Abstract
Objective
Thyroid hormones are involved in metabolic regulation, but the degree to which they affect body weight and body mass index (BMI) in children is unclear. We examined the effect of hypo- and hyperthyroidism on weight and BMI at the time of diagnosis and after appropriate treatment.
Design
Prospective and retrospective case series
Patients
Children referred for thyroid dysfunction were enrolled prospectively if their total or free T4 was elevated with TSH <0.05 mIU/mL (N=57) or if they had a subnormal total or free T4 and TSH >20 (N=29).
Results
Almost all patients had at least 2 classic signs or symptoms including goiter, but hyperthyroid patients had more symptoms. Mean BMI z scores at the time of diagnosis did not significantly differ between the two groups. Males with hyperthyroidism complained of weight loss more frequently and had a lower pretreatment BMI z score than hyperthyroid females. Hypothyroid patients lost a minimal amount of weight by the first follow-up (mean of 0.3 kilograms (kg)) and on average gained weight by the second follow-up visit. In contrast hyperthyroid patients gained a mean of 3.4 kg at the first follow-up visit and a mean of 7.1 kg by the second.
Conclusions
Correction of hypothyroidism resulted in minimal weight loss, suggesting that hypothyroidism does not cause significant weight gain in children. In contrast, correction of the hyperthyroid state had a somewhat greater impact on weight status. These results are consistent with prior reports but surprising given the opposite metabolic effects of hypo- and hyperthyroidism.
Keywords: Hypothyroidism, hyperthyroidism, children, body mass index, weight gain, weight loss, obesity
Introduction
Thyroid hormone serves as one of the major regulators of resting energy metabolism in humans.1 States of thyroid excess lead to an increased metabolic rate and frequently present with weight loss at the time of diagnosis. One might therefore expect to see equivalent weight gain in hypothyroidism as a result of a decreased metabolic rate. Although obesity often leads physicians to measure thyroid hormone concentrations, studies in children suggest that obesity is rarely due to hypothyroidism. In a report from Germany, only 0.3% of 1405 children who presented to an endocrinology clinic for obesity were diagnosed with hypothyroidism.2 The American Academy of Pediatrics guidelines do not recommend routine thyroid testing as part of the evaluation for obesity.3
The impression that hypothyroidism has a major effect on weight gain largely comes from the mid 1900s when thyroid function tests were not available, and patients with severe and long-standing hypothyroidism often developed myxedema with water retention.4 More recent studies of adults have demonstrated minimal if any weight loss after initiating treatment in earlier stages of hypothyroidism, suggesting that negligible weight gain occurred before treatment.4,5,6 Although there is little pediatric data on the subject, a recent study reported minimal weight loss between the time of diagnosis and the first visit after initiating l-thyroxine treatment.7 In contrast, studies of adult hyperthyroid patients have demonstrated significant and sustained weight gain after initiation of treatment;5,8,9 however, there is little pediatric data on this subject.
Assessing whether and to what extent thyroid disease truly affects weight status poses a problem, especially in children. At the time of diagnosis it is difficult to determine when the onset of the disease occurred, particularly because symptoms may develop insidiously. Furthermore, in the pediatric population, weight gain is expected as part of normal growth; thus, a baseline weight prior to disease onset is often hard to establish. As noted above, one option for assessing the effects of thyroid disease on weight is to track BMI change, which takes into account both weight and height change, once treatment for hypo- or hyperthyroidism is initiated.4 In theory, a patient will return to the pre-disease BMI z score once euthyroid status is achieved; however, the time required to return to this baseline weight is unclear.
We report here a cohort of pediatric hyperthyroid and hypothyroid patients diagnosed during a 3 year period at a large urban teaching hospital. We examined signs, symptoms, and weight status at the time of diagnosis and rechecked height and weight at two subsequent follow-up visits to determine how much weight and BMI change occurred with appropriate treatment.
Methods
Study Design and Subject Identification
Children seen at Children's National Medical Center between March 2004 and October 2007 for an initial evaluation of newly diagnosed hyper- or hypothyroidism were included if they were at least 4 years old and the child or the parents could answer the survey questions. Subjects with known celiac disease, type 1 diabetes mellitus, or other conditions affecting weight gain were excluded, although subjects were not screened for undiagnosed diseases. Patients were evaluated by 1 of 4 staff endocrinologists.
Most patients were untreated at the time of the initial visit, although four subjects had been started on treatment in the previous 1–4 weeks and two hyperthyroid patients had been on beta-blockers only. However, in most of these cases, the height, weight, and heart rate just prior to the start of treatment was obtained through the primary care physician's office. Where pre-treatment heart rate was not available, the heart rate at the initial endocrinology visit was not included in the analysis.
All hyperthyroid patients had an elevated serum concentration of total or free T4 and TSH <0.05 mIU/mL. In order to exclude patients with either subclinical or mild hypothyroidism, only hypothyroid patients with serum level of TSH >20 and subnormal total or free T4 were included; 24 of 29 patients had TSH levels >100. Patients with congenital hypothyroidism, surgically or radioiodine-induced hypothyroidism, or transient thyroiditis were excluded.
This study was approved by the Institutional Review Board at Children's National Medical Center.
Evaluation and Data Collection
During the initial evaluation, the presence of a goiter was assessed by the endocrinologist through palpation and inspection, and heart rate was recorded. Weight and height were plotted with an electronic Microsoft Windows ® growth charts program (GrowthCharts version 4.1, copyright 2001–2006 by John T. Cockerham, MD). The program calculated body mass index (BMI) using the standard formula of weight in kilograms (kg) divided by the square of the height in meters. Percentiles and standard deviation (Z) scores for growth parameters were calculated using the appropriate CDC tables, choosing parameters from the nearest half-month of age.10
To document the frequency of the diverse symptoms seen in patients with thyroid disorders, parents and patients were asked a standard set of questions regarding recent onset (within the prior few months) of symptoms commonly seen in hypo- and hyperthyroidism. The list of symptoms (see Table 1) was culled from prior studies11,12,13 and augmented with some symptoms frequently mentioned by patients (e.g. tiring quickly in hyperthyroid patients). Given that some symptoms present in an opposite manner for hyperthyroid versus hypothyroid patients, the first 5 symptoms listed in Table 1 were grouped by opposites; the hyperthyroid patients were asked about the symptom listed on the left side of the first column, and hypothyroid patients were asked about the symptom on the right side of the first column. The sixth symptom was posed as tiring more quickly to the hyperthyroid group and as feeling more fatigued to the hypothyroid group. The next 4 symptoms listed were frequently present in both groups of patients. Finally, the last 6 symptoms were considered to be specific to either hyperthyroid or hypothyroid patients and were only asked of the appropriate group.
Table 1.
Prevalence of Symptoms (hyperthyroid on left; hypothyroid on right)
| Hyperthyroid | Hypothyroid | |
|---|---|---|
| N | 57 | 29 |
| Feeling hotter or sweating more / Feeling colder | 37 (65%) | 14 (48%) |
| Weight loss reported / Weight gain reported | 30 (53%) | 11 (38%) |
| Getting upset more easily / Feeling more depressed | 37 (65%) | 11 (38%) |
| Increased appetite / Decreased appetite | 32 (56%) | 10 (34%) |
| Diarrhea or loose stools / Constipation | 17 (30%) | 6 (21%) |
| Tiring more quickly; feeling more fatigued | 37 (65%) | 19 (66%) |
| More headaches than usual | 26 (46%) | 5 (17%) |
| Difficulty focusing on schoolwork or maintaining grades | 38 (67%) | 7 (24%) |
| Lump in front of neck noted by parents | 19 (33%) | 8 (28%) |
| Trouble sitting still | 38 (67%) | |
| Feeling heart beat too fast or too hard | 31 (54%) | |
| Difficulty breathing or catching breath | 22 (39%) | |
| Eyes more prominent | 20 (35%) | |
| Sleeping more or napping during the day | 13 (45%) | |
| Dry skin | 14 (48%) |
After the initial data collection was completed, data on height and weight for two follow-up visits were extracted from the medical record where available to assess changes in weight and BMI following the initiation of appropriate treatment. Results of thyroid function tests from these visits were also noted. In order to equalize the follow-up intervals for the hyperthyroid and hypothyroid groups, we occasionally used the third visit following diagnosis, rather than the second visit, as the data for the second follow-up visit. In one hyperthyroid case where the interval between diagnosis and the next visit was less than one month, we extracted data from the second visit after diagnosis to use as the first follow-up. In two cases of hypothyroid patients, the first follow-up visit occurred more than 8 months after diagnosis, so we chose to consider that visit as the second follow-up visit. For those few patients who continued to worsen following the start of therapy, as evidenced by worsening thyroid functions, data was not included for follow-up visits.
Statistical Analysis
Two sample t-tests were used to calculate p values in comparisons of population means. The 1-sided Fisher's exact test was utilized to generate p values for comparison of frequencies within 2 populations. Mixed effects multilinear regression models were used to account for the non-normal distribution of the data.
Results
Table 1 displays the study population's frequency of symptoms commonly found in patients with thyroid disease. The sixth symptom (tiring more quickly or fatigue) was found to be a similar complaint in both hyperthyroid and hypothyroid patients, present in about two-thirds of both groups. As noted, most symptoms were reported in one-third to two-thirds of patients; furthermore, almost all patients (except 4 hypothyroid and 1 hyperthyroid) experienced at least 2 and usually at least 3 of these symptoms.
Characteristics of the study patients are summarized in Table 2. As noted in other studies, both hyperthyroidism and hypothyroidism were found much more often in females than in males. A goiter was found on exam in the majority of patients, substantially exceeding the parental reports of swelling at the front of the neck. Among the hypothyroid patients, the one patient with no presenting symptoms and the 3 patients with only 1 symptom all were found to have a goiter. In the hyperthyroid patients, 68% had heart rates above 100 beats per minute, while 41% of hypothyroid patients had heart rates less than 70 beats per minute.
Table 2.
Characteristics at Diagnosis
| Hyperthyroid | Hypothyroid | p value | |
|---|---|---|---|
| n | 57 | 29 | |
| Age in years | |||
| Median (range) | 14.1 (3.8–19.2) | 10.2 (5.7–17.2) | |
| Sex – M(% of total) | 15 (26%) | 8 (28%) | |
| Goiter present | 92% | 66% | |
| Heart rate | |||
| Median (range) | 115 (74–152) | 72 (55–96) | |
| Height in cm | |||
| Mean ± SD | 157.4 ±13.4 | 139.0 ±17.3 | |
| Median (range) | 158.2 (106.5–185.4) | 137.8 (105.5–180.1) | |
| Height z score | |||
| Mean ± SD | 0.45 ± 1.21 | −0.19 ± 1.23 | |
| Median (range) | 0.36 (−1.75 to 3.30) | 0.41 (−3.99 to 1.35) | <0.01 |
| Weight in kg | |||
| Median (range) | 52 (18 to 91.3) | 38 (20.5 to 101.5) | 0.01 |
| Weight z score | |||
| Median (range) | 0.56 (−2.00 to 2.14) | 0.71 (−1.60 to 2.64) | 0.75 |
| BMI | |||
| Median (range) | 20.4 (14.2 to 36.7) | 19.3 (15.0 to 40.7) | 0.68 |
| BMI z score | |||
| Median (range) | 0.42 (−2.07 to 2.27) | 0.91 (−1.53 to 2.58) | 0.11 |
At the time of diagnosis, patients in the hyperthyroid group had a higher mean weight compared to the hypothyroid population, but this was likely due to the fact that the mean age was nearly 3 years older in the hyperthyroid group as evidenced by the similarity in weight when age adjusted norms are used (weight z score). Additionally, the hypothyroid patients were somewhat shorter for age (height z score), which may reflect slowing of growth associated with prolonged hypothyroidism, though there was only one hypothyroid patient in this study with height z score of < 2. The range of both BMI and BMI z scores were quite wide with extensive overlap between the 2 groups, suggesting an overall minimal difference in weight status at the time of diagnosis.
In the hyperthyroid group, males more frequently reported weight loss as a symptom and were found to have a significantly lower BMI z scores than females at the time of diagnosis (Table 3). No differences were found between males and females in the hypothyroid group.
Table 3.
Weight-Related Data for Hyperthyroid Males vs Females
| Males | Females | p-value | |
|---|---|---|---|
| n | 15 | 42 | |
| Mean age in years | 13.9 | 13.25 | |
| Reported weight loss – n (%) | 13 (81%) | 18 (43%) | 0.01 |
| Reported increased appetite – n (%) | 10 (63%) | 22 (54%) | 0.29 |
| BMI z score at diagnosis – mean (range) | −0.53 (−2.1 to 1.7) | 0.54 (−1.8 to 2.3) | <0.01 |
We attempted to determine if the severity of thyroid disease at diagnosis, as assessed by goiter size, heart rate, and degree of abnormality of total or free T4, was predictive of weight z score and BMI z score at the time of diagnosis, but no correlations were found (data not shown).
Weight and BMI data for the first and second follow-up appointments after starting medical treatment are shown in Table 4. First follow-up visit heights and weights were available for 51 of 57 hyperthyroid and 27 of 29 hypothyroid subjects. Second follow-up visit heights and weights were available for 45 hyperthyroid and 22 hypothyroid subjects. Hypothyroid patients on the average lost very little weight at first follow-up (0.3 kg, p=0.45 for comparison of weight at baseline and first follow-up visit). Nine patients gained between 0.2 to 3.6 kg; all of these patients had normalized serum thyroxine levels at this follow-up visit. By the second follow-up visit, the average patient had gained 1.5 kg relative to their diagnosis weight (p=0.07 for comparison of weight between baseline and second follow-up visit; p=0.003 for comparison of weight between first and second follow-up visit), but again there was a wide range of weight changes. Because some of this weight gain may be explained by natural or catch-up linear growth, the BMI z score was used to standardize weight for height and age. After the first visit, the mean BMI z score decreased by an average of 0.24 (p=0.004 for comparison of BMI z score between baseline and first follow-up) and only decreased another 0.05 by the second visit (p=0.90 for comparison of BMI z score between first follow-up and second follow-up; p=0.01 for comparison of baseline to second follow-up), suggesting that there was little or no change in BMI after the first follow-up visit. Three hypothyroid patients had BMI z scores >2 at the time of diagnosis; of these patients, the 2 most overweight (BMI z scores of 2.58 and 2.4) gained 3.1 and 2.7 kg by the second follow-up visit, though both had an insignificant decrease in BMI z score (to 2.55 and 2.29 respectively). The third patient lost 2.4 kg during the first 3 months of treatment and another 6.5 kg over the next 6 months (the greatest weight loss seen in this study), and her BMI z score decreased from 2.21 to 1.85.
Table 4.
Weight Status after Initiating Treatment Compared to Diagnosis
| Hyperthyroid Patients | Hypothyroid Patients | |||
|---|---|---|---|---|
| First Follow-up | Second Follow-up | First Follow-up | Second Follow-up | |
| n | 51 | 45 | 27 | 22 |
| Total time on treatment (months) | ||||
| Median (range) | 2 (1–7)a | 6 (4–14) | 3 (1–6)b | 9 (7–20) |
| Cumulative change in weight (kg) | ||||
| Mean ± SD | 3.4 ± 2.8 | 7.1 ± 4.7 | −0.3 ± 1.7 | 1.5 ± 3.6 |
| Median (range) | 2.9 (−2 to 12) | 6.2 (−1.8 to 17.5) | −0.3 (−3.7 to 3.6) | 2.4 (−8.9 to 6.8) |
| Cumulative % change in weight | ||||
| Median (range) | 6 (−2.9 to 24) | 13 (−2.9 to 35.2) | −0.8 (−7.6 to 9.9) | 6.4 (−13.9 to 18.0) |
| Cumulative change in weight z score | ||||
| Median (range) | 0.16 (−0.16 to 0.86) | 0.35 (−0.19 to 1.41) | −0.17 (−0.73 to 0.31) | −0.10 (−1.08 to 0.31) |
| Cumulative change in BMI z score | ||||
| Mean ± SD | 0.32 ± 0.30 | 0.53 ± 0.45 | −0.24 ± 0.41 | −0.29 ± 0.51 |
| Median (range) | 0.26 (−0.17 to 1.17) | 0.39 (−0.20 to 1.56) | −0.16 (−1.28 to 0.66) | −0.16 (−1.58 to 0.58) |
Only 6 visits occurred more than 4 months after starting treatment
Only 2 visits occurred more than 4 months after starting treatment
p < 0.01 for all comparisons between hyperthyroid and hypothyroid patient groups
In contrast, hyperthyroid patients experienced larger effects on weight at their first follow-up visit and continued to gain weight at the second follow-up visit (mean of 7.1 kg relative to initial weight, 13% of initial weight, or a gain of 0.53 in BMI z score; p<0.01 for all comparisons between baseline and first and second follow-up for weight and BMI z scores). The changes in weight for individual hypo- and hyperthyroid patients on treatment are graphically displayed in Figure 1. The differences between weight and BMI changes between the two groups was consistently statistically significant with all p-values <0.01, as shown in Table 4. Additionally, when comparing absolute weight change irrespective of loss or gain, the change in weight was significantly greater for the hyperthyroid patients between the baseline and first follow up visit (p=<0.0001) and between the first and second follow up visits (p<0.0001) compared to the change experienced in the hypothyroid patients. The magnitude of the change in absolute BMI z score was not different between the two groups at the first follow up visit, although the change was in opposite directions; the absolute change in BMI z score from the first to second follow up visits was significantly greater for the hyperthyroid patients (p=0.01).
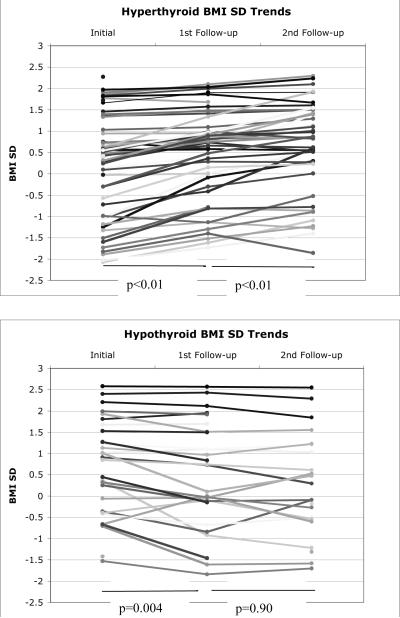
Fig. 1.
BMI z scores for each patient at the initial visit and at the first 2 follow-up visits after initiating treatment. Top: Patients with hyperthyroidism. Bottom: Patients with hypothyroidism.
Not all patients were euthyroid at the time of their follow-up visits as determined by free T4 levels (TSH levels remained suppressed in most hyperthyroid patients even after free T4 normalized). Though in nearly all cases free T4 levels had improved significantly, three of the hypothyroid patients (10%) and 14 of the hyperthyroid patients (27%) had free T4 levels that still remained outside of the normal range. This was either secondary to lack of medication adherence or to initial underdosing of medication. The higher percentage of hyperthyroid patients who did not become euthyroid at the first follow-up visit likely reflects the greater difficulty of controlling hyperthyroidism medically. In a separate analysis, there was a slightly smaller degree of weight gain in the suboptimally treated hyperthyroid patients and a slightly smaller degree of weight loss in the suboptimally treated hypothyroid patients compared to their optimally treated counterparts, but the difference was not statistically significant.
Discussion
Our study examined children with hypo- and hyperthyroidism at the time of diagnosis and then through two follow-up visits. It may seem unusual that we enrolled nearly twice as many hyperthyroid as hypothyroid children in our study, despite a higher frequency of hypothyroidism in the pediatric population. This was likely because many of the hypothyroid patients who were started on treatment have compensated hypothyroidism with normal total or free T4 and variable elevation of TSH; it was decided not to include those subjects, since the symptoms and weight changes we were examining are related to the decrease in free T4 levels rather than the degree of TSH elevation.14,15 In contrast, hyperthyroid patients at diagnosis almost always have several clearly thyroid-related symptoms as well as a very elevated total or free T4.
Even though we studied only moderate-to-severely hypothyroid patients (24 of 29 had TSH >100), they showed on our survey significantly fewer symptoms than our hyperthyroid patients. There were 8 questions on the hyperthyroid survey which received a positive response in over 50%, compared to only one question on the hypothyroid survey (feeling fatigued). It is curious that the same question regarding energy levels received about 65% positive response in both the hyperthyroid and hypothyroid patients, even though one might expect that hyperthyroid patients would report increased energy levels. Fatigue or tiring more quickly has not been reported in other studies of hyperthyroid children.12,13,16,17 Perhaps hyperthyroid patients start the day with more energy but due to their tachycardia and increased metabolic rate, they tire more quickly by the end of the day. Such a hypothesis is supported by the restlessness and hyperactivity described by others.12,17 Alternatively, their difficulty falling asleep at night (which many of our subjects reported, and which has been described in other studies13,16) may contribute to their feeling of tiring more quickly. Two studies have described muscle weakness in hyperthyroid children, which may also contribute to a sense of fatigue.12,17
It is of interest that short stature was not a common finding in our hypothyroid patients, with a mean height z scores of −0.19 and only one patient with a height z score of <−2. However, a few patients had experienced growth deceleration that had not persisted long enough to cause their height z score to fall below −2. This lack of major effects on growth may occur because, in our referral area, primary care physicians are quick to check thyroid tests for a variety of reasons in addition to finding a goiter (i.e. obesity, fatigue, behavior change, hair loss, positive family history of thyroid disease) so that hypothyroid children are unlikely to go undiagnosed for prolonged periods of time. Other papers have reported similar height z scores at the time of presentation of hypothyroidism,7,18 although one study did show a lower height standard deviation in the hypothyroid population compared to euthyroid controls.18
Both hypothyroidism and hyperthyroidism are widely felt to be associated with alterations in weight and BMI, but, as noted earlier, it is difficult to quantify the amount of weight change in children that can be attributed to thyroid dysfunction. Our first approach to this problem was to evaluate weight status, most accurately reflected by BMI z score, at the time of diagnosis of the thyroid problem. One might have predicted that hypothyroid patients would be relatively overweight (strongly positive BMI z score) and that hyperthyroid patients would be relatively underweight (negative BMI z score), reflecting weight gain or loss prior to diagnosis. Surprisingly, the difference in BMI z score between the two groups was minor and not statistically significant, and the range of BMI z scores was wide and highly overlapping between both groups, demonstrating a similar baseline weight status in both populations. A recent retrospective study on the presentation of hypothyroidism in children also found a wide range of BMI z scores at presentation, which did not differ significantly from the BMI z scores of euthyroid patients seen in the same time period.18 Additionally, a study of adults newly diagnosed with thyroid disease did not show any difference in BMI or percent of body fat between the hyperthyroid and hypothyroid groups.6
We then followed the same patients as they started either l-thyroxine therapy or methimazole. One of the problems in analyzing the results is that the patients were not seen on a standard schedule, and so there was a range of time intervals between the initial and first follow-up visits and between the first and second follow-up visits, with the hyperthyroid patients in general seen back more quickly and more often due to the greater need to adjust therapy. We tried to compensate for this by including data from a 3rd follow-up visit if the first visit occurred sooner than 1 month or the second visit occurred sooner than 4 months after starting treatment. It should also be noted that not all patients became euthyroid after the first visit, though this did not affect our overall findings. We evaluated changes in absolute weight, but also in z scores for weight and BMI in order to take into account the influence of age and height on body weight. In order to provide some perspective on the relationship between changes in BMI z scores and weight, we have calculated that a female who is 13.4 years (the average age for our study entry) and of average height and weight for age (160 cm and 50 kg) would gain 5.3 kg and 2.08 BMI units if her BMI z score increased by 0.5. This relationship of weight and BMI z score will be slightly different at different ages.
In the first months of treatment, weight changes were quite variable but on average were modest. Average weight loss in hypothyroid patients was only 0.3 kg, weight z score decreased by 0.17, and BMI z score decreased by only 0.24. Furthermore, by the second follow-up visit, hypothyroid patients showed very minimal and statistically insignificant further decrease in BMI z score and had actually regained some weight, although this weight gain may be explained in part by increased height as evidenced by their stabilization in BMI z score. Thus on average, subjects lost very little weight with treatment of hypothyroidism, which suggests that very little weight gain can be attributable to development of hypothyroidism. This result is consistent with previous reports in adults, and in agreement with the findings of Lomenick, et al, who reported that only 31% of their hypothyroid children lost weight at their first follow-up visit, with a mean loss of 2.3 kg, and that with long-term follow-up there was no significant change in weight and BMI z score compared to baseline.7 At our second follow-up visit, there was on average no further weight loss, suggesting that after the first follow-up visit, pre-disease weight status was largely restored.
In contrast, hyperthyroid patients, half of whom reported weight loss prior to diagnosis, gained an average of 3.4 kg and 0.32 in BMI z score at the first follow-up visit, and continued to gain a statistically significant amount of weight at the second follow-up visit with an average of 7.1 kg of weight gain and an increase of 0.53 in BMI z score compared to diagnosis. This suggests that, unlike hypothyroid patients, weight change is more significant with hyperthyroid patients and correction to baseline is slower. There are several possible explanations for the ongoing weight gain in this group. First, since pre-treatment weight loss with hyperthyroidism was in some cases quite large, correction to baseline weight status may take longer. Second, patients with untreated hyperthyroidism often develop an increased appetite to counter their increased metabolic rate and may not quickly revert to more normal eating patterns. This could explain why some patients gain more than the weight they lost and eventually exceed their pre-disease weights and BMI z scores.
The contrast between the pattern of weight changes in hyperthyroid and hypothyroid patients is perhaps surprising, given that the metabolic changes in these two conditions are polar opposites. Hyperthyroidism causes significant weight loss in some patients due to increased basal metabolic rate, an overall increase in protein degradation and lipolysis, and fat malabsorption.1 However, body composition studies of hyperthyroid adults treated with radioiodine indicate that most of the weight regained during the first year is lean mass, not fat.8 Why, therefore, do we not see equivalent weight gain in untreated severely hypothyroid patients? The reason for this is unclear, but it should be noted that the modest weight gain sometimes seen in severe hypothyroidism is mostly due to water retention.19,20,21 It thus appears that in neither hypothyroidism nor hyperthyroidism are weight changes due to significant changes in the amount of body fat. Animal data also demonstrate differences in metabolic effects of hyper-and hypothyroidism. Hyperthyroid rats demonstrated a larger increase in total energy expenditure and food intake compared to the decrease in these parameters in hypothyroid rats. Although the hypothyroid rats were lower in TEE and food intake than their euthyroid counterparts, the differences were not statistically significant. Furthermore, hyperthyroid rats decreased their rate of inactivity while hypothyroid rats had a non-statistically significantly trend towards increasing inactivity.22 A commentary on this study described the results of hyper- and hypothyroidism as non-mirror images.23
We found that males in the hyperthyroid group exhibited significantly lower BMI z scores at diagnosis and provided a history of weight loss more often than females. A sex difference in weight change in hyperthyroid patients has not been previously reported to our knowledge. No sex differences in weight change were seen in hypothyroid patients. The reasons for such differences are unclear but may reflect a greater increase in caloric intake in females to compensate for the increased metabolic rate, though the percentage of males and females who reported increased appetite was similar. One study has shown lower percent fat mass in males with Graves disease compared to controls but no difference in females.24 There may be gender-specific effects on body composition due to thyroid hormone that have not been elucidated.
Our findings in our hypothyroid patients confirm what others have reported in both children and adults. Based on the minimal weight loss seen with hypothyroidism, even severe hypothyroidism does not appear to cause the degree of overweight that typically generates enough concern to prompt ordering thyroid tests. Although an occasional obese child will prove to have hypothyroidism, this does not appear to occur much more frequently than finding hypothyroidism in normal weight or underweight children, as shown in Figure 1B. The evidence suggests that weight gain alone is not a sufficient reason to order thyroid tests unless accompanied by a goiter or one or more typical symptoms. In a retrospective study of hypothyroid children, a goiter was present in 76% of patients, similar to our findings.18 Weight change is more consistent in hyperthyroid patients, and an unexplained fall in weight should prompt consideration of hyperthyroidism and a search for supporting signs and symptoms. A goiter was found in over 90% of hyperthyroid patients, and the majority had three or more of the classic symptoms noted in Table 1 (particularly heat intolerance, rapid heart rate, declining school performance, and trouble sitting still).
Our study does have several limitations including the retrospective collection of the follow-up data. As mentioned above, the sample size of the groups was uneven due to more stringent inclusion criteria for the hypothyroid subjects. Additionally there was significant loss to follow-up, which further diminished the number of subjects available for analysis; however, given that weight management was not the purpose of the follow-up visits, we think it unlikely that there would be much difference in the weight changes between the included subjects and those lost to follow-up. We also did not achieve euthyroid status on some subjects, but in a subset analysis of those who were euthyroid, the results were very similar. Since thyroid disease affects height as well as weight, BMI may be altered by changes in both parameters. However, weight changes much more rapidly than height, so we expect the change in BMI during the short term follow-up in our study should mostly reflect weight change, not height change. Also, as mentioned above, we only had one patient with significant short stature, suggesting that hypothyroid patients were in most cases referred to endocrinology before the onset of significant growth deceleration. Finally, we have used weight change during treatment with levothyroxine or methimazole as a surrogate marker for weight changes due to hypo- or hyperthyroidism. Such a surrogate measure is necessary because defining the time of disease onset and obtaining a pre-disease weight in a growing child is very difficult. While other studies have also used the same surrogate measures, such methodology does limit the ability to draw absolute quantitative conclusions about weight changes due to thyroid dysfunction.
Future studies could focus on longer-term follow up of these patients. Additionally obtaining data on body composition through air displacement plethysmography or dual energy xray absorptiometry scans could provide interesting information about changes in fat and lean mass during treatment of thyroid disease.
Conclusion
Our data indicate weight loss after starting l-thyroxine treatment is minimal, and assuming treatment returns patients to their baseline, these results suggest that substantial weight gain is not a symptom of hypothyroidism in children. In contrast, hyperthyroid patients do experience weight loss before diagnosis and continue to regain weight many months after starting treatment for their disease. Since metabolically hypothyroidism and hyperthyroidism have opposite effects, it is not clear why the effect of treatment of hyperthyroidism on weight is more significant and prolonged than the effect of treatment of hypothyroidism, but based on other studies, in neither case are weight changes primarily due to changes in body fat.
Acknowledgments
Financial Disclosures: Dr. Crocker is supported by the Intramural Research Program of the NIH, NICHD.
Footnotes
Conflicts of Interest: None
References
- 1.Silva JE. The thermogenic effect of thyroid hormone and its clinical implications. Annals of Internal Medicine. 2003;139:205–213. [PubMed] [Google Scholar]
- 2.Reinehr T, Hinney A, de Sousa G, Austrup F, Hebebrand J, Andler W. Definable somatic disorders in overweight children and adolescents. The Journal of Pediatrics. 2007;150:618–622. doi: 10.1016/j.jpeds.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 3.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120:S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 4.Glass AR, Kushner J. Obesity, nutrition, and the thyroid. Endocrinologist. 1996;6:392–403. [Google Scholar]
- 5.Pears J, Jung RT, Gunn A. Long-term weight changes in treated hyperthyroid and hypothyroid patients. Scottish Medical Journal. 1990;35:180–182. doi: 10.1177/003693309003500609. [DOI] [PubMed] [Google Scholar]
- 6.Brunová J, Kasalický P, Lánská V. The assessment of body composition using DEXA in patients with thyroid dysfunction. Časopis lékařů českých. 2007;146:497–502. [PubMed] [Google Scholar]
- 7.Lomenick JP, El-Sayyid M, Smith WJ. Effect of levo-thyroxine treatment on weight and body mass index in children with acquired hypothyroidism. The Journal of Pediatrics. 2008;152:96–100. doi: 10.1016/j.jpeds.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.de la Rosa RE, Hennessey JV, Tucci JR. A longitudinal study of changes in body mass index and total body composition after radioiodine treatment for thyrotoxicosis. Thyroid: Official Journal of the American Thyroid Association. 1997;7:401–405. doi: 10.1089/thy.1997.7.401. [DOI] [PubMed] [Google Scholar]
- 9.Dale J, Daykin J, Holder R, Sheppard MC, Franklyn JA. Weight gain following treatment of hyperthyroidism. Clinical Endocrinology. 2001;55:233–239. doi: 10.1046/j.1365-2265.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics. Series 11, Data from the national health survey. 2002 May;246:1–190. [PubMed] [Google Scholar]
- 11.Abbassi V, Rigterink E, Cancellieri RP. Clinical recognition of juvenile hypothyroidism in the early stage. Clinical Pediatrics. 1980;19:782–786. doi: 10.1177/000992288001901201. [DOI] [PubMed] [Google Scholar]
- 12.Klein I, Trzepacz PT, Roberts M, Levey GS. Symptom rating scale for assessing hyperthyroidism. Archives of Internal Medicine. 1998;148:387–390. [PubMed] [Google Scholar]
- 13.Sidibé AT, Dembélé M, Diarra AS, Bocoum AI, Mousseni E, Ag Aboubacrine S, Traoré HA, Ag Rhaly A. Hyperthyroidism in children. Experience in internal medicine in Mali. Annales d'endocrinologie. 2007;68:177–180. doi: 10.1016/j.ando.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Evered DC, Ormston BJ, Smith PA, Hall R, Bird T. Grades of hypothyroidism. British Medical Journal. 1973;1:657–662. doi: 10.1136/bmj.1.5854.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansen K, Hansen JM, Skovsted L. Myxoedema and thyrotoxicosis: relations between clinical state and concentrations of thyroxine and triiodothyronine in blood. Acta Medica Scandinavica. 1978;204:361–364. doi: 10.1111/j.0954-6820.1978.tb08455.x. [DOI] [PubMed] [Google Scholar]
- 16.Bossowski AT, Reddy V, Perry LA, Johnston LB, Banerjee K, Blair JC, Savage MO. Clinical and endocrine features and long-term outcome of Graves' disease in early childhood. Journal of Endocrinological Investigation. 2007;30:388–392. doi: 10.1007/BF03346315. [DOI] [PubMed] [Google Scholar]
- 17.Dotsch J, Siebler T, Hauffa BP, Doeker B, Andler W, Bettendorf M, Heinrich U, Gohlke B, Albers N, Willgerodt H, Kiess W. Diagnosis and management of juvenile hyperthyroidism in Germany: a retrospective multicenter study. Journal of Pediatric Endocrinology & Metabolism: JPEM. 2000;13:879–885. doi: 10.1515/jpem.2000.13.7.879. [DOI] [PubMed] [Google Scholar]
- 18.De Vries L, Bulvik S, Phillip M. Chronic autoimmune thyroiditis in children and adolescents: at presentation and during long-term follow-up. Archives of Disease in Childhood. 2009;94:33–37. doi: 10.1136/adc.2007.134841. [DOI] [PubMed] [Google Scholar]
- 19.Kyle LH, Ball MF, Doolan PD. Effect of thyroid hormone on body composition in myxedema and obesity. The New England Journal of Medicine. 1966;275:12–17. doi: 10.1056/NEJM196607072750103. [DOI] [PubMed] [Google Scholar]
- 20.Parving H, Hansen JM, Nielsen SL, Rossing N, Munck O, Lassen NA. Mechanisms of edema formation in myxedema – increased protein extravasation and relatively slow lymphatic drainage. The New England Journal of Medicine. 1979;301:460–465. doi: 10.1056/NEJM197908303010902. [DOI] [PubMed] [Google Scholar]
- 21.Waters AK. Body water compartments and exchangeable body sodium in hypothyroidism. The Journal of Nuclear Medicine and Allied Sciences. 1978;22:43–45. [PubMed] [Google Scholar]
- 22.Klieverik LP, Coomans CP, Endert E, Sauerwein HP, Havekes LM, Voshol PJ, Rensen PCN, Romijn JA, Kalsbeek A, Fliers E. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology. 2009;150:5639–5648. doi: 10.1210/en.2009-0297. [DOI] [PubMed] [Google Scholar]
- 23.Silva JE. Fat and energy economy in hypo- and hyperthyroidism are not the mirror image of one another. Endocrinology. 2010;151:4–6. doi: 10.1210/en.2009-1186. [DOI] [PubMed] [Google Scholar]
- 24.Miyakawa M, Tsushima T, Murakami H, Isozaki O, Takano K. Serum leptin levels and bioelectrical impedance assessment of body composition in patients with Graves' disease and hypothyroidism. Endocrine Journal. 1999;46:665–73. doi: 10.1507/endocrj.46.665. [DOI] [PubMed] [Google Scholar]