Abstract
Objectives
Type 2 diabetes is accepted as a cause of heart failure (HF), but direct cause-effect evidence independent of incident myocardial infarction, hypertension and other coexisting risk factors is less well studied. We tested the hypothesis that diabetes predisposes to HF independently of hypertension and intercurrent myocardial infarction (MI).
Methods
We evaluated 12-year incident HF in 2740 participants (1781 women) without prevalent cardiovascular (CV) or severe kidney disease, at the time of the 1st exam of the Strong Heart Study cohort. Intercurrent myocardial infarction was censored as a competing risk event.
Results
Diabetes was present in 1206 individuals (44%), and impaired fasting glucose (IFG) in 391 (14%). Diabetic participants more frequently had hypertension and central obesity (both p<0.0001). Incident HF was ascertained in 64 participants with normal fasting glucose (NFG, 6%), 26 (7%) with IFG and 201 with diabetes (17%, Hazard ratio [HR]= 4.04 vs. NFG; p<0.0001). In Cox analysis adjusting for age, sex, obesity, central fat distribution, hypertension, antihypertensive medications, prevalent atrial fibrillation, GFR, urinary albumin/creatinine ratio, plasma cholesterol, Hb1Ac, smoking habit, alcohol use, educational level and physical activity, diabetes was associated with a 2-fold greater risk of incident HF than NFG (HR=2.45, p<0.0001). Diabetes maintained 1.5-fold greater risk of HF than NFG (p<0.03) even when intercurrent myocardial infarction (n=221) was censored as a competing risk event, similar to the adjusted HR for HF in hypertension.
Conclusion
Type 2 diabetes is a potent, independent risk factor for HF. Risk of HF in diabetic subjects cannot be fully explained by incident MI and coexisting CV risk factors. Mechanisms directly related to diabetes and impairing cardiac function should be studied and identified.
Keywords: myocardial infarction, cardiovascular risk, risk factors, hypertension, obesity
INTRODUCTION
Among the manifestations of cardiovascular (CV) disease, heart failure (HF) has particular importance because of its increasing incidence, related to both reduced mortality from myocardial infarction and aging of the population. While incident HF is partly attributable to recognized ischemic heart disease, the remaining incidence of HF not directly associated with myocardial infarction is thought to be due to direct effect of major risk factors, such as arterial hypertension, obesity, and type 2 diabetes on myocardial structure and function (1). These risk factors are also highly prevalent in elderly populations without recognized myocardial infarction .
The direct links of arterial hypertension and obesity with HF are well established. However, the influence of type 2 diabetes on incident HF in the absence of preceding myocardial infarction is not yet fully established in populations. While there is strong evidence that type 2 diabetes increases risk of coronary heart disease, especially when associated with other metabolic abnormalities (2), there is little direct evidence that type 2 diabetes can directly cause HF without the mediation of overt coronary heart disease. The American Heart Association web page (http://www.americanheart.org/presenter.jhtml?identifier=324) on HF informs that “…people with diabetes tend to develop hypertension and atherosclerosis from elevated lipid levels in the blood - both of which have been linked to heart failure”. The question of whether diabetes can directly be linked to HF independently of hypertension and other CV risk factors still needs direct demonstration.
Accordingly, we analyzed data from Strong Heart Study cohort members free of prevalent coronary heart disease, to assess whether type 2 diabetes predicts incident HF independently of prevalent and incident myocardial infarction, hypertension and other recognized risk factors.
METHODS
Population
The Strong Heart Study (SHS) is a population-based cohort study of CV risk factors and disease in 4,549 American Indians from 3 communities in Arizona, 7 in Southwestern Oklahoma and 3 in South and North Dakota, extensively described previously and representative of the source populations (3;4). Individuals aged 45 to 74were seen at the first examination (phase 1), conducted between July 1989 and January 1992. Participation rates of all age-eligible tribal members were 72% in the Arizona center,62% in the Oklahoma center, and 55% in the South/North Dakota center. Nonparticipants were similar to participants in age and self-reported frequency of type 2 diabetes but were more likely to be men, smokers and older. No echocardiograms were performed at the time of phase 1 of the study. Duration of diabetes was assessed by questions during collection of clinical history.
For the present analysis, individuals were excluded without classified type 2 diabetes status (n=1) or with prevalent CV diseases including HF [n=179], stroke [n=35], myocardial infarction [n=100] and/or coronary heart disease diagnosed by ECG, coronary angiography, combination of typical symptoms with positive treadmill or imaging stress tests, or need of revascularization procedures [n=151]). All prevalent and incident CV events were confirmed by the Strong Heart Study Mortality and Morbidity Committees, using specified criteria for causes of fatal and nonfatal CV events (5).
In particular, HF was diagnosed when two major, or one major and two minor criteria were present concurrently in the absence of a condition such as end-stage renal failure leading to massive fluid overload. Major criteria were: paroxysmal nocturnal dyspnea or orthopnea, neck vein distention, rales, cardiomegaly, acute pulmonary edema, S3 gallop, venous pressure >16 cm water and hepatojugular reflux. Minor criteria were: ankle edema, night cough, dyspnea on exertion, hepatomegaly, pleural effusion, vital capacity <2/3 of predicted or heart rate ≥120 beats/minute. Weight loss ≥4.5 kg in 5 days in response to treatment could serve as a major or minor criterion.
Because of potential links among type 2 diabetes, severe chronic kidney disease and HF, we also excluded all participants with either serum creatinine >2.5 mg/dL or glomerular filtration rate (GFR) <30 mL/min/1.73 m2. Thus the cohort for the present analysis comprised 2,740 members, including diabetic and non-diabetic adults without prevalent cardiovascular disease or severe chronic kidney disease.
Laboratory tests and definitions of type 2 diabetes
Fasting plasma glucose and lipid profile were measured by standard methods. Glomerular filtration rate (GFR) was estimated by the simplified MDRD formula. Type 2 diabetes (fasting glucose ≥7 mmol/L or antidiabetic treatment) and impaired fasting glucose (6–6.9 mmol/L) were diagnosed by 1997 American Diabetes Association recommendations. Old partition values for impaired fasting glucose were preferred to the recommended new ones (i.e. 5.5 mmol/L) to maximize the chance of identification of prognostic effects. Obesity was classified by 1998 NIH guidelines, as BMI ≥30 kg/m2. Central fat distribution was based on sex-specific waist circumference cutoff points proposed by NCEP-ATPIII. Hypertension was defined by current criteria (blood pressure ≥140/90 mmHg or use of antihypertensive treatment). Physical exercise (hours/week) was assessed, using a validated questionnaire (6), recording the combination of reported leisure and occupational activity over the past year as an estimate of “usual” activity levels (7;8).
Statistical analysis
Data were analyzed using SPSS 12.0 (SPSS, Chicago, IL). Descriptive statistics were obtained using χ2 distributions (with Monte Carlo method for computation of exact 2-tailed p value,) and 2-factor analysis of variance, to display variables of interest, based on glucose profile and incident HF. Serum creatinine, GFR, urinary albumin/creatinine ratio, glycated hemoglobin (Hb1Ac) and education level (assessed as years of school/university instruction) were log-transformed and presented as medians and interquartile ranges. Indicator variables were included for the Arizona, South/North Dakota and Oklahoma field centers.
Event rates in participants with normal or impaired fasting glucose or type 2 diabetes were displayed by Kaplan-Meier plots and log-rank statistics were computed. Log-cumulative hazard functions for incident HF were generated by Cox regression analyses comparing type 2 diabetes and impaired fasting glucose with normal fasting glucose. Cox regression was used to generate unadjusted hazard ratios (HRs) for effect of type 2 diabetes or fasting plasma glucose and, subsequently, to adjust for groups of covariates, including age and sex, established risk factors for HF (obesity, central fat distribution, hypertension, prevalent atrial fibrillation, GFR, urinary albumin/creatinine ratio and plasma cholesterol), Hb1Ac, environmental factors (smoking habit, alcohol use (9), educational level and physical activity) and antihypertensive medications [diuretics, β-blockers, ACE-inhibitors, CA++ channel blockers, other classes] prescribed during the first 4 years of follow-up. The same Cox model also provided adjusted HRs for arterial hypertension. Due to possible cause-effect relationship with incident HF, incident myocardial infarction was censored as a competing risk event in the Cox models (10). Thus, all HRs are also presented accounting for the competing risk event.
RESULTS
Table 1 shows baseline characteristics of the studied population, according to glucose status and incident HF. Participants with incident HF were older, with higher baseline systolic blood pressure and urinary/albumin excretion ratio and more frequently were hypertensive. Baseline diabetes was associated with greater BMI, higher systolic blood pressure and urinary/albumin excretion ratio, a little higher education level and greater prevalence of hypertension and obesity. No significant effect was found for physical activity. No difference in the duration of diabetes was found between diabetic with or without incident HF.
Table 1.
Baseline characteristics of SHS participants with or without incident HF, according to glucose status.
| Normal fasting glucose | Impaired fasting glucose | Type 2 diabetes | ||||
|---|---|---|---|---|---|---|
| No incident HF (n=1079) | Incident HF (n=64) | No incident HF (n=365) | Incident HF (n=26) | No incident HF (n=1005) | Incident HF (n=201) | |
| Age (years)† | 55.58±8.03 | 59.59±8.66 | 55.72±7.72 | 60.57±8.39 | 55.82±7.69 | 57.45±7.31 |
| Women (%) | 41.9% | 32.8% | 38.1% | 34.6% | 32.2% | 27.4% |
| BMI (kg/m2)* | 28.85±5.66 | 29.79±6.59 | 31.59±5.82 | 32.62±7.16 | 32.54±6.14 | 32.51±6.53 |
| Systolic BP (mmHg) *† | 124.18±18.35 | 131.68±18.58 | 128.31±19.25 | 136.07±20.61 | 129.48±18.48 | 132.94±21.29 |
| Diastolic BP (mmHg) | 76.19±10.03 | 76.47±11.53 | 77.85±10.43 | 77.59±8.47 | 77.50±9.75 | 77.51±10.81 |
| Plasma cholesterol (mmol/L) | 5.04±0.98 | 5.12±1.09 | 4.96±0.94 | 5.15±0.98 | 4.89±1.10 | 4.97±0.98 |
| Hb1Ac (mg/dL) | 5.00(4.70–5.41) | 5.15(4.90–5.53) | 5.30(5.00–5.70) | 5.50(5.00–5.68) | 8.60(6.60–10.70) | 9.10(7.07–10.63) |
| Diabetes duration (years) | --- | --- | --- | --- | 6(1–15) | 9(3–15) |
| Creatinine (mmol/L) | 97.24(62.76–130.83) | 83.98(54.81–126.41) | 96.36(68.07–133.48) | 93.70(74.26–105.20) | 87.52(61.00–124.64) | 81.33(50.39–112.27) |
| GFR (mL/min/1.73 m2) | 62.2(44.3–102.5) | 69.42(49.0–108.4) | 59.1(43.2–94.8) | 58.1(53.0–83.0) | 66.7(45.2–102.0) | 71.7(49.9–121.5) |
| Urinary albumin/creatinine*† | 6.34(3.24–12.73) | 5.66(3.40–14.43) | 7.23(3.78–18.25) | 9.94(3.03–19.48) | 30.73(8.69–157.17) | 45.05(12.21–546.48) |
| Education level (years)* | 12.00(10.00–14-00) | 10.00(8.00–12.00) | 12.00(9.00–13.00) | 10.50(8.00–12.50) | 11.00(9.00–12.00) | 11.00(8.00–12.00) |
| Physical activity (hrs/week) | 4.25±13.78 | 6.40±14.27 | 4.09±9.48 | 2.12±4.95 | 4.68±17.35 | 3.52±11.19 |
| Obesity (%)* | 39.3% | 48.4% | 59.7% | 38.5% | 61.4% | 61.7% |
| Hypertension (%)*† | 28.6% | 50.8% | 33.2% | 50.0% | 44.9% | 50.5% |
| Smokers (%) | 39.6% | 29.7% | 32.6% | 53.8% | 23.3% | 28.9% |
| Alcohol drinkers (%) | 47.5% | 39.1% | 40.9% | 38.5% | 35.4% | 29.4% |
| ECG LV hypertrophy (%) | 3.5% | 6.3% | 3.6% | 7.7% | 4.8% | 4.5% |
| Atrial fibrillation (%) | 0.2% | 0.0% | 0.5% | 0.0% | 0.3% | 1.0% |
| Anti-hypertensive drugs: | ||||||
| • Diuretics*† | 8% | 17% | 11% | 23% | 12% | 29% |
| • β-blockers* | 4% | 6% | 3% | 8% | 3% | 1% |
| • ACE-inhibitors*† | 9% | 20% | 11% | 19% | 23% | 29% |
| • Ca++-blockers*† | 7% | 13% | 6% | 12% | 10% | 22% |
Effect of glucose status;
Effect of incident HF;
Former smokers were 33% among participants free of incident HF, and 39% among those with incident HF.
Among diabetic participants, 38% were on oral anti-diabetic medications, 39% on insulin, and 3.4% on both, without difference between those with or without incident HF. Thiazolinediones that might facilitate symptoms of HF were not used in the SHS cohort until late 2000, and even in the following three years of the present follow-up, their use has been very limited. Antihypertensive therapy at the time of 1st SHS exam was recorded in 53% of hypertensive participants who did not have incident HF and in 54% of those with incident HF. Table 1 also shows the distribution of anti-hypertensive medications, in relation to prevalent diabetes and incident HF. Antihypertensive treatment was prescribed more frequently to participants developing HF during follow up, except for β-blockers, and the frequency of prescription increased consistently with the degree of impaired glucose metabolism, except for β-blockers for which frequency of prescription decreased among diabetic participants..
During follow-up (11.9±3.0 years), 291 participants suffered HF and 221 had acute myocardial infarction, 74 of which were fatal (34%). During follow-up, 64 cases of incident HF were ascertained in participants with normal fasting glucose (NFG, 6%), 26 (7%) with IFG and 201 with diabetes (17%). HF occurred in 230 participants in the absence of acute myocardial infarction and in 61 following myocardial infarction.
Type 2 diabetes and incident HF
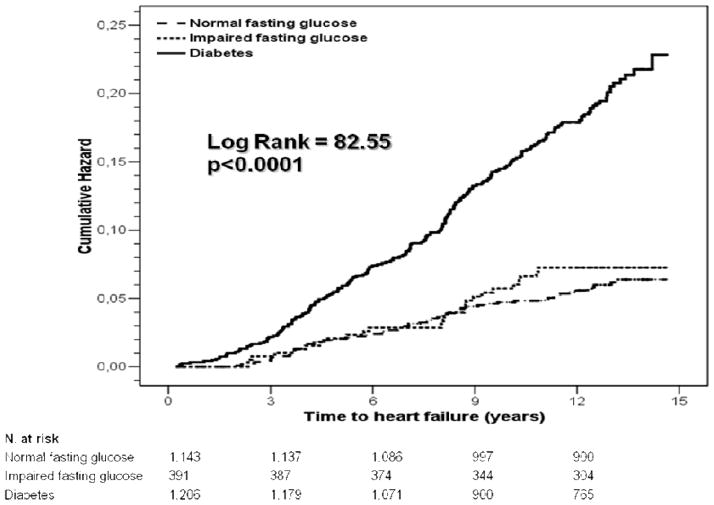
The absolute risk of incident HF was substantially higher (17%) in diabetic participants than in those with impaired fasting (7%) or normal-glucose (6%), without difference between the latter groups (Figure 1). Table 2 shows the unadjusted and adjusted HRs for type 2 diabetes. The unadjusted risk of HF was 4-fold higher in diabetic than non diabetic participants, dropping to 2.6-fold when incident myocardial infarction was considered as competing risk events. Subsequent and additional adjustments for age and sex, risk factors for HF, Hb1Ac, environmental factors and classes of antihypertensive medications, reduced the HR for diabetes, but did not eliminate its significant independent association with incident HF.
Figure 1.
Cumulative rate of incident heart failure in SHS participants with normal (dashed line) or impaired fasting glucose (dotted line) or type 2 diabetes (continuous line).
Table 2.
Cox regression models of incident HF in relation with glucose status (normal fasting glucose, impaired fasting glucose and type 2 diabetes). Results for type 2 diabetes are shown.
| Type 2 diabetes status and hazard for development of HF | ||
|---|---|---|
| Model adjustment | Hazard ratio (95% Confidence Limits) | |
| No competing risk event | MI as Competing risk event | |
| None | 4.04 (3.03–5.41)* | 2.61 (2.10–3.24)* |
| Age and sex | 3.77 (2.82–5.05)* | 2.54 (2.05–3.16) |
| Age, sex, HF risk factors (hypertension, antihypertensive therapy, obesity, central fat distribution, total cholesterol, GFR, urinary albumin/excretion ratio, atrial fibrillation) and HbA1c | 2.14 (1.42–3.21)* | 1.43 (1.04–1.95)† |
| Age, sex, HF risk factors, HbA1c, and environmental factors (smoking, alcohol, educational status, physical activity) | 2.19 (1.45–3.31)* | 1.40 (1.02–1.92)† |
| Age, sex, HF risk factors, HbA1c and environmental factors (smoking, alcohol, educational status, physical activity), classes of antihypertensive medications (diuretics, β-blockers, ACE-inhibitors, CA++ channel blockers, other classes) | 2.45 (1.56–3.86)* | 1.50 (1.13–1.99)‡ |
p<0.0001;
p<0.001;
p<0.03
Table 3 shows the unadjusted and adjusted HRs for hypertension. The unadjusted risk of HF was 2-fold higher in hypertensive than non hypertensive participants. Similar to diabetes, subsequent adjustment for covariates reduced the HR for hypertension, both without or with consideration of the competing incident myocardial infarction. The HRs for hypertension as a predictor of HF was not anymore significant when classes of antihypertensive medications were added to the Cox model of proportional hazard, while a borderline significance was found when myocardial infarction was considered as a competing risk event.
Table 3.
Cox regression models of incident HF in relation with prevalent hypertension.
| Hypertension status and hazard for development of HF | ||
|---|---|---|
| Model adjustment | Hazard ratio (95% Confidence Limits) | |
| No competing risk event | MI as Competing risk event | |
| None | 2.03 (1.61–2.57)* | 2.05(1.70–2.47)* |
| Age and sex | 1.82(1.43–2.32)* | 1.83(1.51–2.22)* |
| Age, sex, HF risk factors (diabetes, antihypertensive therapy, obesity, central fat distribution, total cholesterol, GFR, urinary albumin/excretion ratio, atrial fibrillation) and HbA1c | 1.37(1.06–1.78)† | 1.44(1.17–1.76)* |
| Age, sex, HF risk factors, HbA1c and environmental factors (smoking, alcohol, educational status, physical activity) | 1.45(1.11–1.88)‡ | 1.50(1.21–1.85)* |
| Age, sex, HF risk factors, HbA1c and environmental factors (smoking, alcohol, educational status, physical activity), classes of antihypertensive medications (diuretics, β-blockers, ACE- inhibitors, CA++ channel blockers, other classes) | 1.08(0.79–1.49) | 1.29(1.00–1.67)¶ |
Abbreviations: MI=myocardial infarction HF=heart failure
p<0.0001;
p<0.001
p<0.03;
p=0.05
DISCUSSION
Diabetes is a powerful risk factor for myocardial infarction (3), a very frequent intermediate step in the progression toward heart failure. Although there is wide literature on the association of diabetes with HF, the fact that this association is working also independently of incident myocardial infarction and hypertension has never been demonstrated using statistical approaches that also consider the interference of incident myocardial infarction.
In addition to the well-known associations of HF with hypertension, myocardial infarction and obesity, the present analysis performed in an unselected population, free of overt coronary heart disease, demonstrates that type 2 diabetes is a potent predictor of HF independent of the impact of incident myocardial infarction, itself a common macrovascular complication of diabetes. After considering the competing outcome effect of incident myocardial infarction, diabetic participants had a 2.6 higher 12-year risk of HF than those with normal fasting glucose. The increased risk of HF with diabetes was also independent of effects of other coexisting risk factors, in particular hypertension and central obesity.
Because diabetes, hypertension, central obesity and coronary heart disease often coexist, these factors may act synergistically to produce LV dysfunction, sustain its progression and finally precipitate HF. The observation that diabetes might be a risk factor for HF was recognized as early as the 19th century (11). In 1972, Rubler et al. (12) reported a characteristic cardiomyopathy in diabetic patients with glomerulosclerosis, and early data from the Framingham Heart Study suggested that cardiac death in diabetic participants was not solely related to coronary artery disease (13). Evidence for a cardiomyopathy in familial diabetes has also been reported (14), in most instances in the absence of epicardial coronary lesions. Further evidence of potential effect of diabetes on myocardial mechanics, not mediated by coronary artery disease, included the observation of subtle abnormalities of early LV relaxation in juvenile (15) as well as adult diabetes (16). Coughlin et al. (17) reported a 2-fold greater risk of dilated cardiomyopathy in the presence of history of diabetes, which could not be explained by race, income, cigarette usage, or hypertension. The EPICAL study (18) reported 20% and 33% prevalence of type 2 diabetes in HF patients without or with overt prevalent coronary heart disease, respectively. Aronow and Ahn (19) reported a 34% higher risk of HF associated with diabetes from an elderly cohort, with a large prevalence of coronary heart disease, seen in a health-care facility. In the Cardiovascular Health Study, diabetes was an independent risk factor for HF in participants either with or without coronary heart disease (20). Nichols et al. (21) reported increased incidence of HF in diabetic subjects from a retrospective survey of 17616 electronic medical records (8,460 from diabetic patients). In the United Kingdom Prospective Diabetic Study (UKPDS), the incidence of HF was related to the magnitude of hyperglycemia (22), but no attempt was made to assess the effect of prevalent coronary heart disease. A large number of clinical and epidemiological investigations also reported association between HF and insulin resistance or metabolic syndrome (23–26).
Despite this large body of evidence, there are no studies that have considered the interfering incident myocardial infarction as the main explanation for the strong association between diabetes and HF. In fact, as said before, our study extends these findings, by giving different weight to the follow-up HF in relation to the previous occurrence of myocardial infarction, a potent predictor of HF, by using a relatively novel and conservative statistical approach based on competing risk event estimate (10), under the assumption that the censored case of HF following myocardial infarction was precipitate by it. This method is desirable when participants of cohort studies may experience an event other than the one of interest which alters the probability of experiencing the event of interest.
The development of diabetic cardiomyopathy may reflect biological actions of insulin on the CV system (27) as well as consequences of insulin resistance (28;29). However, the mechanisms of the increased HF rate in diabetic individuals without prior myocardial infarction cannot be established in the present study. One possibility is that unrecognized coronary artery disease plays a major role in precipitating HF. This possibility is supported by an increased rate of “silent” myocardial ischemia with diabetes (30;31). Although we excluded participants with ECG Q-waves indicative of previous myocardial infarction, by systematic Minnesota coding at the 1st Strong Heart Study exam, and included participants with Q-wave-myocardial infarctions detected at the 2nd or 3rd exam as a competing risk event, silent ischemia could not be ruled out.
A second possible mechanism of increased HF with diabetes is the occurrence of LV geometric and functional abnormalities, including increased LV mass and concentric remodelling, characterizing a distinct diabetic cardiomyopathy (32). Further studies have confirmed that diabetes is associated with some degree of concentric LV geometry and subclinical LV systolic dysfunction (33). There is also increasing evidence that diastolic function is compromised in diabetic subjects (34–37), though the interpretation of these findings is debated (38). The alteration of LV filling is at least in part related to abnormal myocardial energy metabolism (39) and to general CV impairment caused by reduced insulin production and loss of insulin sensitivity (27–29;40;41).
A particularly interesting potential mechanism that might compromise myocardial blood supply in diabetic patients is coronary microvascular dysfunction (42;42) that is especially associated with left ventricular hypertrophy (30;43).
Limitations
This study analyzed the longest follow-up from the initial SHS cohort (n=4,549), using data available at the baseline. Echocardiography was not included in the data collection, and this is a limitation of our study, because of the impossibility to merge cardiovascular phenotype with the risk profile emerging from our analysis of the initial cohort. Echocardiograms have been included at the time of the 2nd exam and were performed in a different, less large cohort. The present analysis, however, presents two advantages: 1) it extends from the beginning of the study; and 2) includes all participants of the initial cohort, minimizing the potential survivor bias. Preliminary findings from on-going analyses of the cohort of the 2nd SHS exam suggest that initial LV geometry, systolic and diastolic function are potent predictors of HF among the diabetic SHS participants free of cardiovascular or severe renal disease and independently of incident myocardial infarction (44).
Extension of these findings to other ethnic groups needs to be done with caution, though findings in the SHS may be increasingly applicable to the general population, now that the prevalence rates of obesity and diabetes in other populations in industrialized countries are approaching the levels found in the SHS cohort. At the beginning of the study (1989), the prevalence of overweight in the SHS cohort exceeded national USA averages by 16 to 36 percent. However, during the past 20 years, the prevalence of obesity has increased dramatically among US African-Americans, Hispanics and Caucasians, approaching today the level of American-Indians at the start of the SHS survey (45). Similarly, the prevalence of type 2 diabetes in the SHS cohort at its inception was several times higher than in the general USA and European populations, but impaired glucose tolerance rates were close to those in the general population. Following the obesity epidemic, the prevalence of type 2 diabetes in the other USA ethnic groups and in Europe is rapidly rising (46;47), and the prediction is for about 20 million people with type 2 diabetes in 2025 and over 29 million on 2050 in USA and at least as many in Europe, a 165% increase over the 2000 level (48). Thus, the differences in prevalence of obesity and type 2 diabetes between American Indians and the other ethnicities living in industrialized countries have been attenuated over time and, perhaps more importantly, their adverse consequences already seen in American Indians are now becoming evident in other ethnic groups.
The Strong Heart Study cohort is well-powered for analysis of complications of type 2 diabetes, but few members of this population-based sample have type 1 diabetes. Thus, research in other populations is needed to assess the impact of type 1 diabetes on HF risk.
Conclusion
Type 2 diabetes is a potent risk factor for HF, independent of incident myocardial infarction and coexisting CV risk factors.
Acknowledgments
The authors wish to thank the Indian Health Service, the Strong Heart Study Participants, the Participating Tribal Communities and the Strong Heart Study Center Coordinators for their help in the realization of this project.
Views expressed in this paper are those of the authors and do not necessarily reflect those of the Indian Health Service.
This work has been supported by the National Institutes of Health, Bethesda, MD. [HL41642, HL41652, HL41654, HL65521 and M10RR0047-34]
Footnotes
No authors have conflicts of interest to disclose
Reference List
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52(5):1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 3.Howard BV, Lee ET, Cowan LD, Fabsitz RR, Howard WJ, Oopik AJ, Robbins DC, Savage PJ, Yeh JL, Welty TK. Coronary heart disease prevalence and its relation to risk factors in American Indians. The Strong Heart Study. Am J Epidemiol. 1995;142(3):254–268. doi: 10.1093/oxfordjournals.aje.a117632. [DOI] [PubMed] [Google Scholar]
- 4.Lee ET, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study -- A study of cardiovascular disease in American Indians: Design and methods. Am J Epidemiol. 1990;136:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 5.Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, Howard WJ, Rhoades ER, Robbins DC, Sievers ML, Welty TK. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99(18):2389–2395. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 6.Yurgalevitch SM, Kriska AM, Welty TK, Go O, Robbins DC, Howard BV. Physical activity and lipids and lipoproteins in American Indians ages 45–74. Med Sci Sports Exerc. 1998;30(4):543–549. doi: 10.1097/00005768-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Schulz LO, Harper IT, Smith CJ, Kriska AM, Ravussin E. Energy intake and physical activity in Pima Indians: comparison with energy expenditure measured by doubly-labeled water. Obes Res. 1994;2(6):541–548. doi: 10.1002/j.1550-8528.1994.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 8.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 9.Lu W, Jablonski KA, Resnick HE, Jain AK, Jones KL, Gottlieb AM, Welty TK, Lee ET, Fabsitz RR, Howard BV. Alcohol intake and glycemia in American Indians: the strong heart study. Metabolism. 2003;52(2):129–135. doi: 10.1053/meta.2003.50020. [DOI] [PubMed] [Google Scholar]
- 10.Satagopan JM, Ben Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91(7):1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer J. Über den Zusammenhang des Diabetes Mellitus mit Erkrankungen des Herzens. Zeitschr Klin Med. 1888;14:212–239. [Google Scholar]
- 12.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 14.Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest. 1977;60(4):884–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanderson JE, Brown DJ, Rivellese A, Kohner E. Diabetic cardiomyopathy? An echocardiographic study of young diabetics. Br Med J. 1978;1(6110):404–407. doi: 10.1136/bmj.1.6110.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JE, Robbins DC, Palmieri V, Bella JN, Roman MJ, Fabsitz R, Howard BV, Welty TK, Lee ET, Devereux RB. Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol. 2003;41(11):2022–2028. doi: 10.1016/s0735-1097(03)00403-0. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin SS, Pearle DL, Baughman KL, Wasserman A, Tefft MC. Diabetes mellitus and risk of idiopathic dilated cardiomyopathy. The Washington, DC Dilated Cardiomyopathy Study. Ann Epidemiol. 1994;4(1):67–74. doi: 10.1016/1047-2797(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 18.Zannad F, Briancon S, Juilliere Y, Mertes PM, Villemot JP, Alla F, Virion JM. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL Study. Epidemiologie de l'Insuffisance Cardiaque Avancee en Lorraine. J Am Coll Cardiol. 1999;33(3):734–742. doi: 10.1016/s0735-1097(98)00634-2. [DOI] [PubMed] [Google Scholar]
- 19.Aronow WS, Ahn C. Incidence of heart failure in 2,737 older persons with and without diabetes mellitus. Chest. 1999;115(3):867–868. doi: 10.1378/chest.115.3.867. [DOI] [PubMed] [Google Scholar]
- 20.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 21.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24(9):1614–1619. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 22.Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, Selby JV. Glycemic Control and Heart Failure Among Adult Patients With Diabetes. Circulation. 2001;103(22):2668–2673. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 23.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294(3):334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 24.Thrainsdottir IS, Aspelund T, Thorgeirsson G, Gudnason V, Hardarson T, Malmberg K, Sigurdsson G, Ryden L. The association between glucose abnormalities and heart failure in the population-based Reykjavik study. Diabetes Care. 2005;28(3):612–616. doi: 10.2337/diacare.28.3.612. [DOI] [PubMed] [Google Scholar]
- 25.Butler J, Rodondi N, Zhu Y, Figaro K, Fazio S, Vaughan DE, Satterfield S, Newman AB, Goodpaster B, Bauer DC, Holvoet P, Harris TB, de Rekeneire N, Rubin S, Ding J, Kritchevsky SB. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47(8):1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 26.Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, Sleight P, Teo K. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation. 2007;115(11):1371–1375. doi: 10.1161/CIRCULATIONAHA.106.661405. [DOI] [PubMed] [Google Scholar]
- 27.von Lewinski D, Bruns S, Walther S, Kogler H, Pieske B. Insulin Causes [Ca2+]i-Dependent and [Ca2+]i-Independent Positive Inotropic Effects in Failing Human Myocardium. Circulation. 2005;111(20):2588–2595. doi: 10.1161/CIRCULATIONAHA.104.497461. [DOI] [PubMed] [Google Scholar]
- 28.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105(14):1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 29.Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002;105(15):1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 30.Valensi P, Sachs RN, Lormeau B, Taupin JM, Ouzan J, Blasco A, Nitenberg A, Metz D, Paries J, Talvard O, Leutenegger M, Attali JR. Silent myocardial ischaemia and left ventricle hypertrophy in diabetic patients. Diabetes Metab. 1997;23(5):409–416. [PubMed] [Google Scholar]
- 31.Goraya TY, Leibson CL, Palumbo PJ, Weston SA, Killian JM, Pfeifer EA, Jacobsen SJ, Frye RL, Roger VL. Coronary atherosclerosis in diabetes mellitus: a population-based autopsy study. J Am Coll Cardiol. 2002;40(5):946–953. doi: 10.1016/s0735-1097(02)02065-x. [DOI] [PubMed] [Google Scholar]
- 32.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study) Am J Cardiol. 1991;68(1):85–89. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 33.Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101(19):2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 34.Inoue T, Fujito T, Asahi S, Hoshi K, Sakai Y, Morooka S. Impaired left ventricular diastolic filling occurs in diabetic patients without atherosclerotic coronary artery disease. Am J Med Sci. 1997;313(3):125–130. doi: 10.1097/00000441-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Guglielmi MD, Pierdomenico SD, Salvatore L, Romano F, Tascione E, Pupillo M, Porreca E, Imbastaro T, Cuccurullo F, Mezzetti A. Impaired left ventricular diastolic function and vascular postischemic vasodilation associated with microalbuminuria in IDDM patients. Diabetes Care. 1995;18(3):353–360. doi: 10.2337/diacare.18.3.353. [DOI] [PubMed] [Google Scholar]
- 36.Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2001;87(3):320–323. doi: 10.1016/s0002-9149(00)01366-7. [DOI] [PubMed] [Google Scholar]
- 37.Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24(1):5–10. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]
- 38.Cosson S, Kevorkian JP, Virally ML, Henry P, Laloi-Michelin M, Meas T, Beaufils P, Guillausseau PJ. No evidence for left ventricular diastolic dysfunction in asymptomatic normotensive type 2 diabetic patients: a case-control study with new echocardiographic techniques. Diabetes Metab. 2007;33(1):61–67. doi: 10.1016/j.diabet.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42(2):328–335. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 40.An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291(4):H1489–H1506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- 41.Abe T, Ohga Y, Tabayashi N, Kobayashi S, Sakata S, Misawa H, Tsuji T, Kohzuki H, Suga H, Taniguchi S, Takaki M. Left ventricular diastolic dysfunction in type 2 diabetes mellitus model rats. Am J Physiol Heart Circ Physiol. 2002;282(1):H138–H148. doi: 10.1152/ajpheart.2002.282.1.H138. [DOI] [PubMed] [Google Scholar]
- 42.Guha A, Harmancey R, Taegtmeyer H. Nonischemic heart failure in diabetes mellitus. Curr Opin Cardiol. 2008;23(3):241–248. doi: 10.1097/HCO.0b013e3282fcc2fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galderisi M, de Simone G, Cicala S, De Simone L, D'Errico A, Caso P, de Divitiis O. Coronary flow reserve in hypertensive patients with appropriate or inappropriate left ventricular mass. J Hypertens. 2003;21(11):2183–2188. doi: 10.1097/00004872-200311000-00029. [DOI] [PubMed] [Google Scholar]
- 44.de Simone G, Devereux RB, China, Chinali M, Lee ET, Galloway JM, Howard BV. (abstract) Cardiovascular phenotype predicting incident heart failure in diabetes: The Strong Heart Study. J Am Coll Cardiol. 2008;51(suppl A):A359. [Google Scholar]
- 45.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 46.Gregg EW, Cheng YJ, Narayan KM, Thompson TJ, Williamson DF. The relative contributions of different levels of overweight and obesity to the increased prevalence of diabetes in the United States: 1976–2004. Prev Med. 2007;45(5):348–352. doi: 10.1016/j.ypmed.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 47.British Heart Foundation. Overall prevalence of diabetes. Statistical website, editor. 12-9-2008. http://www.heartstats.org/datapage.asp?id=1106.
- 48.Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001;24(11):1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]