Summary
Memory and attention deficits are common after prefrontal cortex (PFC) damage, yet people generally recover some function over time. Recovery is thought to be dependent upon undamaged brain regions but the temporal dynamics underlying cognitive recovery are poorly understood. Here we provide evidence that the intact PFC compensates for damage in the lesioned PFC on a trial-by-trial basis dependent on cognitive load. The extent of this rapid functional compensation is indexed by transient increases in electrophysiological measures of attention and memory in the intact PFC, detectable within a second after stimulus presentation and only when the lesioned hemisphere is challenged. These observations provide evidence supporting a dynamic and flexible model of compensatory neural plasticity.
Introduction
Brain damage has an immense personal and societal cost yet the neural mechanisms underlying recovery are poorly understood. Damage to the human prefrontal cortex (PFC) results in attention (Barceló et al., 2000; Rossi et al., 2007) and memory deficits (Voytek and Knight, 2010; Tsuchida and Fellows, 2009) with variable levels of recovery observed in individual patients. However, unlike damage to primary motor or sensory cortices which results in overt deficits such as hemiparesis or hemianopsia, long-term deficits in working memory and attention after unilateral PFC damage are often less dramatic. This clinical observation suggests that cognitive processes supported by frontal association cortex are more plastic and likely to recover. Electroencephalographic (EEG) and functional magnetic resonance imaging (fMRI) studies report that neurological patients who have recovered from motor, language, or attention deficits show increases in activity in homologous cortical regions in the non-lesioned hemisphere and in perilesion cortex (Ward et al., 2007; Johansen-Berg et al., 2002; Blasi et al., 2002; Corbetta et al., 2005; He et al., 2007; Nudo, 2007; Chao and Knight, 1998; Rosahl and Knight, 1995). However, cognitive compensation after PFC damage is less understood. In this study we sought to examine whether intact cognitive performance in patients with unilateral PFC damage is mediated by functional compensation by the intact, undamaged frontal cortex.
Neural plasticity is critical for functional recovery after brain damage with improvement possible even 20 years after the initial injury (Bach-y-Rita, 1990). There are several theories of recovery of function, including: cortical compensation by perilesion and intact homologous brain regions (Wundt, 1902) or subcortical (Van Vleet et al., 2003) structures; diaschisis reversal (von Manakow, 1969); unmasking (Lytton et al., 1999); distributed cortical representations (Jackson, 1958); and axonal sprouting and neurogenesis (Carmichael et al., 2001). Many of these theories predate neuroimaging and were based on clinical observations of patients with brain damage. These early theories of recovery logically concluded that recovery must be mediated by intact, undamaged brain regions (Kolb, 1992). Cognitive functions such as working memory and attention are supported by networks of interacting brain regions (Bressler, 1995; Knight, 2007). Given the number of brain regions needed to support visual attention and working memory, it is not unreasonable, given the variety of recovery theories, to hypothesize that recovery could be supported by the entire network. However, the PFC plays an important role in these networks by biasing information flow to favor positive behavioral outcomes (Miller and Cohen, 2001) and may play a privileged role in cognitive compensation.
To examine the nature of cognitive compensation in patients with unilateral PFC damage we conducted two EEG experiments on patients with unilateral PFC lesions in the chronic phase at least one year post-injury. In Experiment 1, six patients with unilateral PFC lesions (Figure 1A) and age-matched controls performed a lateralized visual working memory task (Vogel and Machizawa, 2004; Voytek and Knight, 2010). In Experiment 2 eight patients with unilateral PFC lesions (Figure 1B) and age-matched controls performed a lateralized visual attention task (Yago et al., 2004).
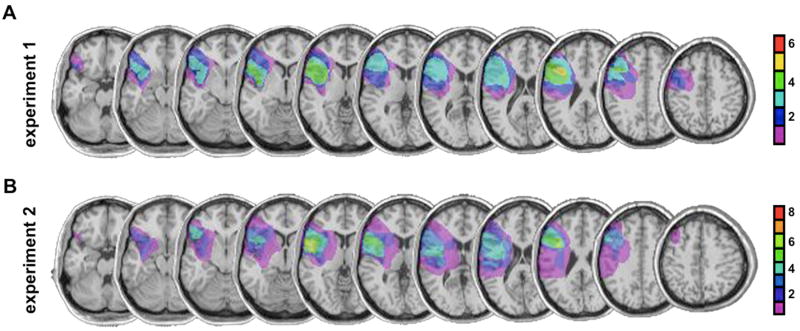
Figure 1. Patient MRIs.
Horizontal MRI slices showing the group-averaged reconstruction of the extent of lesion overlap of the PFC damage in patients from (A) Experiment 1 [n = 6] and (B) Experiment 2 [n = 8]. Color represents number of subjects with a lesion at each specific site. All lesions are normalized to the left hemisphere for comparison. Maximal lesion overlap (>50%) was observed in Brodmann areas 6, 8, 9, and 46 and encompassed portions of the middle and superior frontal gyri. Software reconstructions of the lateral perspective of lesions, determination of lesion volumes, and putative cytoarchitectonic areas damaged were performed using MRIcro (Rorden and Brett, 2000). Note that one of the six subjects from Experiment 1 also participated in Experiment 2.
Previous research on patients with unilateral PFC lesions has demonstrated that patients show behavioral deficits in response to contralesional stimuli in visual attention (Barceló et al., 2000; Yago et al., 2004) and working memory paradigms (Voytek and Knight, 2010). These deficits are associated with a loss of top-down facilitation of visual cortical regions as indexed using scalp EEG. These findings suggest that the separation of visual information by hemifield can emphasize deficits. By making use of two lateralized visual tasks we aimed to take advantage of this lesion by visual-field-of-presentation phenomenon. The design of randomly presenting stimuli to either the intact or damaged hemisphere allowed us to randomly challenge the damaged PFC on a trial-by-trials basis. This technique allows us to make use of a within-subjects design wherein our patients partially serve as their own controls, such that we can examine differences within subjects in response to contralesional versus ipsilesional stimuli.
We hypothesized that cognitive recovery in patients with unilateral PFC damage would be supported by flexible and dynamic compensatory contributions from the intact frontal cortex. That is, the plasticity of frontal association cortex would allow the intact hemisphere to dynamically compensate for the damaged hemisphere. In this model, activity in the intact PFC would increase specifically in response to demands placed on the damaged hemisphere. That is, when behaviorally relevant stimuli are specifically presented to the damaged hemisphere the intact frontal cortex would become more active, in a load-dependent manner, to compensate for the deficits due to the lesion. This is in contrast to a fixed recovery model that might predict that frontal activity would increase with memory or attention load regardless of the hemifield of presentation (see Figure S1A for hypothetical models). Here we show, in two separate patient groups performing two separate PFC-dependent tasks, rapid trial-by-trial increases in neural activity over the intact frontal cortex only when the damaged PFC is challenged. These observations of sub-second dynamic neural activity highlight the role of the intact hemisphere in supporting recovery of function.
Results
Working Memory Experiment
In Experiment 1 we used a lateralized visual working memory task that allowed us to parametrically manipulate the memory load (i.e., 1, 2, or 3 visual objects) delivered to either cerebral hemisphere. As expected, both groups showed a main effect of memory load on behavioral accuracy (d′) such that accuracy decreased with increasing memory load (repeated measures ANOVA, main effect of set size, [F2,20 = 210.41, P < 0.0005], see Figure S2). There was a three-way interaction between group, memory load, and hemifield of stimulus presentation [F2,20 = 11.85, P < 0.0005]. A series of post-hoc analyses examining the effect of group on accuracy suggest that this three-way interaction is driven by an interaction between hemisphere and group [F1,10 = 17.31, P = 0.002] rather than memory load and group [F2,20 < 1.0]. Controls show no interaction between memory load and hemifield [F2,10 = 3.12, P = 0.14], nor a main effect of hemifield on accuracy [F1,5 = 3.28, P = 0.080]. In contrast, PFC patients show an effect of hemifield on accuracy [F1,5 = 29.21, P = 0.003], as well as a load by hemifield interaction [F2,10 = 15.65, P = 0.001]. This interaction is driven by decreased performance for contralesional stimuli at memory loads one [one-tailed paired samples t-tests, p = 0.002] and two [p = 0.013] with performance equalizing between hemifields at three-item loads [p = 0.14].
This task elicits a lateralized neural event-related potential (ERP) during the delay period. This contralateral delay activity (CDA) is focused over extrastriate cortex and is modulated by the number of items that are currently being maintained in working memory (Vogel and Machizawa, 2004; Vogel et al., 2005; Voytek and Knight, 2010). For controls, we replicated the finding that CDA amplitude increases as memory load increases [F2,10 = 9.75, P = 0.004] and that CDA amplitude was equivalent for each hemisphere (set-by-laterality interaction: [F2,10 < 1.0]; Figure S3A1,2). However, while the PFC patients showed a similar load increase in CDA amplitude for ipsilesional stimuli [F2,10 = 4.77, P = 0.035], this load effect was absent when the memory array was presented contralateral to the lesioned hemisphere (contralesional hemifield, Figure S3B1,2; [F2,10 < 1.0]). Notably, patient CDA amplitude for contralesional stimuli are of larger amplitude despite their lack of memory load specificity. In a two-way post hoc analysis comparing control CDA for right hemifield stimuli to patient CDA for contralesional stimuli we found a main effect of group that corroborates this observation [F1,10 = 7.43, P = 0.021], though there was no interaction between group and load [F2,20 = 1.30, P = 0.29]. Although amplitudes are larger in patients, absolute CDA amplitude is a poor predictor of behavioral performance; rather, it is the slope of the CDA load effect that tracks behavior (Vogel and Machizawa, 2004; Drew and Vogel, 2008).
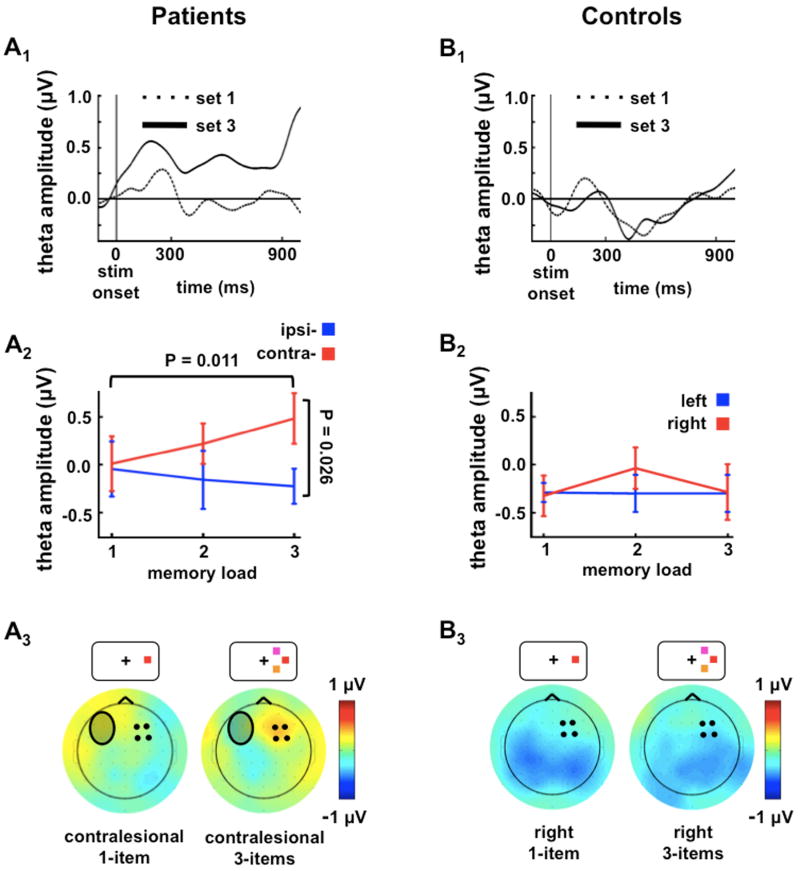
Working memory paradigms generate increased frontal theta (4-8 Hz) oscillatory EEG activity (Raghavachari et al., 2001; Bastiaansen, et al., 2002), and here we focus our frontal analyses on the theta band during the memory delay period (see Experimental Procedures for other band analyses which were non-informative). While controls showed negligible frontal theta activity over either hemisphere, patients showed sustained frontal theta activity (600-900 ms) only over their intact hemisphere. This frontal theta activity increased as a function of memory load for contralesional stimuli (Figures 2A1 and 2A2; [F1, 5 = 10.45, P = 0.023]), but was absent for ipsilesional stimuli [F1, 5 < 1.0], resulting in an interaction in the PFC group between set size and visual field for sustained frontal theta over the intact PFC (Figure 2A2; [F1, 5 = 12.07, P = 0.018]) that was not seen in controls (Figures 2B1 and 2B2; [F2, 10 < 1.0]) nor over the lesioned cortex (Figure S4; [F2, 10 = 1.05, p = 0.39]). This pattern of results cannot be accounted for by eye movement differences between groups or conditions (see Experimental Procedures) and a source analysis suggests that this anterior theta may have a PFC source (Figure S5).
Figure 2. Experiment 1: Frontal Load-Dependent Compensation During Visual Working Memory.
(A1-A3) Patient and (B1-B3) age-matched control data showing load dependence of frontal theta activity. (A1, B1) Frontal theta waveforms are measured from the intact frontal region represented by the black dots in the scalp topographies in (A3) and (B3) and show theta amplitudes for one- (dashed lines) and three-item (solid lines) memory arrays over the frontal sites. (A1) Time course of the sustained frontal theta load dependence measured over the intact frontal cortex when the lesioned hemisphere is challenged.
(A2, B2): Frontal theta amplitude and standard error by memory load and hemifield of stimulus presentation. (A2) Compensatory theta in patients is largest over intact frontal sites and increases with memory load in response to contralesional stimuli. (B2) In age-matched controls there is no frontal theta activity difference between one- and three-item or left and right memory arrays. Error bars denote SEM.
(A3, B3): Scalp topographies of the difference in theta for contralesion minus ipsilesion (right minus left) activity for three-item memory loads. (A3) The scalp topography highlights the increased theta in response to contralesional memory load. The shaded oval represents the relative scalp location of the patients' lesions. (B3) There are no load-dependent activity changes over frontal sites in controls.
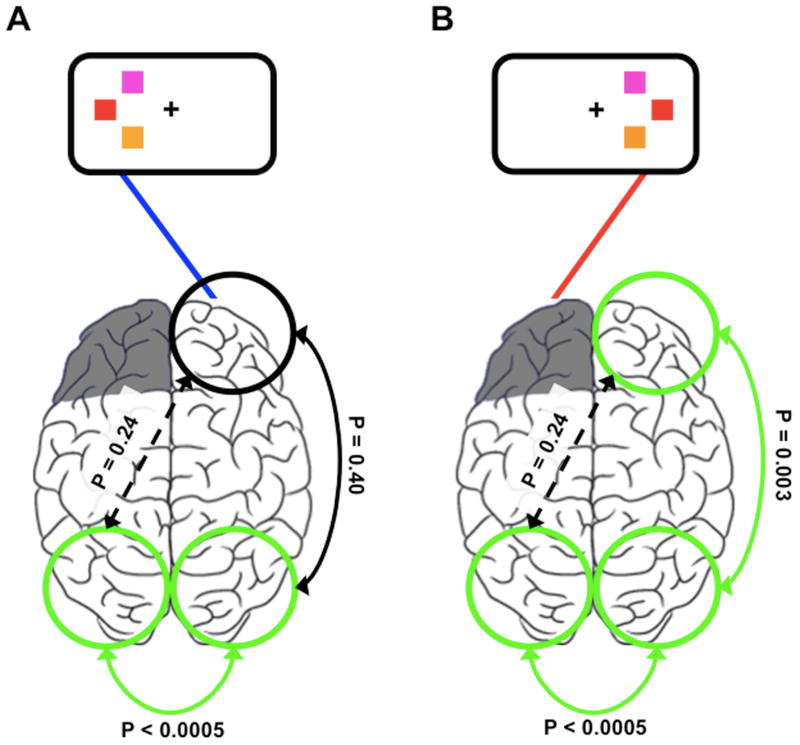
Our hypothesis that intact frontal theta increases are related to memory function necessitates that information from the visual cortex from the lesioned hemisphere crosses to the intact hemisphere for processing by the intact PFC. To examine such information flow we looked at correlations between early visual ERPs (N1 amplitude from 100-200ms) between visual hemispheres. Consistent with the notion that visual information crosses transcallosally between visual hemispheres, N1 amplitude is correlated in both hemispheres in both conditions (Pearson correlation across all trials, all subjects; ipsilesional: r = 0.62, P < 0.0005; contralesional: r = 0.68, P < 0.0005). In contrast, for contralesional stimuli only, N1 magnitude of the intact hemisphere and intact frontal theta amplitude are also correlated, partialling out the effects of N1 magnitude of the damaged hemisphere (contralesional: ρ = 0.076, P = 0.003; ipsilesional: ρ = 0.007, P = 0.40) across trials (Figure 3). Intact frontal theta and N1 magnitude from the damaged hemisphere are uncorrelated partialling out the effects of N1 magnitude from the intact hemisphere (contralesional: ρ = 0.019, P = 0.24; ipsilesional: ρ = 0.019, P = 0.24). In a sliding-window correlation analysis we observed that, in response to an ipsilesional stimulus there is no correlation between N1 amplitude from the intact hemisphere and intact frontal theta at any time point.
Figure 3. Experiment 1: Posterior visual activity is correlated with compensatory frontal theta.
(A, B): Consistent with information crossing transcallosally from the visual cortex contralateral to the stimulus over to the opposite hemisphere, N1 amplitude between the ipsi- and contralesional visual cortices is highly correlated across all trials. Unilateral PFC lesions are represented in grey.
(A): In response to ipsilesional stimuli, N1 amplitude between both visual cortices is highly correlated, however there is no correlation between N1 magnitude and frontal theta across trials.
(B): Similar to ipsilesional stimuli, in response to contralesional stimuli, N1 amplitudes are highly correlated between visual cortices. In contrast however, later compensatory frontal theta amplitude is correlated with N1 magnitude only within the intact hemisphere. These results suggest that early visual components are related to later compensatory frontal theta activity consistent with the hypothesis that information enters the visual cortex of the damaged hemisphere and crosses to the intact hemisphere for processing to support working memory.
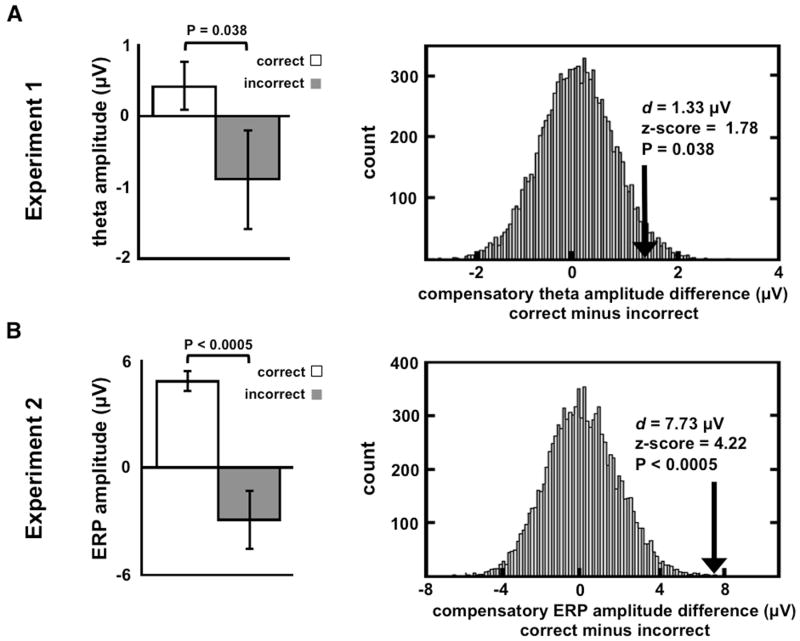
However, this analysis reveals that for contralesional stimulation, N1 amplitude in the intact hemisphere predicts late frontal theta activity in that hemisphere during the time window of interest (600-900ms; see Figure S6). These findings suggest that the degree of compensatory frontal theta activity is contingent upon the fidelity of the visual information that crosses from the damaged to the intact hemisphere. Importantly, frontal compensatory theta activity in response to contralesional stimuli was larger for correct trials when compared to incorrect trials, (Figure 5A; [P = 0.038 for 3-item load]) supporting the contention that theta activity is related to correct performance and indexes second to second functional compensation.
Figure 5. Compensatory Activity and Standard Error During Correct Versus Incorrect Trials.
Left panels show means for correct and incorrect trials, right panels show distributions of differences from resampling statistics (see Methods). (A) Sustained frontal theta amplitudes over intact PFC in patients are larger during correct trials than during incorrect trials in response to three-item contralesional stimuli. Error bars denote SEM. (B) Frontal ERP amplitudes over intact cortex in patients in response to correctly identified contralesional targets are larger than for incorrect trials.
Attention Experiment
To test whether the observed compensatory neural activity over the intact frontal cortex generalizes across PFC-dependent cognitive functions, we analyzed data from a lateralized visual attention experiment conducted in patients with unilateral PFC lesions (Yago et al., 2004; Figure 1B). Subjects viewed a rapid stream of stimuli presented to the left or right visual fields while attending to one hemifield and responding to infrequent targets embedded within a stream of frequent non-target stimuli (see Experimental Procedures for details). Patients were impaired in detecting contralesional targets (repeated measures ANOVA, group-by-hemifield of presentation interaction on arcsine transformed percent correct, [F1, 17 = 7.62, P = 0.013]; controls, 95.7% and 94.7% correct for left and right targets, [P = 0.65]; patients 94.7% and 87.9% correct for ipsi- and contralesion targets, respectively, [P = 0.027]; one-tailed paired-samples t-tests). However, even though the task placed heavy demands on sustained attention, performance in both hemifields was well above chance (one-sample t-tests for both hemifields, [P < 0.0005]). As in experiment 1, preserved behavioral performance was evident despite the fact that the PFC lesion markedly reduced neural responses over visual cortices ipsilateral to the PFC lesion during correct trials (Barceló et al., 2000; Yago et al., 2004).
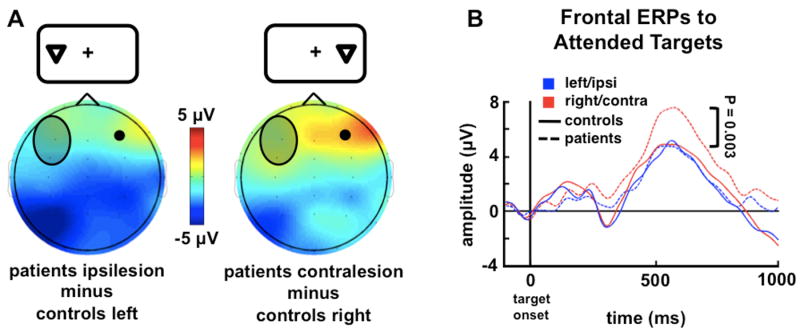
In contrast to controls, the patients' P1 (60 - 160 ms) and P3 (450 - 650 ms) components of the extrastriate ERP were attenuated in the lesioned hemisphere in response to contralesional targets (P1: [P = 0.003]; P3: [P = 0.009]; all between-group comparisons are one-tailed independent sample t-tests) replicating the pattern of attenuated extrastriate activity observed in Experiment 1. Similar decrements have been shown in fMRI studies of aphasic patients with PFC lesions during word learning wherein visual cortical activity in the hemisphere ipsilateral to the lesion was decreased relative to controls (Blasi et al., 2002). A different pattern emerged in the frontal neurophysiological data. The PFC group showed no target-related electrophysiological differences over the intact frontal cortex compared to controls (Figure 4A, left panel; [P = 0.63]) in response to ipsilesional stimuli. However, a late frontal positivity (450-650 ms) increased in amplitude in the intact hemisphere in patients in response to contralesional stimuli compared to controls (Figure 4A, right panel and Figure 4B; [P = 0.003]). Just as in Experiment 1, this enhanced electrophysiological activity in patients in response to contralesional targets was absent on error trials (Figure 5B; [P < 0.0005]). There were no differences in intact frontal oscillatory activity in this target detection task (see Experimental Procedures).
Figure 4. Experiment 2: Frontal Load-Dependent Compensation During Visual Attention.
(A): Late frontal positivity (450-650 ms) in patients is enhanced over the intact PFC and attenuated over the extrastriate in the damaged hemisphere compared to controls in response to attended targets presented contralateral to the side of the lesion. Topographies show average frontal positivity differences—patient minus control difference waves—in response to left/ipsilesional (left panel) or right/contralesional (right panel) targets. The shaded oval represents the relative scalp location of the patients' lesions.
(B): Frontal ERPs show the time course of activity over the intact PFC in comparison to controls. The dashed blue line represents the response to ipsilesion target stimuli. The dashed red line shows the enhanced activity over intact PFC when stimuli are delivered contralesionally. The ERP waveforms are measured from the intact frontal region represented by the black circle in (A). Error bars denote SEM.
Discussion
Our results provide evidence that the intact, non-lesioned hemisphere dynamically compensates for the damaged PFC when the damaged hemisphere is challenged with either memory or attentional loads. In a paper examining alterations in cortical activity related with normal, healthy aging (Davis et al., 2008), two criteria were established as necessary for cortical activity differences in older adults to be more likely to be regarded as “compensatory”. First, novel activity increases not seen in normal controls must be associated with correct behavioral outcomes. Second, deficits in processing by one region must be associated with increases in activity in the putative compensatory region. Consistent with the first criterion, increases in activity over the intact PFC are enhanced on correct trials in both of our experiments. With regard to the second criterion, our experimental designs allowed us to preferentially challenge the damaged hemisphere in patients with unilateral PFC damage. We show that PFC patients have top-down working memory and attention deficits for contralesional stimuli reflected by decreased electrophysiological responses in the posterior visual cortex. The fact that we observe increased activity over the intact PFC, which correlated with posterior visual activity specifically when the damaged hemisphere is challenged, satisfies the second criterion.
Thus, we suggest that the observed neural pattern supports a mechanism of compensation whereby the intact hemisphere plays a dynamic and flexible role in mediating the cognitive functions impaired by unilateral PFC injury. In both experiments PFC damage resulted in marked attenuation of neural activity in the extrastriate cortex ipsilateral to PFC damage, yet the patients performed well above chance even when stimuli were delivered to the impaired field. Our findings account for this behavioral/electrophysiological discrepancy by providing evidence that the intact frontal cortex is assuming control of the task on a sub-second time scale. That is, although patients show attenuated responses in ipsilesional visual cortex, these decreases are accompanied by rapid increases in activity over intact frontal cortex (Figure S1B).
The electrophysiological increases we observed over the intact frontal cortex varied with load and predicted behavior as evidenced by their increased neural activity during correct compared to incorrect task performance. We did not observe any such electrophysiological changes when stimuli were presented ipsilesionally. This extends findings in motor recovery where selective disruption of the intact motor cortex using transcranial magnetic stimulation increases simple reaction times (Johansen-Berg et al., 2002). Here we expand the findings of motor recovery to the cognitive domain and further demonstrate a dynamic compensation model that contrasts with a fixed compensation model. By using lateralized memory and attention tasks to alternately challenge the damaged or intact cerebral hemispheres we highlight intrahemispheric electrophysiological deficits in top-down visual working memory and attention processing. Furthermore, by taking advantage of the temporal resolution of EEG we show that neural compensation occurs rapidly as task demands increase compensatory requirements.
In Experiment 1, theta power over intact frontal cortex increased with memory load when the damaged hemisphere was challenged. Frontal theta amplitude has been previously shown to be modulated by memory load and is proposed to represent active maintenance of the visual stimuli within the PFC (Jensen and Tesche, 2002). In Experiment 2, late frontal activity, linked to attentional allocation, increased over the intact cortex in response to targets presented only to the damaged hemisphere. If these effects were purely modulated by task difficulty we would expect load-dependent increases in frontal activity in either the control group or in response to ipsilesional stimuli. Neither pattern was observed.
Although we found robust, lateralized theta delay period activity in Experiment 1 in PFC patients when the damaged hemisphere was challenged, we note that we observed no frontal theta activity in normal controls nor in the patients when the lesioned hemisphere was not challenged. Several scalp and intracranial EEG studies have found that frontal theta activity increases with memory load (Raghavachari et al., 2001; Onton et al., 2005). In scalp EEG this usually manifests as a midline frontal theta increase. Notably, these studies most often make use of a Sternberg or n-back paradigm in which multiple items are presented in succession, or in delayed match to sample paradigms similar to ours but across longer (3-10 sec) delays. Single-unit, intracranial electrophysiology, and fMRI studies also show similar PFC delay-period activity, however these studies often also make use of successive visual presentation and/or longer delays. Sternberg and n-back paradigms with successive item presentation may require more fronto-striatal resources to filter out irrelevant distractors (McNab and Klingberg, 2008) and may not directly reflect only simple visual template maintenance. It has also been shown that frontal theta does not emerge at delays under 1.5 seconds in tasks similar to ours (Griesmayr et al., 2010). We were forced to use a short delay to mitigate eye movements in the control and patient groups since we employed a lateralized visual-field design. Thus, it is not surprising we do not observe theta at our short delay intervals.
The fact that we observe frontal theta activity in our patient group across a relatively short delay and with a relatively low memory load may reflect a shift in the threshold at which large groups of PFC neurons are recruited to perform the task. That is, the fronto-parietal network involved in maintaining a template of the visual stimulus during the delay period may be less prefrontally dependent in normal controls across a short delay, with fewer PFC neurons participating in active stimulus maintenance. However, in patients with unilateral PFC lesions, the frontoparietal network in the intact hemisphere behaves normally for ipsilesional stimuli; that is, at short delays and low loads the PFC is relatively inactive at a level observable in scalp EEG. However, that same network in the intact hemisphere becomes active at a much lower time/load threshold in response to contralesional stimuli, reflecting a dynamic compensatory process to assist the damaged hemisphere. Also of note is the fact that the compensatory activity we observe in our patients in Experiment 2 is relatively late and may reflect post-decision processes. While this may be true in the context of a single-trial, over the course of an entire task post-decision processes related to the increased frontal EEG activity may lead to improved performance. This design requires subjects to maintain an internal representation of the target stimulus across the entire task, and these late potentials may reflect a reinforcement of the template. While we cannot directly support this assertion, the fact that intact frontal activity is associated with correct performance is in agreement with the argument that this activity reflects a compensatory mechanism.
Models of anatomical connectivity changes in response to unilateral PFC lesions show that fronto-parietal connectivity is drastically reduced within the damaged hemisphere, as is fronto-frontal connectivity between the damaged and intact hemispheres (Alsott et al., 2009). Thus, in order for subjects to correctly perform our lateralized visual working memory task, the most likely route through which the necessary information can be processed and maintained during the delay period is across the posterior corpus callosum. That is, at an early stage post-stimulus onset, visual information must cross from visual cortex in the damaged hemisphere to the intact hemisphere for processing by the intact PFC. This idea is corroborated by our finding that early visual potentials are correlated across hemispheres, and that these early potentials correlate with later frontal theta amplitude within the intact hemisphere only when the damaged hemisphere is challenged (Figure 3). Of note, it has been shown that visual information typically transfers across the callosum in 15-20 ms (Rugg et al., 1984).
We propose that the visual information delivered to the contralesional hemisphere is transferred trans-callosally to the intact hemisphere where the intact PFC assumes task control as needed on a trial-by-trial basis. Support for this contention is provided by studies in non-human primates revealing that top-down PFC control over visual cortex during memory retrieval relies on callosal information transfer (Hasegawa et al., 1998; Tomita et al., 1999). Our results show that the neural changes observed in movement recovery after motor cortex damage (Ward et al., 2007; Johansen-Berg et al., 2002) expand to cognitive domains and apply to a dynamic model of memory and attention compensation by the intact, undamaged cortex. We demonstrate that brain recovery can manifest itself as transient changes in information processing occurring on a sub-second timescale after the injured brain has been challenged to perform, supporting a dynamic and flexible model of neural plasticity.
Experimental Procedures
Subjects
All subjects gave informed consent approved by the University of California, Berkeley Committee for Protection of Human Subjects and the Department of Veterans Affairs Northern California Health Care System Human Research Protection Program. In Experiment 1 we tested six patients (three male) with unilateral PFC damage due to stroke (two right hemisphere, average lesion volume 59 cm3). Age for the patients (mean 57 years) and education (mean 15 years) were matched by our six controls such that each control was within ±5 years of age and ±3 years of education to their matched patient ([p > 0.05] between groups for age and education). PFC subjects were in the chronic stroke phase (5-12 years post-stroke at the time of study). Details for subjects included in Experiment 2 are reported in a previous manuscript (Yago et al., 2004).
Data collection
Subjects were tested in a sound-attenuated EEG recording room. In Experiment 1, EEG was collected using a 64+8 channel BioSemi ActiveTwo amplifier (Metting van Rijn et al., 1990) sampled at 1024 Hz. In Experiment 2, EEG was collected from 32 scalp electrodes and sampled at 512 Hz. Horizontal eye movements (HEOG) were recorded at both external canthi; vertical eye movements (VEOG) were monitored with a left inferior eye electrode and superior eye or fronto-polar electrode. In both experiments, subjects were instructed to maintain central fixation and responded using the thumb of their ipsilesional hand. All data were referenced offline to the average potential of two earlobe electrodes and analyzed in MATLAB® (R2008b, Natick, MA) using custom scripts and the EEGLAB toolbox (Delorme and Makeig, 2004) and SPSS® (Rel. 16, Chicago: SPSS Inc.). Electrodes in patients with right hemisphere lesions ([n = 2] for each experiment) were swapped across the midline allowing us to plot scalp topographies wherein lesions are normalized to the left hemisphere.
Behavioral Tasks
The behavioral paradigm used in Experiment 1 was slightly modified from the procedures used in Vogel and Machizawa (2004). We modified this design such that subjects were visually presented with one, two, or three colored squares. These squares were presented for 180 ms and only appeared in one visual hemifield at a time. After a 900 ms delay, a test array of the same number of colored squares appeared in the same spatial location. Subjects were instructed to manually respond to indicate whether or not the test array was the same color as the initial memory array. Every subject completed 8-10 blocks of 60 trials each resulting in 80-100 trials per subject per condition (2 visual hemifields × 3 memory loads for 6 total conditions). All other features of the task (color template, eccentricity, stimulus size, etc.) are identical to Vogel and Machizawa (2004). Behavioral accuracy was assessed by normalizing percent correct responses for each subject using a d′ measure of sensitivity.
The behavioral paradigm used for Experiment 2 has been described in detail previously (Yago et al., 2004), but in brief, subjects were rapidly presented (107 ms presentation; 200, 800, or 1000 ms interstimulus interval) with a series of non-target standard stimuli [p = 0.7], target stimuli [p = 0.2], or neutral novel stimuli [p = 0.1] to either the left or right visual field ([p = 0.5] for each hemifield). On separate blocks of trials, subjects manually responded to targets presented only to the left or only to the right visual hemifield. For both experiments PFC patients responded with their ipsilesional hand to reduce the influence of motor deficits on responses.
EEG Analyses
ERP analyses were performed on bandpass filtered (0.1-30 Hz) data resampled to 256 Hz using a 100 ms pre-stimulus baseline. Blinks and saccades were identified on raw VEOG and HEOG channels respectively and verified with scalp topographies. Events with incorrect or no response, blinks, or saccades were removed from all analyses except where otherwise stated. For time-frequency analyses, the absolute value of the Hilbert transform of bandpass filtered raw EEG was used to extract frequency band analytic amplitudes (frequency-domain Gaussian kernel multiplication; Gaussian standard deviation was 10% of the center frequency resulting in full width half maximum of 0.2355 of the center frequency). These frequency band analytic time series were then subjected to normal event-related analyses.
In Experiment 1, in patients, there was no load dependence on HEOG [F2, 10 < 1.0] or VEOG [F2, 10 = 1.40, P = 0.29] activity. There were no differences for three-item arrays between patients and controls for HEOG [P = 0.43] or VEOG [P = 0.25] activity, or in patients for three-item ipsilesional versus contralesional HEOG [P = 0.94] or VEOG [P = 0.52]. To test the specificity of the theta compensatory effect we examined broadband ERP, alpha (8-12 Hz), and beta (12-18 Hz) frontal delay activity over intact PFC in Experiment 1 in a series of post-hoc analyses. Patients showed no set-by-laterality interactions for frontal ERP or for alpha or beta frequencies ([F1,5 < 1.0] for all analyses), nor was there an effect of load over intact cortex for contralesional stimuli for ERP [F1,5 < 1.0], alpha [F1,5 < 1.0], or beta [F1,5 = 1.25, P = 0.32] bands during the time window of interest.
In Experiment 2 there were no differences between patients and controls in VEOG [P = 0.88] or HEOG [P = 0.59] activity (mean activity during late frontal activity time windows; two-sample t-tests). We examined theta, alpha, and beta activity in patients over intact cortex for Experiment 2. There was no attention effect of laterality on compensatory measures of oscillatory activity over the intact PFC during the frontal positivity time window for theta, alpha, or beta bands ([F1,7 < 1.0] for all analyses).
Because there was an imbalance in the number of patients with right hemisphere versus left hemisphere lesions in each group there is some concern that the effects of interest may be driven by differences in hemispheric function rather than specifically reflecting compensation for the lesioned cortex. While we did not have enough power to examine left/right hemispheric lesion differences among our patient groups, we do not see any trend toward differences among patients with left or right hemisphere lesions. In Experiment 1 the four patients with left hemisphere lesions show intact frontal theta increases from one- to three-item arrays of -0.15, 0.40, 0.63, and 0.94 μV and the two patients with right hemisphere lesions show increases of 0.57 and 0.44 μV. In Experiment 2 the six patients with left hemisphere lesions show ERP increases for contralesion stimuli over ipsilesion stimuli of 2.00, 2.04, 2.83, 2.17, 4.31, and 1.57 μV and the two patients with right hemisphere lesions show increases of 4.00 and 0.92 μV.
Resampling Statistics
Because patients had many more correct than incorrect trials, in order to more accurately calculate the significance of any mean amplitude difference between correct and incorrect trials we calculated the real mean difference (d) between correct (c) and incorrect (i) trials for Experiment 1 theta [d = 1.33μV] and Experiment 2 ERP amplitude [d = 7.73 μV]. For each experiment separately we pooled all correct and incorrect trial compensatory amplitudes for patients and then randomly selected nc and ni amplitudes. We then calculated a difference between these surrogate data and repeated this process 10,000 times. For each experiment this provided a distribution of surrogate mean differences from the actual data from which we could calculate the probability (z-score) and one-tailed significance (P-value) of finding such an amplitude difference if the correct and incorrect labels were uninformative.
Highlights.
Unilateral PFC lesions cause top-down attention and memory deficits
The intact PFC rapidly and flexibly compensates for the damaged hemisphere
Compensatory activity increases as demands to the damaged hemisphere increase
Compensatory activity is related to correct behavioral outcomes
Supplementary Material
Acknowledgments
This work was supported by the American Psychological Association Diversity Program in Neuroscience (5-T32-MH18882) to B.V., and the National Institute of Neurological Disorders and Stroke (NS21135 and PO40813) to R.T.K. and (NS21135-S1) to B.V. We thank L. Tseng for assistance with data collection, A.S. Kayser for technical assistance, C. Clayworth for lesion reconstruction, and D. Scabini for patient delineation.
Footnotes
Supplemental Information: Supplemental Information includes six figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alstott J, Breakspear M, Hagmann P, Cammoun L, Sporns O, Friston K. Modeling the impact of lesions in the human brain. PLoS Comput Biol. 2009;5:1–12. doi: 10.1371/journal.pcbi.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach-y-Rita P. Brain plasticity as a basis for recovery of function in humans. Neuropsychologia. 1990;28:547–554. doi: 10.1016/0028-3932(90)90033-k. [DOI] [PubMed] [Google Scholar]
- Barceló F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Posthuma D, Groot PF, de Geus EJ. Event-related alpha and theta responses in a visuo-spatial working memory task. Clin Neurophysiol. 2002;113:1882–1893. doi: 10.1016/s1388-2457(02)00303-6. [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder A, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36:159–170. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Large-scale cortical networks and cognition. Brain Res Brain Res Rev. 1995;20:288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J Cogn Neurosci. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA?The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Drew T, Vogel EK. Neural measures of individual differences in selecting and tracking multiple moving objects. J Neurosci. 2008;28:4183–4191. doi: 10.1523/JNEUROSCI.0556-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesmayr B, Gruber WR, Klimesch W, Sauseng P. Human frontal midline theta and its synchronization to gamma during a verbal delayed match to sample task. Neurobiol Learn Mem. 2010;93:208–215. doi: 10.1016/j.nlm.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Hasegawa I, Fukushima T, Ihara T, Miyashita Y. Callosal window between prefrontal cortices: Cognitive interaction to retrieve long-term memory. Science. 1998;281:814–818. doi: 10.1126/science.281.5378.814. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder A, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Jackson JH. A study of convulsions. In: Taylor J, editor. Selected Writings of John Hughlings Jackson. Staples; London: 1958. [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth M, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT. Neural networks debunk phrenology. Science. 2007;316:1578–1579. doi: 10.1126/science.1144677. [DOI] [PubMed] [Google Scholar]
- Kolb B. Mechanisms underlying recovery from cortical injury: Reflections on progress and directions for the future. In: Rose FD, Johnson DA, editors. Recovery from Brain Damage. Plenum Press; New York: 1992. [DOI] [PubMed] [Google Scholar]
- Lytton WW, Williams ST, Sober SJ. Unmasking unmasked: Neural dynamics following stroke. Prog Brain Res. 1999;121:203–218. doi: 10.1016/s0079-6123(08)63075-7. [DOI] [PubMed] [Google Scholar]
- Mcnab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Metting van Rijn AC, Peper A, Grimbergen CA. High-quality recording of bioelectric events. Part 1. Interference reduction, theory and practice. Med Biol Eng Comput. 1990;28:389–397. doi: 10.1007/BF02441961. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. NeuroImage. 2005;27:341–56. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rosahl SK, Knight RT. Role of prefrontal cortex in generation of the contingent negative variation. Cereb Cortex. 1995;5:123–134. doi: 10.1093/cercor/5.2.123. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Bichot NP, Desimone R, Ungerleider LG. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci. 2007;27:11306–11314. doi: 10.1523/JNEUROSCI.2939-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg M, Lines CR, Milner AD. Visual evoked potentials to lateralized visual stimuli and the measurement of interhemispheric transmission time. Neuropsychologia. 1984;22:215–225. doi: 10.1016/0028-3932(84)90064-2. [DOI] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Lesion evidence that two distinct regions within prefrontal cortex are critical for n-back performance in humans. J Cogn Neurosci. 2009;21:2263–2275. doi: 10.1162/jocn.2008.21172. [DOI] [PubMed] [Google Scholar]
- Van Vleet TM, Heldt SA, Pyter B, Corwin JV, Reep RL. Effects of light deprivation on recovery from neglect and extinction induced by unilateral lesions of the medial agranular cortex and dorsocentral striatum. Behav Brain Res. 2003;138:165–178. doi: 10.1016/s0166-4328(02)00246-2. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Mccollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- von Monakow C. Die lokalisation im grosshirn und der abbau der funktion durch kortikale herde. In: Pribram KH, editor. Mood, States and Mind. Penguin Books; London: 1969. [Google Scholar]
- Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proc Natl Acad Sci U.S.A. 2010;107:18167–18172. doi: 10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, Greenwood RJ, Rothwell JC. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wundt W. Outlines of Psychology. 2nd. Engelmann; Leipzig: 1902. [Google Scholar]
- Yago E, Duarte A, Wong T, Barceló F, Knight RT. Temporal kinetics of prefrontal modulation of the extrastriate cortex during visual attention. Cogn Affect Behav Neurosci. 2004;4:609–617. doi: 10.3758/cabn.4.4.609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.