Preformed donor-specific T-lymphocytotoxic antibody (DSTAb) has an adverse effect on patients with orthotopic liver transplantation (OLTx), yet some liver allografts were tolerated with cytotoxic antibody-mediated rejection.1–3 To study such an antibody effect further, we monitored posttransplant DSTAb for patients who received OLTx across a positive T-lymphocyte crossmatch.
MATERIALS AND METHODS
This study consisted of 40 patients who received primary liver transplantation across a positive T-lymphocyte crossmatch at the University of Pittsburgh Medical Center, between November 1989 and December 1991. Initial immunosuppression comprised cyclosporine A (4 cases) or FK 506 (36 cases), in combination with a low-dose (17 cases) or high-dose steroid (23 cases). The protocol for Combined steroid therapy was the following: low dose, 20 mg/d of methylprednisolone given on the day of transplantation and continued during the first 2 weeks posttransplant, then decreased to 10 mg/d. In high-dose steroid therapy, methylprednisolone was started at 200 mg/d in the operating room, and decreased daily by 40 mg until reaching 20 mg/d. All patients were monitored for their DSTAb level in serum samples collected every week for 2 months following OLTx. The lymphocyte cytotoxicity test was performed with dithiothreitol (DTT)-treated sera according to the National Institutes of Health standard procedure, and the reactivity of DSTAb was decided by the end point of cytotoxic titer using 2-fold diluted serum up to 1:32. Actuarial graft survival was computed by the life-table method, and patient death, as well as graft removal, was considered graft failure, regardless of the reason.
RESULTS
According to posttransplant DSTAb titer, 40 patients were divided into two groups: group 1 consisted of 25 patients who became negative in the first 2 months posttransplant, and group 2 comprised 15 patients who had an increased or stable titer over a 2-month period following OLTx. Figure 1 illustrates the graft outcomes of these two groups. The survival rate of group 2 dropped markedly during the first 3 weeks posttransplant, with graft survival rates of 53.3%, 40.0%, and 33.3% at 1, 2, and 3 months, respectively. On the other hand, the graft survival rates of Group 1 were 96.0%, 88.0%, and 88.0% at 1, 2, and 3 months, respectively, which were higher than those of Group 2.
Fig 1.
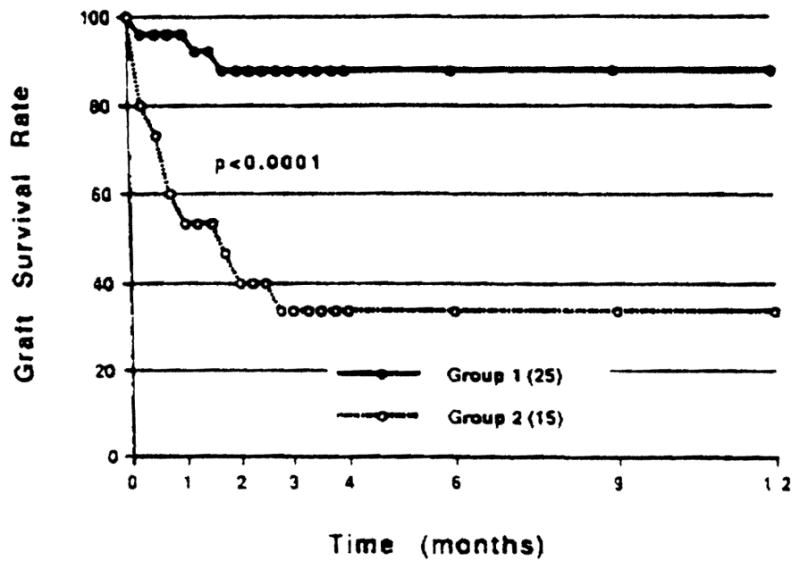
Effect of posttransplant donor-specific T-lymphocytotoxic antibody (DSTAb) on liver graft outcome.
DISCUSSION
Although a detrimental effect was observed for patients who received orthotopic liver transplantation across a positive crossmatch, our earlier study revealed that this effect was minimal compared to that observed in kidney transplant recipients.1–3 As a result, the liver has been considered invulnerable from an antibody-mediated injury. However, this study clearly indicated that if a patient possessed a persistent DSTAb during the early posttransplant period, the transplant outcome was significantly low. In fact, a 3-month survival rate as low as 33% was observed in such recipients. These data suggest that the transplanted liver could also be a target of the antibody injury, and the presence of posttransplant donor-specific antibody seems to be more substantial than the pretransplant antibody. If this were the case, efforts should be made to reduce the posttransplant antibody titer for patients with a positive crossmatch. Although an extended study is required, our preliminary data reveal that a high dose of steroid administration is effective in reducing posttransplant DSTAb in patients with pretransplant antibody,4 which would relieve the transplanted liver from an antibody-mediated injury.
References
- 1.Takaya S, Bronsther O, Iwaki Y, et al. Transplantation. 1992;53:400. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura K, Yagihashi A, Iwaki Y, et al. Transplant Proc. 1991;23:3021. [PMC free article] [PubMed] [Google Scholar]
- 3.Yagihashi A, Kobayashi M, Noguchi K, et al. HLA. Oxford, England: Oxford University Press; 1991. in press. [Google Scholar]
- 4.Takaya S, Iwaki Y, Starzl TE. Transplantation. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


