Abstract
Ca2+-Calcineurin-NFAT signaling plays a major role in promoting pathological cardiac hypertrophy. Heineke et al. (2010) show that CIB1 strongly enhances calcineurin activation and cardiac hypertrophy upon pathological stress, likely by functioning as a scaffold protein that exposes calcineurin to the L-Type Ca2+ channel and the sarcolemma.
Growth of the adult mammalian heart can be physiological (during postnatal development and in response to pregnancy and exercise) or pathological (in the context of a cardiac disease). In either case, growth of the heart is achieved primarily by increasing the size (hypertrophy) of pre-existing cardiomyocytes, with little or no contribution of cardiomyocyte proliferation. While physiological heart growth increases cardiac performance to match metabolic demands, pathological heart growth is ultimately maladaptive (Hill and Olson, 2008) and can culminate in heart failure, the inability of the heart to supply sufficient blood flow and one of the leading causes of death in industrialized countries. Physiological and pathological hypertrophy are mediated largely through different extra- and intracellular signaling pathways. Amongst them, a Ca2+-calcineurin-NFAT signaling cascade has been identified as a crucial node where inputs from several signaling pathways are integrated to promote pathological hypertrophy (Molkentin et al., 1998).
In a beautifully conducted study in Nature Medicine, Heineke and colleagues (Heineke et al., 2010) shed new light on how calcineurin activation can be sustained by Ca2+ signals in a cellular environment where cytosolic Ca2+ concentrations continuously fluctuate to induce cardiomyocyte contraction.
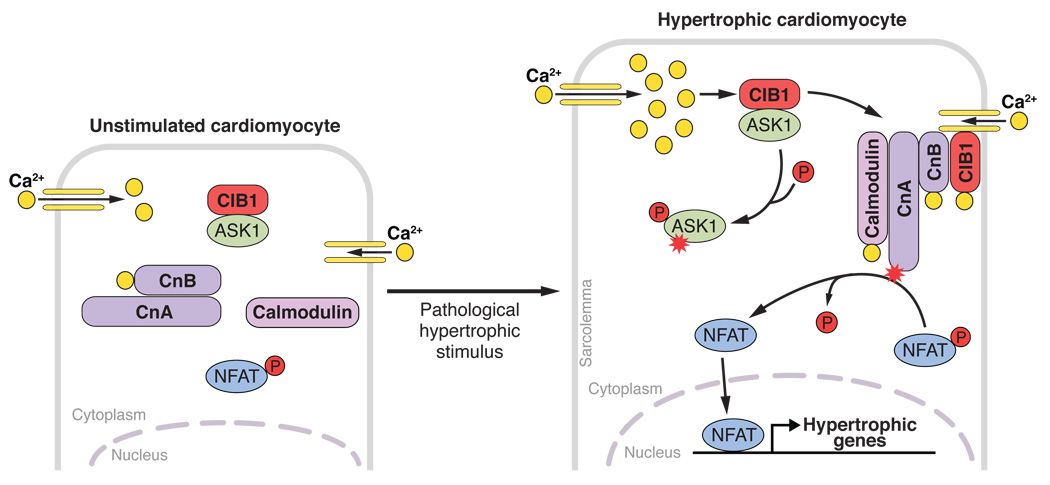
Calcineurin is a serine-threonine protein phosphatase that comprises a catalytic A subunit (CnA), which can be bound by two small EF-hand calcium-binding proteins, the regulatory calcineurin B subunit (CnB) and calmodulin (Crabtree and Olson, 2002). CnB binds CnA at low calcium concentrations; in contrast, calmodulin binds to CnA only at higher cytosolic calcium concentrations, for example during cardiac stress, and thereby activates the phosphatase activity of CnA by inducing a conformational change that separates an autoinhibitory domain from the active enzymatic site. Active calcineurin dephosphorylates nuclear factor of activated T cells (NFAT), which results in translocation of NFAT from the cytoplasm to the nucleus, where it promotes hypertrophic gene expression (figure).
Model of CIB1-induced activation of calcineurin-NFAT signaling.
Upon pathological cardiac stress, cytosolic Ca2+ concentration increases, due to enhanced Ca2+ influx through the L-Type Ca2+-channel (yellow). Elevated intracellular Ca2+ concentrations lead to Ca2+ binding of CIB1, inducing a conformational change of this scaffold protein that likely replaces ASK1 with calcineurin (Cn) as a binding partner. Unbound ASK1 might get activated by autophosphorylation and promote apoptosis, while the Ca2+-CIB1-calcineurin complex interacts with the sarcolemma. This seems to be a prerequisite to allow calcineurin activation by binding of Ca2+ loaded calmodulin. Activated calcineurin dephosphorylates NFAT resulting in its nuclear translocation, induction of hypertrophic gene expression and eventually cardiomyocyte hypertrophy. ASK1, apoptosis signal-regulating kinase1; CIB1, Ca2+ and integrin-binding protein-1; CnB, calcineurin B (regulatory calcineurin domain); CnA, calcineurin A (catalytic calcineurin domain), NFAT, nuclear factor of activated T cells
Heineke and colleagues show now that calcineurin activation is enhanced upon compartmentalization of calcineurin to the sarcolemma, the cell membrane of the cardiomyocyte, possibly by directly exposing the calcineurin protein complex to Ca2+ influx through the L-type Ca2+-channel. They further show that binding of calcineurin to the sarcolemma is promoted through a scaffold protein, Ca2+- and integrin-binding protein-1 (CIB1, calmyrin), that is specifically upregulated during pathological, but not physiological cardiac hypertrophy. Only upon pathological stress, CIB1 localizes to the sarcolemma of murine and human cardiomyocytes, where it can bind to L-Type Ca2+-channels and seems to function as an anchor that facilitates binding of CnB to the sarcolemma (figure).
Cib1 knockout mice undergoing thoracic aortic constriction (TAC) surgery, a model of pathological hypertrophy, in which the heart is exposed to acute hypertension secondary to ligation of the thoracic aorta, had a blunted hypertrophic response, reduced induction of hypertrophic marker genes and less fibrosis compared to wild-type mice. Conversely, inducible overexpression of CIB1 in the hearts of adult transgenic mice enhanced cardiac hypertrophy in response to TAC, which was reversed in a CnA knockout background. These results suggest that pharmacological blocking of CIB1 could be a means to specifically suppress pathological cardiac hypertrophy. However, since CIB1 is expressed in a wide variety of tissues, CIB1 inhibitors likely would have to be cardiac-specific to circumvent side effects in other tissues such as impaired angiogenesis following ischemia, impaired thrombosis or male sterility, all of which were observed in Cib1 knockout mice (Naik et al., 2009; Yuan et al., 2006; Zayed et al., 2007).
Two calcineurin inhibitory drugs, cyclosporin A (CsA) and tacrolimus/FK506 have been used since the 1980s to suppress T-cell immunity and prevent graft rejection in organ transplantation. By blocking calcineurin in the heart, they are believed to also suppress pathological cardiac hypertrophy (at least in mice). However, CsA also causes kidney damage and hypertension, a primary cause of cardiac hypertrophy, which further highlights the need to develop cardiac-specific inhibitors of calcineurin-NFAT signaling.
It is unclear whether the CIB1-CnB interaction also plays a role in other tissues. However, if the CIB1-CnB interaction were cardiac-specific, inhibition of this interaction might be a promising pharmacologic approach. One the other hand, it is also unclear whether the observed pro-hypertrophic effects of CIB1 were mediated solely via binding to calcineurin, since CIB1 also interacts with several other signaling molecules, although those interactions have not been shown to occur in cardiomyocytes. Nevertheless, inactivation of pathological calcineurin-NFAT signaling by specific disruption of the CIB1-CnB interaction might be advantageous compared to direct inhibition of calcineurin, since Cib1 knockout mice do not seem to have a basal cardiac phenotype, while cardiac-specific deletion of CnB resulted in impaired cardiac function during postnatal cardiac development (Schaeffer et al., 2009).
Recently, CIB1 was also shown to interact with apoptosis signal-regulating kinase1 (ASK1) (Yoon et al., 2009), which is believed to regulate cardiomyocyte apoptosis (Liu et al., 2009). CIB1 binding of ASK1 blocks autophosphorylation of ASK1 and thereby its activation. In response to an increase in intracellular Ca2+, CIB1 dissociates from ASK1, inducing its activation in neurons. One could speculate that under basal conditions CIB1 also binds and inhibits ASK1 in cardiomyocytes. Ca2+ binding of CIB1 upon a stress-induced Ca2+ increase would then function as a switch that actives ASK1 and calcineurin simultaneously by replacing ASK1 with CnB as a binding partner for CIB1.
In summary, Heineke et al. (Nature Medicine, 2010) identified CIB1 as a crucial pro-hypertrophic activator of calcineurin-NFAT signaling. Inhibition of CIB1-dependent calcineurin activation might be an interesting pharmacologic approach to treat pathological cardiac hypertrophy, however development of such inhibitors could be challenging since CIB1 does not possess an intrinsic enzymatic activity but, instead, works through protein-protein interaction. While the plethora of regulatory inputs into the calcineurin-NFAT pathway offers many opportunities (and challenges) to the therapeutic manipulation of this signaling axis in the setting of pathological cardiac hypertrophy, for now, prevention of pathological hypertrophy by rigorous treatment of risk factors such as arterial hypertension, aortic stenosis or coronary artery disease remains the best means to treat pathological hypertrophy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109 Suppl:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Heineke J, Auger-Messier M, Correll RN, Xu J, Benard MJ, Yuan W, Drexler H, Parise LV, Molkentin JD. CIB1 is a regulator of pathological cardiac hypertrophy. Nat Med. 2010;16:872–879. doi: 10.1038/nm.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sargent MA, York AJ, Molkentin JD. ASK1 regulates cardiomyocyte death but not hypertrophy in transgenic mice. Circ Res. 2009;105:1110–1117. doi: 10.1161/CIRCRESAHA.109.200741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik MU, Nigam A, Manrai P, Millili P, Czymmek K, Sullivan M, Naik UP. CIB1 deficiency results in impaired thrombosis: the potential role of CIB1 in outside-in signaling through integrin alpha IIb beta 3. J Thromb Haemost. 2009;7:1906–1914. doi: 10.1111/j.1538-7836.2009.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer PJ, Desantiago J, Yang J, Flagg TP, Kovacs A, Weinheimer CJ, Courtois M, Leone TC, Nichols CG, Bers DM, Kelly DP. Impaired contractile function and calcium handling in hearts of cardiac-specific calcineurin b1-deficient mice. Am J Physiol Heart Circ Physiol. 2009;297:H1263–H1273. doi: 10.1152/ajpheart.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KW, Cho JH, Lee JK, Kang YH, Chae JS, Kim YM, Kim J, Kim EK, Kim SE, Baik JH, Naik UP, Cho SG, Choi EJ. CIB1 functions as a Ca(2+)-sensitive modulator of stress-induced signaling by targeting ASK1. Proc Natl Acad Sci U S A. 2009;106:17389–17394. doi: 10.1073/pnas.0812259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Leisner TM, McFadden AW, Clark S, Hiller S, Maeda N, O'Brien DA, Parise LV. CIB1 is essential for mouse spermatogenesis. Mol Cell Biol. 2006;26:8507–8514. doi: 10.1128/MCB.01488-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed MA, Yuan W, Leisner TM, Chalothorn D, McFadden AW, Schaller MD, Hartnett ME, Faber JE, Parise LV. CIB1 regulates endothelial cells and ischemia-induced pathological and adaptive angiogenesis. Circ Res. 2007;101:1185–1193. doi: 10.1161/CIRCRESAHA.107.157586. [DOI] [PubMed] [Google Scholar]