1999;128:945–952. doi: 10.1038/sj.bjp.0702889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris CL, Fraser DA, Morgan BP. Tailoring anti-complement therapeutics. Biochem Soc Trans. 2002;30:1019–1026. doi: 10.1042/bst0301019. [DOI] [PubMed] [Google Scholar]
- 40.Patel H, Smith RA, Sacks SH, Zhou W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J Am Soc Nephrol. 2006;17:1102–1111. doi: 10.1681/ASN.2005101116. [DOI] [PubMed] [Google Scholar]
- 41.Smith GP, Smith RA. Membrane-targeted complement inhibitors. Mol Immunol. 2001;38:249–255. doi: 10.1016/s0161-5890(01)00047-5. [DOI] [PubMed] [Google Scholar]
- 42.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]