Abstract
Autophagy is an evolutionarily conserved catabolic pathway of lysosome-dependent turnover of damaged proteins and organelles. When nutrients are in short supply, bulk removal of cytoplasmic components by autophagy replenishes depleted energy stores, a process critical for maintaining cellular homeostasis. However, prolonged activation of autophagic pathways can result in cell death. Longstanding evidence has linked the stimulation of lysosomal pathways to pathologic cardiac remodeling and a number of cardiac diseases, including heart failure and ischemia. Only recently, however, has work begun to parse cyto-protective autophagy from autophagy that contributes to disease pathogenesis. Current thinking suggests that the effects of autophagy exist on a continuum, with the eliciting triggers, the duration and amplitude of autophagic flux, and possibly the targeted intra cellular cargo as critical determinants of the end result. Deciphering how autophagy participates in basal homeostasis of the heart, in aging, and in disease pathogenesis may uncover novel insights with clinical relevance in the treatment of heart disease.
Introduction
Heart disease is the leading cause of death in the industrialized world [1], a powerful statistic that is expected to extend soon to nations around the world. For example, 5 million Americans currently suffer from chronic heart failure, a syndrome with mortality of approximately 50% at 5 years. From a global perspective, heart disease is likely the most important noninfectious health problem ever to afflict humankind.
In many instances, heart disease of diverse sorts culminates in heart failure, a syndrome wherein the heart is unable to meet the metabolic demands of the body. In its pathogenesis, heart failure is the final result of many different insults to the myocardium, each eliciting an array of responses, some adaptive and others maladaptive [2]. The resulting cardiac growth and remodeling is sometimes beneficial, facilitating the response to exercise, postnatal development, or pregnancy. In other instances, as in the setting of hypertension or myocardial infarction, cardiac remodeling is maladaptive, predisposing to arrhythmia and contractile dysfunction. In yet other instances, such as prolonged bedrest or weightlessness, the heart responds with significant decreases in mass. Among the mechanisms activated by cardiac stress and contributing to these remodeling events, recent work has pointed to cardiomyocyte autophagy as a major element.
The Process of Autophagy
The term autophagy was coined by Nobel laureate Christian de Duve to describe a process whereby the cell degrades, or “eats” (phagy), part of itself (auto). In this process, cytoplasmic contents, such as organelles or ubiquitinated protein aggregates, are sequestered in specialized vacuoles termed autophagosomes and subsequently delivered to the lysosome for degradation [3,4,5••] (Fig. 1). Autophagy is ubiquitous in eukaryotic cells, being conserved from yeast to human. Activation of autophagy serves as a means of providing nutrients to sustain vital cellular functions during conditions of starvation. Autophagy can also serve to eliminate misfolded and dysfunctional proteins, and it is the only means whereby a cell can rid itself of obsolete or defective organelles. Additionally, autophagy participates in the cellular response to invading microorganisms and in antigen presentation during the immune response.
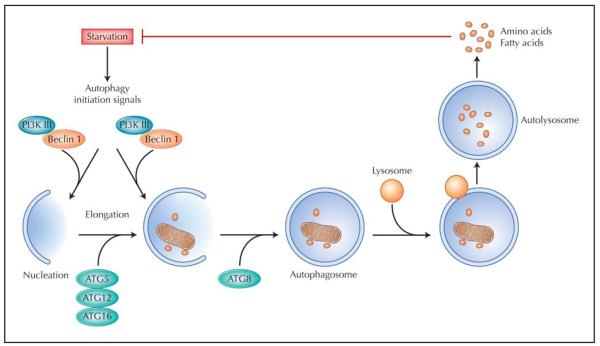
Figure 1.
The process of autophagy. A variety of stresses, such as starvation, trigger the autophagy process through the Class III PI3K/Beclin 1 complex. This complex initiates formation of isolated double membranes (the membrane nucleation process) and subsequent membrane elongation. The membrane then fuses on itself, forming the distinctive double-membrane autophagosome. Autophagosomes ultimately fuse with lysosomes, and their cargo is degraded, providing fuel and elemental building blocks to preserve vital cellular functions. ATG—autophagy-related genes; PI3K— phosphatidylinositide-3-kinase.
A number of triggers are capable of eliciting an autophagic response. These include nutrient deprivation, as discussed above, and growth factor withdrawal. These stimuli activate class III phosphatidylinositide-3-kinase (PI3K), a protein that forms a complex with the rate-limiting autophagy protein, Beclin 1. Activation of this pathway, in turn, initiates autophagosome formation by transporting other autophagy proteins to the pre-autophagosomal membranes to form an isolation membrane (phagophore), a process called nucleation. With the participation of other autophagy-related gene (ATG) products, such as ATG5, ATG12, and ATG16, the phagophore expands to engulf cytoplasmic contents, and the edges of the phagophore fuse. The resulting double membrane structure (autophagosome) ultimately fuses with a lysosome to form an autolysosome, where the sequestered material is digested and then released in the form of elemental building blocks (amino acids, fatty acids) to sustain the cell under conditions of stress.
There are three major types of autophagy: macroautophagy, microautophagy, and chaperone-mediated autophagy. Of these, macroautophagy is the most widely studied and best understood. This article focuses exclusively on macroautophagy (hereafter termed simply autophagy) and summarizes recent findings, which have unveiled important insights that could ultimately lead to novel treatment strategies for diverse heart diseases.
Autophagy and Cell Death
Morphologic classification categorizes cell death into four different types [6]:
Type 1 cell death, also known as apoptosis, is characterized by nuclear condensation and fragmentation.
Type 2 or autophagic cell death is marked by massive accumulation of autophagosomes.
Type 3 cell death (necrosis) is a global event within the cell, in which the plasma membrane and the cytoplasm are extensively dismantled.
Type 4 cell death is termed mitotic catastrophe and is manifested by micronucleation and multinucleation during or shortly after a failed mitosis.
The labeling of type 2 cell death as autophagic connotes a detrimental role for the autophagic process, but the differentiation between autophagy-induced cell death and death with autophagic features is debated [7]. One reason this distinction has defied easy resolution is that autophagy is a prominent adaptive response to a variety of cellular stresses [5••]. For example, autophagy enhances clearance of toxic Huntingtin protein fragments within neurons and promotes disposal of mutant α-1-antitrypsin in hepatocytes (the accumulation of which causes liver inflammation and carcinogenesis) [8,9]. Similar beneficial roles of autophagy are evident in the immune system, where autophagy functions as a defense mechanism and delivers cytosolic proteins for major histocompatibility complex (MHC) II presentation [10,11]. There is also strong evidence that autophagy functions as an anti-aging mechanism in lower organisms such as Caenorhabditis elegans, as well as in mammals [12-15]. But whereas a large body of evidence demonstrates convincingly that autophagy may be adaptive, sustained autophagy can be maladaptive, leading to cell death [16••]. This duality in function is especially evident in hearts under stress, as discussed below.
Autophagy in Normal Heart Function and During Aging
The myocardium is comprised of long-lived, postmitotic myocytes; as a result, the heart has very limited, though measurable, regenerative capacity. It is thus tempting to speculate that autophagy is particularly important in cardiomyocytes in order to maintain myocyte function and viability, as it provides a means for intracellular self-renewal, energy repletion, and substrate recycling through the degradation of dysfunctional or misfolded proteins and aged or damaged organelles. Not surprisingly, disruption of this housekeeping role of the autophagy–lysosome degradation pathway can lead to adverse effects in the heart. For example, lysosomal storage diseases are characterized by defects in the autophagy-lysosome pathway. Among them, Danon disease results from a deficiency of the lysosome-associated membrane protein-2 (LAMP-2) that leads to inability to digest cargo delivered to the lysosome. Affected patients develop cardiomyopathy, skeletal myopathy, and variable cognitive delays [17,18]. Intracellular accumulation of macromolecules occurs prominently in these conditions; at the same time, abnormal mitochondria also accumulate, likely contributing to the maladaptive phenotype [19,20].
Another example of the importance of basal autophagy in the heart is in desmin-related cardiomyopathies (DRCMs), a group of disorders that arise from mutations in proteins such as desmin, myotilin, and dystrophin, as well as αB-crystallin, a protein chaperone that facilitates proper processing of desmin [21]. Mutation of the gene coding for αB-crystallin produces a phenotype that includes protein aggregation, myofibrillar disarray, contractile dysfunction, and sudden cardiac death. Work from our group demonstrated a robust autophagic response in DRCM myocytes [22]. Further, we found that blunting autophagy in mice harboring an αB-crystallin mutation dramatically hastened the progression of heart failure and accelerated mortality [22].
Autophagy also plays a role in cardiac aging. In the heart, aging is associated with the development of multiple disorders, a process that is clearly multifactorial. That being said, intracellular accumulation of damaged macromolecules and mitochondria is a consistent finding [23]. Mitochondria, a major target of autophagy, account for up to 40% of the myocyte volume and provide 90% of the high amount of energy consumed by the myocardium. Besides providing adenosine triphosphate, mitochondria play key roles in the generation and neutralization of reactive oxygen species and in the regulation of important cellular signaling functions including calcium handling and cellular apoptosis. In cardiac myocytes, the production of reactive oxygen species from mitochondria increases with age and leads, in turn, to more mitochondrial damage [24]. Indeed, mitochondria of aged cells are generally enlarged, with evidence of structural damage such as loss of cristae [23]. Elimination of these damaged and dysfunctional mitochondria through autophagy is critical to ensure proper cell function. However, autophagy may be unable to keep up with these events during aging, resulting in progressive accumulation of deleterious changes within cardiomyocytes, contributing to gradual functional declines and increased susceptibility to disease. Interestingly, knock-in mice expressing defective mitochondrial DNA polymerase accumulate mutations in their mitochondrial DNA and exhibit phenotypes of premature aging, including premature weight loss, alopecia, kyphosis, reduced fertility and lifespan, osteoporosis, anemia, and enlarged hearts [25].
A number of reports directly link autophagy to aging. For example, in Drosophila, overexpression of the autophagy gene Atg8 in the nervous system extends lifespan by 50% [26]. Furthermore, genetic studies in both worms and flies show that autophagy-related genes are required for the powerful lifespan-extending effects of caloric restriction [27,28]. In mammals, caloric restriction elicits prominent features of upregulated autophagy [29]. Thus, some evidence points to a relationship between the activation of autophagy and the rate at which tissues age.
Autophagy in the Heart Under Stress
Whereas autophagy is an important process in the regulation of basal cellular homeostasis, it is robustly upregulated in a variety of disease states. In the heart, increased autophagic activity in cardiomyocytes has been described in the setting of multiple forms of cardiovascular stress, including starvation, chronic ischemia, ischemia/reperfusion injury, and pressure overload. The function of autophagy in these conditions is poorly understood: Does it serve a pro-survival function or contribute to disease pathogenesis, cell death, or both? Current research supports the notion that autophagy can have both beneficial and detrimental roles in the myocardium depending on the level of autophagic activation and the context in which it is induced [30]. For example, upregulation of autophagy in the setting of mild ischemia provides a protective effect that promotes functional recovery of these cardiac myocytes [31], presumably by recycling cellular constituents to generate the free amino acids and fatty acids needed to maintain energy production, by removing damaged organelles, and by preventing accumulation of protein aggregates. However, there is also strong evidence that load-induced autophagy contributes to myocyte death and cardiac dysfunction [16••]. At present, the key elements that differentiate “good autophagy” from “bad autophagy” are not known, but possibilities include the specific triggering events, the intracellular substrates being degraded, and/or the degree and duration of autophagic activation. Efforts to tease out these mechanisms are complicated by the inability to distinguish between cell death mediated by autophagy and cell death following autophagy that failed in an effort to prevent cellular demise.
Autophagy in load-induced cardiac remodeling
The heart is an organ of remarkable plasticity [2]; cardiomyocytes are capable of robust increases and decreases in size in response to ever-changing environmental influences. In the setting of hemodynamic stress, such as that which occurs in hypertension or following myocardial infarction, the heart undergoes a compensatory hypertrophic growth response. Left unchecked, this hypertrophic response culminates in ventricular dilatation, diminished contractile performance, and a clinical syndrome of heart failure. For some years, autophagy has been implicated in the pathophysiology of heart failure of diverse etiology. In a model of surgically induced pressure-overload cardiac hypertrophy in vivo, our group has reported that autophagic activity increases rapidly after thoracic aortic constriction, peaks at 72 hours, and is maintained at elevated levels for at least 3 to 4 weeks [16••]. We also observed that the degree of autophagic activity correlates with the magnitude of hypertrophic growth and with the rate of transition to heart failure. Consistent with this observation, cardiomyocytes engineered to overexpress Beclin 1 manifested an amplified autophagic response to biomechanical stress, coupled with heightened pathologic remodeling [16••]. In contrast, heterozygous disruption of the gene coding for Beclin 1 led to a blunted autophagic response to a variety of stressors, and load-induced remodeling was attenuated [16••]. Together, these data are consistent with a large literature in rodent models demonstrating that load-induced hypertrophy is pathologic and is not required to maintain cardiac function [32,33]. It is thus conceivable that under conditions of elevated afterload, autophagy is maladaptive partly as a result of its facilitating greater and more sustained hypertrophic growth. By contrast, completely abolishing autophagy (both basal and disease-activated) by inactivation of the Atg5 gene triggers rapid deterioration of the myocardium [34••]. In an effort to reconcile these observations, we have proposed a model in which basal levels of autophagy in the heart serve critical life-sustaining functions, whereas load-induced increases in autophagy are detrimental [30].
Autophagy in heart failure
Cardiac hypertrophy is a major risk factor for heart failure [35,36] and very often precedes its development [37]. As a result, it is not surprising that cardiac hypertrophy is an independent risk factor for cardiovascular mortality [38]. Numerous structural changes occur during the progression of hypertrophy and failure, including myocyte growth, deposition of interstitial fibrosis, cellular ischemia, and cell death, all of which contribute ultimately to cardiac dysfunction and failure. Ventricular decompensation is ultimately associated with thinning of the ventricular walls via a combination of proteolysis and death of myocytes.
Several theories exist regarding the mechanisms underlying this hypertrophy-to-failure transition. Among these are progressive activation of neurohumoral systems, a blood supply that is inadequate to meet the demands of the thickened ventricular wall, functional changes in the contractile molecular machinery, remodeling of the myocardial interstitium with deposition of excess extracellular matrix, elevated levels of reactive oxygen species, chronic infl ammation, and cardiomyocyte apoptosis [39]. However, the exact mechanisms remain poorly understood. That being said, recent studies have implicated autophagy in the progression of heart failure [30,40]. Anecdotal evidence in support of this notion has also been uncovered in cases where patients with cardiomyopathy of unknown cause manifest characteristic features of autophagy-induced cell death, including marked accumulation of autophagosomes in cardiac myocytes [41].
In a model of dilated cardiomyopathy in which transgenic mice express the human diphtheria toxin (DT) receptor in cardiac myocytes, injection of DT caused myocyte death and subsequent heart failure through the autophagic pathway [42]. Further, it has been shown that elevated autophagic activity is apparent in failing myocardium associated with both valvular and ischemic heart disease. [43,44] A study by Kostin et al. [44] reported that in human idiopathic dilated cardiomyopathy, the prevalence of autophagic, apoptotic, and necrotic cells was 0.08%, 0.002%, and 0.06%, respectively. However, it is worth emphasizing again that these findings do not distinguish between different causes of cell death, cellular events serving simply as fi nal common pathways (but which were elicited by diverse untoward triggering events), or failed pro-survival mechanisms.
Autophagy in ischemic heart disease
Activation of lysosomal pathways was reported more than 30 years ago in neonatal hearts exposed in vitro to hypoxia and glucose deprivation followed by reoxygenation with glucose restoration [45]. Since then, many studies have reported increased autophagic activity in the settings of ischemia and ischemia/reperfusion [31,46-48]. For example, a study of Langendorff-perfused rabbit hearts subjected to ischemia and subsequent reperfusion revealed upregulation of autophagy when the ischemic time was limited to 40 minutes [46]. With prolonged ischemia (60 minutes), enlarged lysosomes were detected during reperfusion, suggesting impairment of the autophagy-lysosome pathway. In fact, these investigators reported a correlation between increased autophagy and the functional recovery and salvage of the myocardium after ischemia/reperfusion injury [46]. Conversely, extended ischemia that led to an impairment of the autophagy-lysosome pathway correlated with irreversible damage and contractile dysfunction. More recently, enhanced autophagy has been observed in chronically ischemic human and swine myocardium, as well as in rodent models of ischemia and reperfusion [31]. Interestingly, areas of the heart with increased autophagy displayed fewer apoptotic cells, suggesting that the induction of autophagy may suppress apoptotic cell death [31].
Whereas it is clear that autophagy is upregulated in both ischemia and ischemia/reperfusion, it is less apparent whether that upregulation is cardioprotective. As noted above, considerable evidence supports the notion that autophagy protects the heart during ischemic stress. However, most of these studies were performed in vitro, and the ischemic stress was relatively mild. In contrast, other studies have demonstrated that inhibition of ischemia/reperfusion–induced autophagy by either 3-methyladenine (3-MA) or by genetic knockdown of Beclin 1 leads to enhanced cardiac myocyte survival in vitro [49]. Further, a study in vivo suggested that autophagy is protective in ischemia but detrimental in reperfusion [50••]. In this model, autophagy is beneficial during the “starved” state of ischemia. By contrast, reperfusion provokes robust mitochondrial dysfunction, oxidative stress, and protein misfolding, each of which is capable of eliciting profound increases in autophagy. Further, the energy required for the autophagic process is suddenly replenished when the match between oxygen supply and demand is restored, further amplifying the autophagic response, possibly into zones where autophagy is pathologic. Consistent with this model, markedly elevated autophagic activity has been observed during reperfusion [50••]. Further, blunting autophagy during reperfusion by Beclin 1 haploinsufficiency was associated with significant reductions in infarct size and myocyte apoptosis [50••]. Similar results were reported in a model of ischemic brain injury, where knocking down expression of the autophagy gene Atg7 was protective [51]. Together, these findings highlight the complex—and possibly opposite—roles that autophagy is likely to play in tissue ischemia and ischemia/reperfusion injury.
Conclusions
Autophagy is a regulated, highly conserved, and functionally complex process that participates in basal cellular homeostasis, aging, and virtually all forms of heart disease. In some contexts, autophagy is cardioprotective; in others, it participates in (and contributes to) disease pathogenesis. Emerging evidence suggests that autophagy is pathologic when activated to excess, but abrogation of all autophagic activity in the cell is deleterious. Clearly, much work remains to be done to dissect the continuum over which autophagy functions. On one hand, functionally inadequate autophagy leads to accumulation of toxic proteins, protein aggregates, and defective organelles, compromising cellular function and eventually leading to cell death. On the other hand, excessive autophagy, such as that occurring in the setting of pressure overload, is maladaptive and may possibly trigger cell death. Between the extremes when too little or too much autophagy is pathologic, appropriate levels of autophagic flux promote cellular homeostasis, stress responsiveness, and cell survival. Careful dissection of this fascinating biology will be required to envision gainful, therapeutic manipulation of autophagy in patients with heart disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL-075173; HL-080144; HL-090842), American Heart Association (0640084N), and the American Heart Association–Jon Holden DeHaan Foundation.
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 3.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.••.Levine B, Kroemer G. Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell Death Differ. 2009;16:1–2. doi: 10.1038/cdd.2008.139. This review summarizes the major recent advances in autophagy in the contexts of disease and aging.
- 6.Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perlmutter DH. Autophagic disposal of the aggregation-prone protein that causes liver inflammation and carcinogenesis in alpha-1-antitrypsin deficiency. Cell Death Differ. 2009;16:39–45. doi: 10.1038/cdd.2008.103. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez MG, Master SS, Singh SB, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 11.Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hars ES, Qi H, Ryazanov AG, et al. Autophagy regulates ageing in C. elegans. Autophagy. 2007;3:93–95. doi: 10.4161/auto.3636. [DOI] [PubMed] [Google Scholar]
- 13.Alvers AL, Wood MS, Hu D, et al. Autophagy is required for extension of yeast chronological life span by rapamycin. Autophagy. 2009;5:847–849. doi: 10.4161/auto.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvers AL, Fishwick LK, Wood MS, et al. Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae. Aging Cell. 2009;8:353–369. doi: 10.1111/j.1474-9726.2009.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamerdinger M, Hajieva P, Kaya AM, et al. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28:889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.••.Zhu H, Tannous P, Johnstone JL, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. This paper demonstrates that cardiomyocyte autophagy is activated by pathologic hemodynamic stress and that this autophagic response is maladaptive, contributing to disease progression.
- 17.Maron BJ, Roberts WC, Arad M, et al. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. JAMA. 2009;301:1253–1259. doi: 10.1001/jama.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruivo R, Anne C, Sagne C, Gasnier B. Molecular and cellular basis of lysosomal transmembrane protein dysfunction. Biochim Biophys Acta. 2009;1793:636–649. doi: 10.1016/j.bbamcr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Verity MA. Infantile Pompe’s disease, lipid storage, and partial carnitine deficiency. Muscle Nerve. 1991;14:435–440. doi: 10.1002/mus.880140509. [DOI] [PubMed] [Google Scholar]
- 20.Yu W, Gong JS, Ko M, et al. Altered cholesterol metabolism in Niemann-Pick type C1 mouse brains affects mitochondrial function. J Biol Chem. 2005;280:11731–11739. doi: 10.1074/jbc.M412898200. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Osinska H, Klevitsky R, et al. Expression of R120G-alphaB-crystallin causes aberrant desmin and alphaB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- 22.Tannous P, Zhu H, Johnstone JL, et al. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2008;105:9745–9750. doi: 10.1073/pnas.0706802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terman A, Dalen H, Eaton JW, et al. Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Exp Gerontol. 2003;38:863–876. doi: 10.1016/s0531-5565(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 24.Juhaszova M, Rabuel C, Zorov DB, et al. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 26.Simonsen A, Cumming RC, Brech A, et al. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 27.Hansen M, Chandra A, Mitic LL, et al. A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toth ML, Sigmond T, Borsos E, et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–338. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- 29.Wohlgemuth SE, Julian D, Akin DE, et al. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 30.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circ Res. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan L, Vatner DE, Kim SJ, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill JA, Karimi M, Kutschke W, et al. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 33.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 34.••.Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. This paper demonstrates that complete elimination of cardiomyocyte autophagy is detrimental to cardiac homeostasis under basal conditions and alters the myocyte response to pressure overload stress.
- 35.Mathew J, Sleight P, Lonn E, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 36.Rame JE, Dries DL. Heart failure and cardiac hypertrophy. Curr Treat Options Cardiovasc Med. 2007;9:289–301. doi: 10.1007/s11936-007-0024-3. [DOI] [PubMed] [Google Scholar]
- 37.Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am J Physiol Heart Circ Physiol. 2005;289:H8–H16. doi: 10.1152/ajpheart.01303.2004. [DOI] [PubMed] [Google Scholar]
- 38.Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 39.Hilfiker-Kleiner D, Landmesser U, Drexler H. Molecular mechanisms in heart failure: focus on cardiac hypertrophy, inflammation, angiogenesis, and apoptosis. J Am Coll Cardiol. 2006;48:56–66. [Google Scholar]
- 40.Zhu H, Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Methods Enzymol. 2009;453:343–363. doi: 10.1016/S0076-6879(08)04017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugimoto S. A novel vacuolar myopathy with dilated cardiomyopathy. Autophagy. 2007;3:638–639. doi: 10.4161/auto.4931. [DOI] [PubMed] [Google Scholar]
- 42.Akazawa H, Komazaki S, Shimomura H, et al. Diphtheria toxin-induced autophagic cardiomyocyte death plays a pathogenic role in mouse model of heart failure. J Biol Chem. 2004;279:41095–41103. doi: 10.1074/jbc.M313084200. [DOI] [PubMed] [Google Scholar]
- 43.Shimomura H, Terasaki F, Hayashi T, et al. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 44.Kostin S, Pool L, Elsasser A, et al. Myocytes die by multi ple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 45.Sybers HD, Ingwall J, DeLuca M. Autophagy in cardiac myocytes. Recent Adv Stud Cardiac Struct Metab. 1976;12:453–463. [PubMed] [Google Scholar]
- 46.Decker RS, Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol. 1980;98:425–444. [PMC free article] [PubMed] [Google Scholar]
- 47.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 48.Hamacher-Brady A, Brady NR, Logue SE, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 49.Valentim L, Laurence KM, Townsend PA, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 50.••.Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. This study provides strong evidence for differing effects of autophagy in different stages of ischemic heart disease.
- 51.Koike M, Shibata M, Tadakoshi M, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]