Abstract
The slow (<1 Hz) oscillation, along with its alternating UP and DOWN states in individual neurons, is a defining feature of the EEG during slow-wave sleep. Although this oscillation is well preserved across mammalian species, its physiological role remains unclear. Electrophysiological and computational evidence from cortex and thalamus now indicates that the slow oscillation UP states and the ‘activated’ state of wakefulness are remarkably similar dynamic entities. This is consistent with behavioural experiments suggesting that slow oscillation UP states provide a context for the replay and possible consolidation of previous experience. In this scenario, the T-type Ca2+ channel-dependent bursts of action potentials that initiate each UP state in thalamocortical neurons, might act as triggers for synaptic and cellular plasticity in corticothalamic networks.
The function of sleep is a mystery that has long fascinated biologists and is still today the matter of intense debate [1]. One of the most prominent features of sleep in mammals is the occurrence of the slow (<1 Hz) sleep oscillation that dominates slow-wave sleep (SWS) (Box 1). This oscillation is extremely similar in different species [2-12], suggesting that it might play well defined, conserved roles. Fairly recently, behavioral experiments have indicated that SWS might be related to memory consolidation [11-17], and that the basis of such consolidation might be the slow sleep oscillation itself [12, 14-18]. In this review we explore this issue from an electrophysiological and computational modeling point of view. Specifically, we re-assess various electrophysiological measurements of the waking (or wake-like) state and compare them with those obtained for the UP state of the slow (<1 Hz) sleep oscillation in the specific brain structures involved in its generation. The extremely close similarity of both single neuron and network dynamics during these different scenarios is compatible with the results of behavioural experiments indicating that during SWS selected epochs of prior experience are episodically replayed and consolidated.
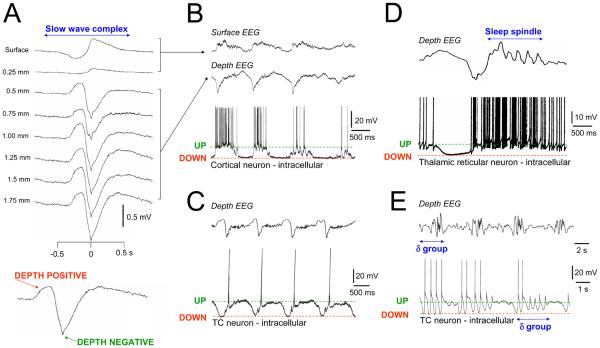
Box 1. The EEG slow (<1 Hz) oscillation and its relationship to neuronal activity and other types of sleep rhythms.
A. One cycle of the EEG slow (<1 Hz) oscillation at different cortical depths. Note the reversal in polarity between 0.25 and 0.5 mm. The bottom panel shows an enlarged slow wave from a depth recording with depth positive and negative components clearly indicated. B. Simultaneous surface EEG, depth EEG and intracellular recording from a cortical pyramidal neuron during the slow oscillation. The depth positive EEG component is associated with neuronal silence (DOWN state) whereas the depth negative component is related to neuronal depolarization (UP state). C and D. TC and TRN neurons also show UP and DOWN states in association with the EEG depth negative and positive components respectively. Note the stereotypical nature of the UP and DOWN states and how each UP state commences with a T-type Ca2+ channel-mediated burst. Sleep spindles tend to be grouped by the slow oscillation and typically occur immediately following the depth negative peak (see D). They are closely associated with rhythmic firing during the early part of the UP state in TRN neurons. Although sleep spindles are primarily generated within the TRN, their widespread spatiotemporal coherence is brought about by corticothalamic feedback [65]. E. Bursts of EEG delta (1-4 Hz) rhythms recurring within the frequency of the slow oscillation. These rhythms can be traced back to the activity of some TC neurons which can exhibit brief epochs of intrinsic rhythmic bursting rather than a silent down state. Again, although delta frequency activities originate primarily from intrinsic thalamic oscillators, these oscillators are synchronised by corticothalamic feedback [29]. In C and D, EEG and intracellular recording were performed simultaneously whereas the upper and lower traces in E are from separate recordings. All data are from the cat. Modified and reproduced with permission from [61] (A), [33] (B and C), [66] (D) and [21, 22] (E)
Local field potentials
Recordings of extracellular electrical activity using low-impedance electrodes. They provide a signal similar to the EEG, but sampling more local populations (of the order of 1 mm) in cortex.
Alpha (α) rhythms
EEG oscillations at ~10 Hz (range 8-13 Hz) that primarily occur during relaxed wakefulness. In humans they are most pronounced at occipital sites and are most prominent when the eyes are closed.
Sleep spindles
Brief bursts (typically lasting 0.5-1.5 s) of rhythmic EEG activity at ~7-14 Hz. Sleep spindles are characteristic of early sleep and are commonly associated with individual slow wave complexes (see above).
In vitro models of the slow oscillation
A slow oscillation can be reliably generated in isolated slices of neocortex. This oscillation is an emergent property of networks of cortical neurons with the UP state being generated by recurrent excitation that is balanced and regulated by inhibitory networks [24]. The down state arises when these mechanisms fail but also appears to be shaped by activity-dependent (i.e. Ca2+, Na+ and ATP-dependent) K+ channels [24, 42]. A recovery from this active disfacilitation can eventually lead to a new UP state.
In thalamic slices, individual TC and TRN neurons can generate a slow oscillation intrinsically without the need for any network input. This occurs when leak K+ currents are reduced below a certain threshold [43, 44], a feat that can be easily achieved by tonically activating mGluRs as likely occurs in the whole brain. In both TC and TRN neurons the UP and DOWN states are primarily generated by the “switching” on and off, respectively, of the T-type Ca2+ “window” current (see Refs. [9] and [43-47] for further details).
The dynamics of single neurons during ‘activated’ states and SWS
We start by reviewing the essential cellular correlates of the ‘activated’ brain state, which corresponds to attentive wakefulness, and examine how they qualitatively compare to those of SWS. Brain activation is invariably associated with a so-called “desynchronized” electroencephalogram (EEG), which consists of low-amplitude fluctuations at relatively high frequencies (>15 Hz) [5, 6] (Fig. 1A, right panel). This state is associated with tonic and apparently irregular firing of cortical neurons [19], the membrane potential of which has been shown in naturally waking animals to be persistently depolarized and to fluctuate around −60 mV [5, 6] (Fig. 1A, right panel).
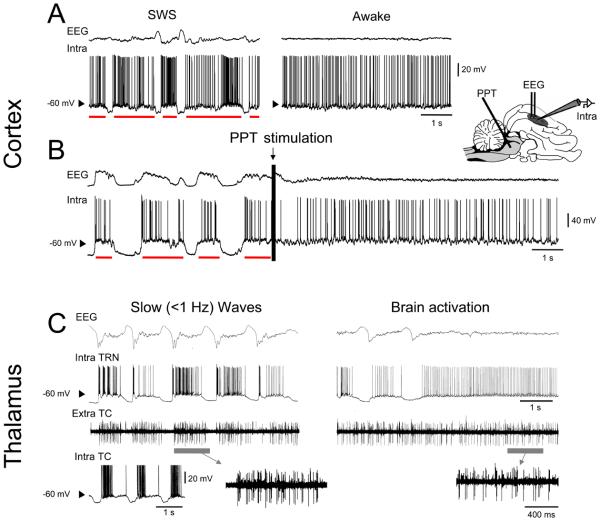
Figure 1. Cortical and thalamic activity in the cat during slow-wave oscillations and activated states.
A. Intracellular activity of cortical neurons during natural wake and sleep states. During wakefulness (right panel) the depth EEG (top trace) is desynchronized and the membrane potential fluctuates around a depolarized level close to −60 mV. During SWS, the activity alternates between depolarized periods (the UP states, red bars) similar to wakefulness, and hyperpolarizations (the DOWN states) synchronous with depth EEG slow waves (left panel). B. Transformation of UP/DOWN state dynamics to activated states under ketamine-xylazine anesthesia. Brief electrical stimulation of the pedunculopontine tegmentum (PPT, indicated schematically in the cat brain on the right) induces a transition from the typical UP/DOWN state dynamics to a long lasting EEG-activated state (see arrow above trace), where the membrane potential seems to become ‘locked’ in the UP state. C. Simultaneous recording of depth EEG, intracellular activity from a TRN neuron and extracellular multi-unit TC neuron firing in the ventral lateral (VL) nucleus (further enlarged below) during both slow waves (left panel) and a period leading to transient brain activation (right panel) (obtained from the cat). The inset to the bottom left illustrates the intracellular nature of the slow oscillation in TC neurons. Note the close similarity in TRN neuron activity and VL firing between the slow oscillation UP states and continuous brain activation. Note also that the reversed polarity of the EEG recording in B, as compared to A and C, is due to it being obtained with a surface electrode and is explained in Box. 1). Modified and reproduced with permission from [5] (A), [31] (B), and [32] (C).
During SWS, the overriding activity of the EEG is the so-called slow (<1 Hz) sleep rhythm or slow (<1 Hz) sleep oscillation [2, 3, 20] (Fig. 1A, left panel). This slow oscillation comprises rhythmically repeating, large amplitude biphasic waves (i.e. slow waves) and is responsible for temporally grouping all other types of sleep-related EEG patterns such as spindle waves (7-14 Hz) and delta oscillations (1-4 Hz) into clearly demarcated recurring sequences [3, 20] (Fig. 1A, left panel) (Box 1). As in the activated state, the intracellular correlate of the slow (<1 Hz) oscillation in individual cortical neurons is also characterized by periods of irregular tonic firing and a membrane potential that rapidly fluctuates around −60 mV [5, 6] (Fig. 1A, left panel). Unlike the activated state, however, such periods are regularly interrupted by large, stereotypical hyperpolarizations that occur in tandem with the depth positive component of the EEG slow waves [5, 6] (Fig. 1A, left panel) (Box 1). These distinct depolarized and hyperpolarized periods are usually referred to as UP and DOWN states, respectively. Thus, even through this cursory evaluation of intracellular recordings it is apparent that the ‘activated’ state of wakefulness and the UP states of the slow (<1 Hz) sleep oscillation correspond to broadly similar membrane potential dynamics.
Interestingly, several anesthetic regimes, including urethane and ketamine-xylazine, can also lead to a slow oscillation in the EEG of a variety of species [10, 21-28], with correlated UP and DOWN states in individual cortical neurons that are very similar to those which occur during natural sleep [10, 21-24, 27, 28] (Fig. 1B, left portion of trace). Indeed, whilst the slow oscillations induced by anesthesia might not show all the dynamic variability of those present during normal sleep, they capture many important aspects of natural sleep oscillations and have therefore proven to be an indispensable tool [3]. During anesthetic treatment, brain activation can be mimicked by stimulation of the ascending arousal system [29-32]. For example, under ketamine-xylazine anesthesia, brief electrical stimulation of the pedunculopontine tegmentum (PPT) typically induces a prolonged period (about 20 sec) of desynchronized EEG activity which is paralleled by a continuous depolarized state of the membrane potential (Fig. 1B, right portion of trace), similar to that observed during natural waking [30-32] (Fig. 1A, right portion of trace). Again, broad qualitative similarities between this stimulation-induced ‘activated’ state and the UP states of the slow oscillation are clearly evident, with the PPT stimulation appearing to ‘lock’ the membrane potential into an extended UP state.
The increased stability of intracellular recordings during the anesthetic-induced slow oscillation has proved vital for assessing the behaviour of neurons in key subcortical structures that are critically involved in influencing the dynamics of sleeping and wake states [21, 32, 33]. Specifically, intracellular recordings from both thalamocortical (TC) and thalamic reticular nucleus (TRN) neurons have revealed a pattern of UP and DOWN states during the slow oscillation that are similar to, and synchronized with, those in cortical neurons [21, 32, 33] (Fig. 1C), and which correspond closely with the activity of thalamic neurons observed during natural sleep. Thus, the slow oscillation UP states in TC and TRN neurons are characterized by seemingly irregular tonic firing and/or a membrane potential which fluctuates around −60 mV, whereas the DOWN state is manifest as a rhythmically recurring, stereotypical hyperpolarizing event (Fig. 1C, left panel). Also, in close similarity to cortical neurons, the activity of both types of thalamic neurons during brain activation becomes apparently ‘locked’ in a depolarized state (Fig 1C, right panel), being characterized by continuous firing and/or membrane potential fluctuations that are superficially indistinguishable from those that occur during the slow oscillation UP states [21, 32, 33] (Fig 1C, left panel). The qualitative similarities between the cellular correlates of the ‘activated’ state and the UP states of the slow (<1 Hz) sleep oscillation therefore appear to be a ubiquitous feature of neuronal dynamics in corticothalamic networks.
Similarities in cortical network dynamics between the ‘activated’ state and the UP states of the slow oscillation
The correspondence between activated states and the slow oscillation UP state in the cortex is not only apparent at the rank of single cells but can also be found at the level of the EEG and local field potentials (LFPs) (Box 1). Firstly, the typical desynchronized EEG pattern of arousal is evident locally in the EEG during UP states, both in the naturally sleeping animal (Fig. 1A, left panel) and during anesthesia (Fig. 1B, left portion of trace). A second and stronger indication of the similarities comes from studies using multiple bipolar electrodes in awake and naturally sleeping cats [34], where it has been shown that the desynchronized EEG patterns that are present during wakefulness are not only irregular temporally, but more generally, are characterized by low and fluctuating spatiotemporal coherence in local field potentials (LFPs) (Fig. 2A, left panel). The LFP signals at neighboring electrodes (1 mm distance) alternate between periods of high and low coherence (H.C. and L.C., respectively, in Fig 2A), as quantified by the correlation excursions (Fig. 2B, left panel). However, these fluctuations in coherence are local, since the excursions of correlations are greatly diminished between more distant pairs of electrodes [34-37] (Fig. 2B, left panel). Extremely similar results are obtained for the UP states of the slow oscillation during natural sleep (Fig. 2A, right panel), indicating that the spatiotemporal dynamics of these UP states, as viewed through the EEG and LFPs, are essentially indistinguishable from those of wakefulness.
Figure 2. Similar dynamic states during wakefulness and the UP state of SWS.
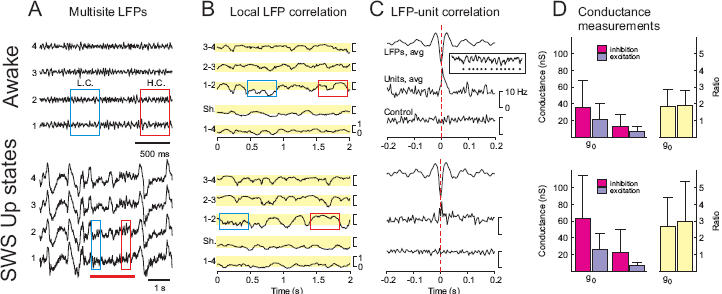
A. Local field potential (LFP) activity in cat parietal cortex during wakefulness (left) and SWS (right). Four electrodes (traces 1 to 4) were placed in cortical depth along a line in the anterior-posterior axis of the suprasylvian gyrus with 1 mm interelectrode distance. The red boxes indicate a period where LFP activity was highly coherent (H.C.) between two of the adjacent electrodes, whereas the blue boxes indicate a period of low coherence (L.C.). During SWS, the UP states were identified by locally desynchronized EEG activity (indicated by the red bar). B. Local correlation dynamics in LFP activity. Correlations between adjacent electrodes calculated in small time windows (100 ms) show fluctuations between high and low values. The color boxes indicate the degree of correlation during the corresponding periods delineated in A. Distant electrodes (1-4) did not show any significant correlation. C. Correlation between unit firing and LFP activity. The wave-triggered average shows that the negative LFP deflections (detected using a thresholding procedure [34]; see example in inset) are correlated with an increased firing in both wakefulness and the UP states of SWS. The control indicates the absence of relation after randomizing spike times. D. Conductance measurements in wake and UP states of SWS by matching intracellular activities to stochastic models. The general pattern of mean conductance (g0) and conductance fluctuations (σ) values was similar in wake and SWS, but the absolute values were different. Modified and reproduced with permission from [34] (A-C), and [38] (D).
Extracellularly-recorded cortical neurons also display similar dynamics during activated states and slow oscillation UP states. It has been known for some time that the mean firing rate of cortical neurons during wakefulness and SWS is in the same range [19] (Fig. 2C), a fact that is especially evident if one specifically analyzes the UP states of the slow oscillation [5, 34]. However, the dynamic similarity not only concerns the mean rate, but also the temporal patterns of discharge and the respective timing between different cells. Although such entities are more difficult to characterize, a convenient, albeit subjective, way to appreciate such information is by transforming the spike patterns into audio signals by assigning a specific note to each neuron. Allowing for the presence of “concerted silences” during SWS due to the slow oscillation DOWN states, the melodies produced by wakefulness and SWS are remarkably similar (such audio material can be downloaded from http://www.archive.org/details/NeuronalTones).
Importantly, the similarity between cortical UP states and activated states is not only evident in EEG/LFP dynamics and single cell firing, but also extends to the relationship between them. Performing wave-triggered averages of spiking activity, by averaging the periods of neuronal firing around the negative peaks of the LFP (see inset in Fig. 2C), reveals that the depth-negative EEG component is correlated with an increased probability of unit firing, both in the awake state and during the slow oscillation UP states (Fig. 2C).
From the recent intracellular investigation of SWS [5], the membrane potential dynamics of cortical neurons were decomposed into excitatory and inhibitory conductance components [38]. Such an analysis demonstrates that in the majority of cortical cells, inhibition is stronger than excitation, both at the level of mean conductances, as well as at the level of conductance variations (as quantified by the standard deviation σ of the conductance; see Fig. 2D). This pattern is seen for both wakefulness and the UP states of SWS. However, there is a significant difference between the absolute values measured during the two states, with SWS generally showing higher conductances (Fig. 2D). Nevertheless, both states have a qualitatively similar ratio of excitatory-to-inhibitory conductance, in which both the mean inhibitory conductance and its associated fluctuations are larger on average compared to excitatory contributions (Fig. 2D). This resemblance also extends to the spike-triggered average conductance patterns in the two states [38].
Finally, additional indirect evidence that cortical slow oscillation UP states and wakefulness stem from similar network dynamics comes from modeling studies. Biophysical models with realistic cellular properties and synaptic interactions have simulated cortical slow oscillation patterns with UP and DOWN states that are based on recurrent interactions between networks of excitatory and inhibitory neurons [39-41]. These models have established that minimal parameter changes enable the transition from the UP/DOWN states of the slow oscillation to a sustained UP state, with electrophysiological features consistent with the experimental measurements. Thus, several independent lines of experimental and modeling evidence indicate that cortical network dynamics during slow oscillation UP states are extremely similar to those present during wakefulness or brain activation.
Similarities between a persistently depolarized state and the UP state of the slow oscillation in TC and TRN neurons
Both the cortical and thalamic slow (<1 Hz) oscillations can be reproduced using in vitro models. In cortical slices, the slow oscillation is reliant on a modified artificial cerebrospinal fluid containing a reduced Ca2+ concentration, and is generated primarily by network-dependent mechanisms [24, 42] (Box 1). In thalamic slices, on the other hand, the slow oscillation in both TC and TRN neurons is generated mainly by intrinsic mechanisms [9, 43-46] (Box 1). These oscillations have extremely similar properties to those observed in thalamic cells during natural sleep [45, 47], and become apparent when activation of modulatory cortical input is mimicked through either electrical stimulation of corticofugal afferents or the persistent pharmacological activation of the metabotropic glutamate receptors that are postsynaptic to these afferents [9, 43-46]. The intrinsic nature of the slow oscillation in thalamic neurons offers a unique opportunity to examine the similarities between the slow oscillation UP states and a continuously depolarized state (as occurs in natural wakefulness and brain activation, see Fig. 1C), because these two scenarios can be invoked at will by simply varying the amount of steady injected current during an intracellular recording (Figs. 3A1, A2 and B). For example, we can compare the interspike interval (ISI) distributions between a condition where thalamic neurons fire continuously in the absence of injected current (Fig. 3, right column) and one where they are hyperpolarized to produce intermittent epochs of firing during the slow oscillation UP states (Fig. 3, left column). Such a comparison reveals that the mode and the essential form of the ISI distributions are extremely similar, although their coefficient of variation (CV) is always larger for UP state-generated firing episodes than for the ‘activated’ state. Interestingly, the typical rate of firing of TRN neurons (~40 Hz) (Fig. 3B) in both these conditions is always much higher than that of TC neurons (~10 Hz) [9, 43, 45] (Fig. 3A1 and A2). This, in turn, suggests a dominance of inhibitory over excitatory activity in the thalamus during both brain activation and the slow oscillation UP state, in a similar manner to that revealed for cortical neurons (see above, and Fig. 2D).
Figure 3. Close quantitative similarities between the UP state of the slow oscillation and a persistently depolarized state in TC and TRN neurons.
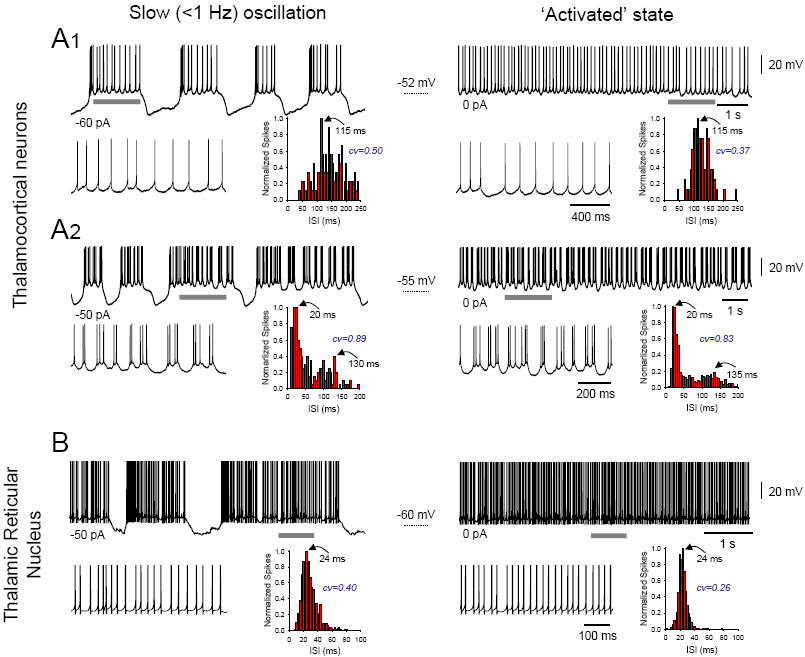
A. Left column: intrinsic slow (<1 Hz) oscillations recorded intracellularly from tonically hyperpolarized TC neurons in the cat lateral geniculate nucleus in vitro exhibiting either simple tonic firing (1, −60 pA) or high-threshold (HT) bursting (2, −50 pA) during the UP state (see expanded sections to the lower left). The corresponding (interspike interval) ISI distribution histograms shown to the lower right of the main traces are computed from several consecutive UP states. Right column: spontaneous activity of the neurons depicted in the left column, but in the absence of hyperpolarizing current, consists of continuous tonic firing (1) and continuous HT bursting (2) (see expanded sections to the lower left). The corresponding ISI distribution histograms are shown to the lower right and reveal an extremely similar pattern to that observed during the slow oscillation UP states. B. Left column: intrinsic slow oscillation recorded intracellularly from a tonically hyperpolarized (−50 pA) cat TRN neuron in vitro exhibiting high frequency tonic firing during the UP state (see expanded section to the lower left). The corresponding ISI distribution histograms shown to the lower right is computed from several consecutive UP states. Right column: spontaneous activity of the same neuron in the absence of hyperpolarizing current consists of continuous tonic firing (see expanded sections to the lower left). Again, the corresponding ISI distribution histograms is shown to the lower right and reveals an extremely similar pattern to that observed during the slow oscillation UP states. Adapted from [9] (A1), [43] (A2), and [45] (B).
In most TC neurons, continuous firing during sustained depolarization consists solely of tonic single action potential firing (Fig. 3A1, right panel), and correspondingly, the slow oscillation UP state also shows straightforward single action potential firing [9, 43] (Fig. 3A1, left panel). In a small number of TC neurons, however, firing during sustained depolarization comprises rhythmic (~3-15 Hz) high-threshold (HT) bursting [9, 43] (Fig. 3A2, right panel), and correspondingly, the slow oscillation UP state also produces epochs of HT bursting (Fig. 3A2, left panel). Because these HT burst generating neurons might act as pacemakers for wake-related alpha (α) (8-13 Hz) rhythms [47-49] (Box 1), a distinct possibility is that they also function to generate brief epochs of α frequency activity during the UP state of the slow oscillation in SWS [50, 51].
The slow oscillation UP states as micro-wake ‘fragments’
We have presented an assortment of data from the corticothalamic system that converges to establish that, at both the single cell and neuronal network levels, the fully activated brain state and the slow oscillation UP states are dynamically very similar. An attractive interpretation of this is that individual corticothalamic UP states provide micro-wake-like contexts which facilitate specific types of neuronal interaction. In particular, they might provide brief epochs of network dynamics which aid the transfer of memory traces between short term storage sites in the hippocampus and long term memory space in the neocortex. Within this framework, the subtle differences that do exist between the dynamics of continuous waking and the slow oscillation UP states may act to alter the direction of information flow between cortical areas whilst maintaining individual neurons in a depolarized and active state that is conducive to information processing. This modified information streaming might also be aided by the sleep related changes that occur in various neuromodulator levels, and particularly in acetylcholine (Ach) [52]. These ideas are certainly consistent with the results of recent behavioural studies showing that firing sequences from cell assemblies in both the neocortex and hippocampus are compressed and reactivated concurrently with the generation of the cortical slow oscillation UP state [12; see also 13, 53-56]. They also link well with human studies showing that i) local increases in the average power density of slow oscillations follow specific learning tasks and are related to a subsequent improvement in task performance [14], and ii) artificially boosting slow oscillations via the transcranial application of oscillating fields enhances the retention of hippocampus-dependent declarative memories [16].
Given that the dynamics of the slow oscillation UP states are so similar to those of continuous wakefulness, why do they not lead to conscious experience during SWS? One possibility is that current methods and approaches overlook some vital variables and are simply too coarse to detect the essential differences between what constitutes conscious and non-conscious dynamics. Alternatively, it could be that the differences in dynamics between the activated state and the UP states (i.e. discrepancies in neuronal conductances and CVs, compression of firing sequences etc.) are sufficient to determine whether full awareness takes place or not. In this sense, perhaps the most obvious and intuitive candidate to explain the behavioural disparity between wakefulness and sleep is the increased level of inhibition present during the UP state compared to the wake state (Fig 2D). Another possibility is that conscious awareness might require an uninterrupted stream of ‘activated’ neuronal dynamics lasting more than the few seconds of an UP state, perhaps so that certain types of long-range neuronal interactions during high-frequency (20-80 Hz) activity, which have been suggested to break down during sleep [57], can be fully established and maintained [58]. This is partly backed up by noting that during sleep, the most lucid experiences occur during the rapid eye movement (REM) phase when the intermittent hyperpolarizations (i.e. DOWN states) of the slow oscillation are absent [5]. However, it should also be noted here that not all studies support the idea that long range interactions are diminished during sleep [15].
T-type Ca2+ channel-mediated bursts in TC neurons as a trigger for UP states and synaptic plasticity
If individual UP states contain segments of prior wake-related dynamics, an attractive hypothesis is that these segments are determined and selected during wakefulness through the ongoing remodeling of cortical [59] or corticothalamic attractors by sensory input [18]. Such attractors might then be preferentially activated in sleep during the slow oscillation UP state [18], particularly in response to a strong thalamic signal which is a highly effective way to trigger internally-defined cortical ensemble dynamics [60, 61]. This is noteworthy because a conspicuous property of the slow oscillation in TC neurons is the presence of a robust T-type Ca2+ channel-mediated burst at the commencement of each UP state [9, 21, 32, 33, 43] (Figs. 4) (Box 1). Since these highly prominent TC neuron bursts invariably precede the UP state transitions in cortical neurons in vivo (see Fig. 9 in ref. [33])., they might be the key network triggers that are ultimately responsible for initiating a new epoch of re-activated corticothalamic dynamics. Supporting evidence for this comes from the finding that in cortical networks which lack thalamic input, i.e. cortical slabs [62] or slices [24], the slow oscillation DOWN state is considerably longer than when thalamic input is present. In addition, recordings in the intact brain demonstrate that the firing rate of a large proportion of cortical neurons is transiently elevated at the start of each UP state [27, 34] (Fig. 4), which is consistent with a robust, stereotypical excitatory input. Thus, although spontaneous UP and DOWN states can emerge in isolated cortical networks [24, 42] (Box 1), there is strong evidence that in the intact brain cortical UP states might be triggered by thalamic input.
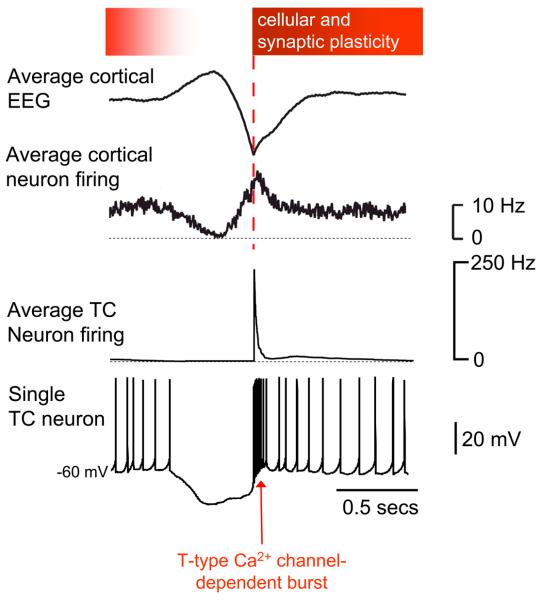
Figure 4. Key events of the slow oscillation cycle in thalamic and cortical neurons and their potential relation to plasticity.
During the positive phase of the depth EEG slow wave (top trace), neurons in both the thalamus (bottom two trace) and cortex (second trace from the top) are silent. This scenario is terminated by the powerful T-type Ca2+ channel-dependent bursts in TC neurons. In turn these bring about widespread neuronal synchronization and Ca2+ entry which provide advantageous conditions for inducing cellular and synaptic plasticity. The degree to which cortical and thalamic neurons are susceptible to plasticity is indicated by the intensity of shading in the bar at the top of the figure. Whilst the opportunity for plasticity is likely to be most evident at the commencement of the UP state it might also persist later into the UP state when wake-related neuronal activity might be replayed and consolidated. Average cortical EEG and neuron activity modified and reproduced with permission from [34].
In addition to acting as network triggers, T-type Ca2+ channel-mediated bursts might also play an important role in facilitating plasticity. The Ca2+ entry associated with these bursts, both directly (i.e. in thalamic neurons) and indirectly (i.e. in cortical neurons), could open the gate to subsequent modifications of synaptic strength and/or intrinsic excitability (Fig. 4), as previously proposed for sleep spindle oscillations (Box 1) in cortex [63, 64]. Moreover, the large increase of firing, that occurs at the start of the UP state in cortical neurons, and associated LFPs are synchronous over distances of several millimeters [34]. This highly synchronous nature of neuronal activity during the early part of the UP state might further contribute toward bringing about cellular and synaptic plasticity. These suggestions are consistent with the demonstrations that the slow oscillation UP state is critically related to memory consolidation in humans [15] and cell assembly reactivation in rats [13, 53-56].
Concluding remarks
Electrophysiological and modeling data show that the UP states of the slow (<1 Hz) sleep oscillation are dynamically equivalent to the activated state of wakefulness. This is in agreement with a number of behavioural investigations, which indicate that awake activities are replayed and possibly consolidated during SWS. The prominent T-type Ca2+ channel-mediated bursts in TC neurons might act as key network triggers that ensure the synchronous start of slow oscillation UP states across related cortical territories. The highly synchronized and increased firing that is present in the initial portion of the UP state in different neuronal components of corticothalamic networks might represent an important element in bringing about cellular and synaptic plasticity.
Clearly, a number of outstanding questions remain: (1) Why are the corticothalamic dynamics of slow oscillation UP states so similar to those of continuous wakefulness and why, given this similarity, is the slow oscillation associated with a lack of consciousness? (2) What are the precise neuronal rudiments that define a lack of consciousness during sleep? Are our techniques too coarse to determine this or is it simply that UP states are too short to sustain conscious experience? (3) What are the basic cellular mechanisms underlying SWS-specific learning and memory consolidation?
Acknowledgments
This review is dedicated to the memory of Mircea Steriade. Work in our labs is supported by The Wellcome Trust (grants 71436, 78311 and 78403, to VC and SWH) and by the CNRS, Human Frontier Science Program and European Community (AD and MR). Additional information regarding other published work from the Crunelli and Destexhe labs are available at http://www.thalamus.org.uk and http://cns.iaf.cnrsgif.fr, respectively.
References
- 1.Hobson JA. Sleep is of the brain, by the brain and for the brain. Nature. 2005;437:1254–1256. doi: 10.1038/nature04283. [DOI] [PubMed] [Google Scholar]
- 2.Achermann P, Borbely AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 3.Steriade M, Amzica F. Slow sleep oscillation, rhythmic K-complexes, and their paroxysmal developments. J Sleep Res. 1998;7(Suppl 1):30–35. doi: 10.1046/j.1365-2869.7.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 4.Molle M, et al. Grouping of Spindle Activity during Slow Oscillations in Human Non-Rapid Eye Movement Sleep. J Neurosci. 2002;22:10941–10947. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steriade M, et al. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 6.Timofeev I, et al. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci U S A. 2001;98:924–929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, et al. Lack of delta waves and sleep disturbances during non-rapid eye movement sleep in mice lacking alpha1G-subunit of T-type calcium channels. Proc Natl Acad Sci U S A. 2004;101:18195–18199. doi: 10.1073/pnas.0408089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirota A, et al. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu L, et al. Nucleus- and species-specific properties of the slow (<1 Hz) sleep oscillation in thalamocortical neurons. Neuroscience. 2006;141:621–636. doi: 10.1016/j.neuroscience.2006.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolansky T, et al. Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. J Neurosci. 2006;26:6213–6229. doi: 10.1523/JNEUROSCI.5594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eschenko O, et al. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26:12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 13.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 14.Huber R, et al. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 15.Molle M, et al. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci U S A. 2004;101:13963–8. doi: 10.1073/pnas.0402820101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 17.Huber R, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 18.Battaglia FP, et al. Firing rate modulation: a simple statistical view of memory trace reactivation. Neural Netw. 2005;18:1280–1291. doi: 10.1016/j.neunet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Hobson JA, McCarley RW. Cortical unit activity in sleep and waking. Electroencephalogr Clin Neurophysiol. 1971;30:97–112. doi: 10.1016/0013-4694(71)90271-9. [DOI] [PubMed] [Google Scholar]
- 20.Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82:671–686. doi: 10.1016/s0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- 21.Steriade M, et al. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steriade M, et al. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steriade M, et al. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 25.Bokor H, et al. Selective GABAergic control of higher-order thalamic relays. Neuron. 2005;45:929–940. doi: 10.1016/j.neuron.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 26.Hahn TT, et al. Phase-locking of hippocampal interneurons' membrane potential to neocortical up-down states. Nat Neurosci. 2006;9:1359–1361. doi: 10.1038/nn1788. [DOI] [PubMed] [Google Scholar]
- 27.Haider B, et al. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isomura Y, et al. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Steriade M, et al. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991;11:3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steriade M, Amzica F, Nunez A. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol. 1993;70:1385–400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph M, et al. Characterization of synaptic conductances and integrative properties during electrically induced EEG-activated states in neocortical neurons in vivo. J Neurophysiol. 2005;94:2805–2821. doi: 10.1152/jn.01313.2004. [DOI] [PubMed] [Google Scholar]
- 32.Steriade M, et al. Synchronization of fast (30-40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci. 1996;16:392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–62. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Destexhe AD, et al. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckhorn R, et al. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 36.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steriade M, Amzica F. Intracortical and corticothalamic coherency of fast spontaneous oscillations. Proc Natl Acad Sci U S A. 1996;93:2533–8. doi: 10.1073/pnas.93.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudolph M, et al. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J Neurosci. 2007 doi: 10.1523/JNEUROSCI.4652-06.2007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bazhenov M, et al. Model of thalamocortical slow-wave sleep oscillations and transitions to activated States. J Neurosci. 2002;22:8691–8704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Compte A, et al. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 2003;89:2707–2725. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez FP, Destexhe A. Simulating cortical network activity states constrained by intracellular recordings. Neurocomputing. 2004;58:285–290. [Google Scholar]
- 42.Cunningham MO, et al. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci U S A. 2006;103:5597–5601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hughes SW, et al. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 44.Crunelli V, et al. The ‘window’ T-type calcium current in brain dynamics of different behavioural states. J Physiol. 2005;562:121–129. doi: 10.1113/jphysiol.2004.076273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blethyn KL, et al. Neuronal basis of the slow (<1 Hz) oscillation in neurons of the nucleus reticularis thalami in vitro. J Neurosci. 2006;26:2474–2486. doi: 10.1523/JNEUROSCI.3607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crunelli V, et al. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium. 2006;40:175–190. doi: 10.1016/j.ceca.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes SW, et al. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron. 2004;42:253–268. doi: 10.1016/s0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- 48.Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–372. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- 49.Hughes SW, Crunelli V. Just a phase they're going through: The complex interaction of intrinsic high-threshold bursting and gap junctions in the generation of thalamic alpha and theta rhythms. Int J Psychophysiol. 2006 doi: 10.1016/j.ijpsycho.2006.08.004. In Press, doi:10.1016/j.ijpsycho.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacFarlane JG, et al. Periodic K-alpha sleep EEG activity and periodic limb movements during sleep: comparisons of clinical features and sleep parameters. Sleep. 1996;19:200–204. doi: 10.1093/sleep/19.3.200. [DOI] [PubMed] [Google Scholar]
- 51.Karadeniz D, et al. EEG arousals and awakenings in relation with periodic leg movements during sleep. J Sleep Res. 2000;9:273–277. doi: 10.1046/j.1365-2869.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 52.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends in Cognitive Sciences. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 53.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 54.Nadasdy Z, et al. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Battaglia FP, et al. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molle M, et al. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol. 2005;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]
- 57.Massimini M, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 58.Roelfsema PR, et al. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature. 1997;385:157–161. doi: 10.1038/385157a0. [DOI] [PubMed] [Google Scholar]
- 59.Cossart R, et al. Attractor dynamics of network UP states in the neocortex. Nature. 2003;423:283–288. doi: 10.1038/nature01614. [DOI] [PubMed] [Google Scholar]
- 60.MacClean JN, et al. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 61.Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 2001;82:671–686. doi: 10.1016/s0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- 62.Timofeev I, et al. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 63.Contreras D, et al. Intracellular and computational characterization of the intracortical inhibitory control of synchronized thalamic inputs in vivo. J Neurophysiol. 1997;78:335–350. doi: 10.1152/jn.1997.78.1.335. [DOI] [PubMed] [Google Scholar]
- 64.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 65.Contreras D, et al. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–4. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 66.Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490:159–179. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]