Abstract
Glycolysis, a central metabolic pathway, harbors evolutionary conserved enzymes that modulate and potentially shift the cellular metabolism on requirement. Pyruvate kinase, which catalyzes the last but rate-limiting step of glycolysis, is expressed in four isozymic forms, depending on the tissue requirement. M2 isoform (PKM2) is exclusively expressed in embryonic and adult dividing/tumor cells. This tetrameric allosterically regulated isoform is intrinsically designed to downregulate its activity by subunit dissociation (into dimer), which results in partial inhibition of glycolysis at the last step. This accumulates all upstream glycolytic intermediates as an anabolic feed for synthesis of lipids and nucleic acids, whereas reassociation of PKM2 into active tetramer replenishes the normal catabolism as a feedback after cell division. In addition, involvement of this enzyme in a variety of pathways, protein–protein interactions, and nuclear transport suggests its potential to perform multiple nonglycolytic functions with diverse implications, although multidimensional role of this protein is as yet not fully explored. This review aims to provide an overview of the involvement of PKM2 in various physiological pathways with possible functional implications.
Keywords: metabolism, enzyme catalysis, cancer, mutation, PKM2, Bloom syndrome
Introduction
The primary function of pyruvate kinase (PK; EC 2.7.1.40) enzyme is to catalyze the transphosphorylation from phosphoenolpyruvate (PEP) to ADP as the last step of glycolysis to generate ATP.1,2 Depending upon the metabolic requirement, the enzyme is expressed in four different isozymic forms, L, R, M1, and M2, in mammalian tissues, which differ in their regulatory properties.3,4 The M2, L, and R isozymes show homotropic cooperative activation with PEP and heterotropic cooperative activation with FBP.5,6 The M1 isozyme is regulated by neither P-enolpyruvate nor Fru-1,6-P2 because of its intrinsic active conformation in the R-state.7 The M1 and M2 isozymes are produced from a single gene locus by mutually exclusive alternative splicing.8 In human M1 and M2 isozymes, the exon that is exchanged because of alternative splicing encodes 56 amino acids, in which a total of 22 amino acids differ within a length of 45 residues. The residues located in this region form the major intersubunit contact domain.9 The distinguishable kinetic properties of the M1 and M2 isozymes are attributed to these amino acid substitutions. It has been shown by X-ray crystallographic analyses and computer modeling that the corresponding regions of their polypeptides participate directly in the intersubunit contact, which is responsible for the intersubunit communication required for allosteric cooperativity.5,10 PK has been largely conserved throughout evolution. The enzyme is usually a homotetramer composed of four identical subunits, and each subunit consists of four domains: the A-, B-, and C-domains and the N-terminal domain. The structure of human PKM2 was determined in complex with inhibitors.9 In mammalian cells, PK activity is regulated by two different mechanisms: one at the level of expression and the other through allosteric regulation. The catalytic site usually constitutes a small part of the enzyme, but allosteric control is transmitted over a long range, thus increasing the number of possible residues involved in regulation. The allosteric transition in PK involves mutual rotations of the A- and C-domains within each subunit and the subunit within the tetramer.11 The residues at the subunit interfaces have the critical function of relaying the allosteric signal from and to the catalytic and regulatory sites.
PKM2 is a ubiquitous prototype enzyme present in all tissues during the embryonic stage and is gradually replaced by other isozymic forms in specific tissues during development. Although the primary function of PKM2 is to catabolize glucose, it is possibly involved in many other nonglycolytic functions too. In addition to its localization in nucleus,12–14 it interacts with a variety of biological molecules (Table I) such as HERC1 (homologous to the E6-AP (UBE3A) carboxyl terminus domain and RCC1-like domain-1 for intracellular membrane trafficking),18 signaling proteins such as A-Raf,15 cytoplasmic-PML (promyelocytic leukemia) protein,14 and pantothenate kinase 4,24 and transcription factors such as octamer-4,22 SUMO ubiqutin E3 ligase,29 thyroid hormone,33,34 somatostatin,13 and lysophosphatidic acid (LPA).31 It is also known to interact with pathogenic E7 protein of HPV (human papillomavirus),19,35 NS5B of hepatitis C virus,17 PP60v-src-tyrosine kinase,26 and Opa (opacity associated) protein of Staphylococcus.23 The diverse interaction of PKM2 supposedly places this molecule at a principal position of complex cellular pathways, although there is dearth in understanding this association and its relevance in cellular physiology. Incidentally, the overall contributions in PKM2 physiology have just highlighted its role in tumor progression36 ignoring its other possible diverse impacts, which could be easily speculated by its known interactions and subcellular localization, possible after observing the aberrant form of PKM2 in a pathological condition of Bloom syndrome (BS).37 Our further study on the aberrant mutant forms of PKM238 has allowed us to propose in this review the possible multiple roles the enzyme could play in cellular physiology and in the genetic background of a syndrome, BS, where the mutants of PKM2 were first found.39
Table I.
Interacting Partners of PKM2 and Their Proposed Biological Importance
| S. no. | Interacting proteins | Method/s used | Physical alterations | Functional alterations (dimer/tetramer ratio) | Potential biological relevance | Ref. |
|---|---|---|---|---|---|---|
| 1 | A-Raf (Raf kinase isozyme) | IP, Co-IP | Phosphorylation at Ser residue | Modulates PKM2 dimer/tetramer ratio hence the activity accordingly | Regulation of glycolysis (Go and stop mechanism) | 15 |
| 2 | Break point cluster region (BCR)-ABL fusion Tyr kinase | In vitro kinase assay | Phosphorylation at Y105 residue | Inhibits tetramerization | Promotes cancer metabolism | 16 |
| 3 | ETV6-neurotrophic Tyr kinase receptor-3 | In vitro kinase assay | Phosphorylation at Y105 residue | Inhibits tetramerization | Promotes cancer metabolism | 16 |
| 4 | Fibroblast growth factor receptor-1 (FGFR-1) | In vitro kinase assay | Phosphorylation at Y105 residue | Subunit dissociation to dimerization; FBP release and activity reduction | Facilitates Warburg effect | 16 |
| 5 | Fms-related Tyr kinase-3 ITD mutant | In vitro kinase assay | Phosphorylation at Tyr residue | Inhibits tetramerization | Promotes cancer metabolism | 16 |
| 6 | Hepatitis C virus-NS5B (RNA polymerase) | GST pull down, Co-IP | Binds to PKM2 | Not known | Possibly promotes pathogenesis by helping viral RNA synthesis | 17 |
| 7 | HECT domain of HERC-1 | IF, GST pull down | Binds to amino acid residues from 406 to 531 of PKM2 | Not known | Possibly regulates GTP production for guanine nucleotide exchanger or intracellular membrane trafficking | 18 |
| 9 | Human papillomavirus-16 E7 | Y2H, GST-pull down, IP | Dissociation of PKM2 tetramer into inactive dimer | Dimerization and PKM2 activity inhibition | Transforming mechanism of viral oncoprotein (HPV-E7) | 19 |
| 10 | JAK-2 mutant (Val617Phe) | In vitro kinase assay | Phosphorylation at Y105 residue | Inhibits tetramerization | Promotes cancer metabolism (IL3-dependent nuclear localization; cell division) | 12,16 |
| 11 | IgE receptor (ITAM region of δ chain) | Y2H, GST-pull down, IP | Phosphorylation at tyrosine residue involving SrcK, PI3K | Activity reduction | Possibly facilitates the mast cell degranulation | 20,21 |
| 12 | Octamer-4 | AC-MALDI-TOF, Co-IP, GST pull down | Binds to amino acid residues from 307 to 521 of PKM2 | Not known | Possibly modulates transactivation potential of the Oct-4 positively | 22 |
| 13 | N. gonorrhea opacity- associated protein (Opa) | Y2H, IF | Not known | Possibly alters host ATP production | Possibly helps in pathological establishment by acquisition of host C source of N. gonorrhea | 23 |
| 14 | Pantothenate kinase-4 (PANK-4) | Y2H, GST-pull down, Co-IP, IF | Not known | Not known | Possibly regulates glycolysis and coenzyme-A biosynthetic flux, hence TCA cycle | 24 |
| 15 | PKC δ protein kinase C isozyme | 2D Gel EF, IP | Phosphorylation at Ser residue | No change in activity or dimer/tetramer ratio | Possibly regulates PKM2 stability | 25 |
| 16 | Promyelocytic leukemia (PML) tumor suppressor protein | Co-IP, GST-pull down | Not known | Reduces activity of PKM2 tetramer, not dimer | Possibly help in promoting cancer metabolism or genomic instability | 14 |
| 17 | Pp60v-src | IP | Dissociation of PKM2 tetramer into dimer | Dimerization and PKM2 activity inhibition | Transforming mechanism of Rouse Sarcoma virus | 26,27 |
| 18 | SOCS3 | IP, GST pull down | Not known | Reduces ATP production | Defective antigen presentation; immune evasion by tumor cells | 28 |
| 19 | SUMO-E3 ligase (PIAS3) | Y2H, GST-pull down, IP | Binds to amino acid residues from 1 to 348 of PKM2 | Not known | Sumoylation and nuclear translocation | 29 |
| 20 | Tumor endothelial Marker-8 (TEM-8) | IP | Binds to amino acid residues from 379 to 385 of PKM2 | Not known | Interaction with PKM2 (released by tumor cells) possibly promotes angiogenesis | 30 |
| Other interacting partners: | ||||||
| 1 | Somatostatin | FPLC-MS | Not known | Not known | Nuclear localization of PKM2; caspase- independent apoptosis | 13 |
| 2 | Lysophosphatidic acid (LPA) | LAC-MS, IP, ITC | Increased α-helical content, dissociation to inactive dimer | Reduced activity | Possibly facilitates cell division | 31 |
| 3 | Fructose-1,6- bis-phosphate | XRD | Brings conformational changes, induces tetramerization | Triggers allosteric signal transduction, increases activity | Promotes catabolism | 9 |
| 4 | Phospho-tyrosine peptides | LC-MS/MS | Modulates allosteric pocket conformation | FBP release and activity reduction | Essential for cell growth and anabolism | 16,32 |
| 5 | T3 (thyroid hormone) | In vitro binding assay | Stabilizes PKM2 monomer, inhibits FBP-induced tetramerization | PKM2 activity inhibits to ∼5% | Reduced glycolysis, facilitates T3-dependent O2 consumption | 33 |
Y2H, Yeast 2 hybrid; Co-Ip, coimmunoprecipitation; IP, immunoprecipitation; LC-MS/MS, microcapillary reversed-phase tandem mass spectrometry; FPLC, fast protein liquid chromatography; 2D EF, two-dimensional gel electrophoresis; ITC, isothermal titration calorimetry; XRD, X-ray diffraction; AC-MALDI-Tof, affinity chromatography-matrix-assisted laser desorption ionization-time of flight; GST pull down, glutathione-S-transferase pull down assay.
Human PKM2: a metabolic regulator
Warburg's proposal of upregulation of glycolysis in cancer even in the presence of O2 had highlighted the exclusive importance of glycolytic pathway in dividing cells.40,41 A dividing cell has dual dependence on glycolysis for: (i) energy and (ii) the glycolytic intermediates (phosphometabolites) required as precursors for the synthesis of nucleic acids, amino acids, and lipids.42 This dual dependence ensures the activation of synthetic processes, only when the source of energy (glucose) is sufficient in microenvironment to maintain the metabolic homeostasis of the cell. Because accumulation of synthetic precursors and availability of energy from glycolysis are mutually exclusive (one at a time), the dividing cell efficiently coordinates both pathways in a cyclic manner, where PKM2 plays a major role because of its positioning at the last step of glycolysis.43 Hence, expression of PKM2 isoform in a dividing cell is a metabolic requirement, and its presence at the last step of glycolysis decides the fate of glucose carbons to channel either in synthetic pathway (nucleogenic) or for energy production. The destined ATP is produced by maintaining a default active tetramer state of the enzyme (PKM2). However, when the cell senses the requirement of precursors, especially during cell division, the activity of this enzyme is downregulated (by subunit dissociation) in a reversible manner to block the glycolytic flux toward pyruvate production. This allows accumulation of the glycolytic intermediates used further as synthetic precursors of nucleic acid, lipid, and amino acid synthesis.44 However, dissociation of PKM2 tetramer into dimer depletes the cellular ATP concentration and activates AMP-activated protein kinase (AMPK).45 We suggest that this may have dual advantage because activated AMPK is known to activate p53,46,47 which in turn activates TIGAR (TP53-induced glycolysis and apoptosis regulator).48 The TIGAR protein blocks phosphofructokinase, which probably could save consumption of ATP at this step and channelize more glycolytic intermediates to the anabolic pathway, involving PKM2 (Fig. 1).
Figure 1.
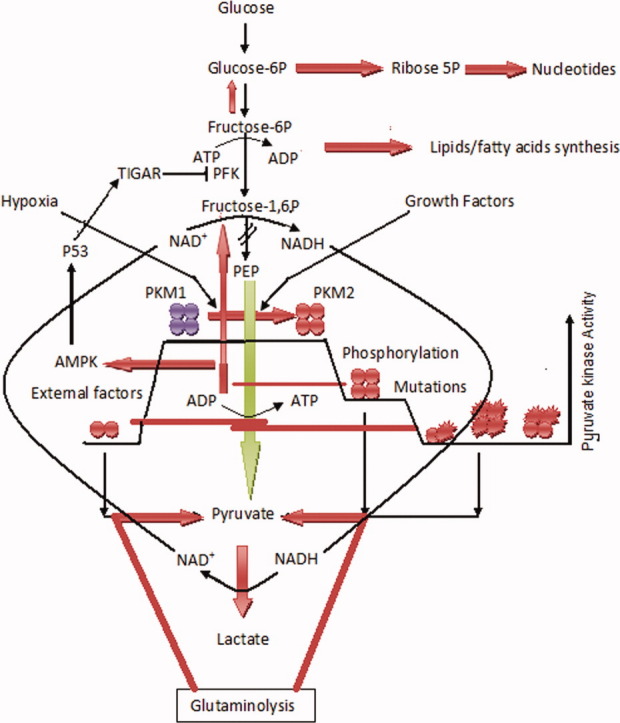
Biological implications of reexpression of M2 isoform of PK enzyme in cells: Downregulation of the enzyme activity by either phosphorylation or dissociation into dimer blocks the pyruvate production and leads in turn to an accumulation of the synthetic precursors to activate nucleic acid and lipid biosynthesis, required for cell division. The pyruvate concentration is compensated by glutaminolysis process. However, the reduced cellular ATP amount as a result of PKM2 inactivation possibly activates TIGAR protein through AMPK-p53 pathway, supplementing similar process by blocking PFK (phosphofructokinase).
The subunit dissociation (tetramer to dimer) is a well-known process for activity downregulation when the availability of FBP is low under physiological conditions.43 Binding of FBP is known to tetramerize the enzyme, whereas its release causes dissociation to dimer. However, as in vitro purified protein is a homotetramer even in the absence of FBP,37 the exact mechanism of dimerization/tetramerization under physiological condition is yet not known. Also, how the factor regulating the oscillating concentration of FBP in cells is yet to be studied. In a phosphor-peptides library screening, it has been observed that some Tyr phosphorylated peptides interact with PKM2 at a site near to FBP-binding pocket and can affect FBP binding. Followed by it, it was seen that FGFR-dependent phosphorylation of PKM2 at Y105 causes its dimerization by the release of FBP leading to Warburg effect.16 Nevertheless, there are many oncogenic proteins that over physical interaction with PKM2 cause its phosphorylation and inactivation.16,19,27
The dissociation of PKM2 into dimer is a reversible process in normally dividing cells as the dimers assemble to high-affinity tetramers and recover with full enzymatic activity later to produce energy (ATP) again. However, the tumor cells incidentally need a permanent supply of glycolytic intermediates,42,43,49,50 where a permanent downregulation of PKM2 activity (by dimer formation) is observed to favor rapid cell proliferation. In short, the dynamic equilibrium between tetramer and dimer maintains a balance between anabolic and catabolic phases of cell metabolism. Recently, the importance of PKM2 in cell growth and cancer progression was highlighted using in vivo mouse model, where the authors also observed an accumulation of lactate in cells overexpressing PKM2. This observation again indicates the metabolic shift in favor of tumor progression, reviving the classical Warburg effect.32,36
The role of PKM2 in tumor development was earlier indicated by the fact that many oncogenic viral pathogens during evolution have chosen PKM2 for their phenotypic effect by inducing its dissociation into dimer after physical interaction.19,51 Some proteins known for cellular growth and proliferation such as A-Raf15,52 and PML protein14 are known to downregulate PKM2 activity by interacting with it. Interaction of PKM2 with growth factor receptor like FGFR-1 (fibrocytes growth factor receptor-1), receptor tyrosine kinase like FlT3 and JAK-2, and oncogenes like BCR-ABL further support to the proposed potential.16 LPA, a mitogenic factor, also interacts with PKM231; recently, Oct4 (octamer-4), a homeodomain transcription factor expressed in normal embryonic stem cells, has been reported as PKM2-interacting partner. Oct 4 is involved in stem cell self-renewal and its knockdown is reported to induce cell differentiation.53 A physical interaction of PKM2 with Oct4 probably indicates their auxiliary function to induce cell division and tumor sustenance under malfunctioning conditions, especially when PKM2 is already known to promote cancer of adult germ cells.22 We suggest that inactive PKM2-dependent phosphometabolite pooling not only supplements nucleogenic metabolic activity but also provides extra advantage to the sustenance of a tumor, possibly by accumulating molecules like 2,3-bisphosphoglycerate (BPG), which in turn bind to deoxyhemoglobin, releasing more oxygen from oxyhemoglobin in tissues.54,55 The increased concentration of BPG has been observed as a result of adaptation in people living at higher altitudes56,57 with low oxygen pressure. PKM2 activity in cancer cells probably serves as a mechanism with dual advantage of nucleic acid synthesis and protection from hypoxia, features associated with tumors. PKM2 has also been hypothesized earlier to promote angiogenesis by binding to TEM-8 (tumor endothelial factor 8).30
Adding another dimension to the study, our laboratory has characterized two independent missense heterozygous mutations in human PKM2 (Fig. 2) from a BS patient and a cell line.39,58 Both the mutations (H391Y and K422R) were present at intersubunit contact domain of the enzyme (Fig. 3) and showed a differential biochemical and functional impact on the protein (Table II). Interestingly, the mutant PKM2 proteins maintained their homotetrameric state till they were expressed independently and showed compromised activity in comparison to wild protein,37 favoring the cell growth and polyploidy.38 When wild and mutant proteins were coexpressed (to mimic in vivo heterozygous state with biallelic expression), it was observed to result in cross monomer (wild-mutant monomer) interaction, producing less active heterodimer and -tetramer forms (Fig. 4).38 The formation of wild-mutant heterodimers had a potential to disturb the dimer:tetramer equilibrium in a dividing cell to promote cell growth in an uncontrolled manner.38 In addition, the structural rigidity attained by H391Y mutant in its tetrameric state suggested adaptation of the protein under possible stressful condition of tumor microenvironment. The case of naturally occurring aberrant PKM2 unraveled an alternative pathway of activity downregulation and provided an explanation for a possible inherent tendency of tumor sustenance in BS.38
Figure 2.

Nucleotide (A) and protein (B) sequence of human PKM2 cDNA highlighting the mutations, H391Y and K422R (red color). The nucleotide sequence was retrieved from NCBI Reference Sequence: NM_002654.3.
Figure 3.
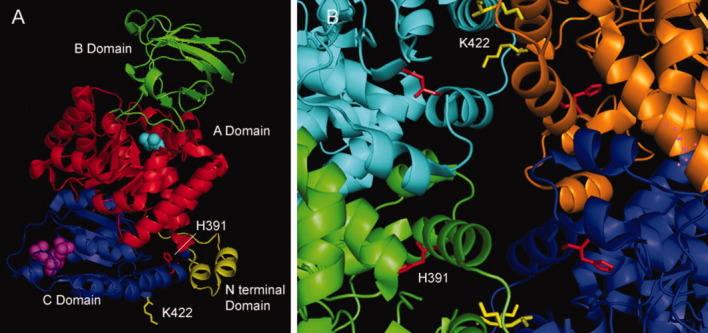
A: A monomer of human PKM2 (PDB id; 1T5a) displaying all four domains with bound PEP structural homolog (Cyan) and FBP (magenta) and His 391 (red) and Lys 422 (yellow) residues. B: An enlarged view of intersubunit contact domain of homotetramer of human PKM2 with the unique position of both the residues (sites of mutation) which had a differential impact over the structural and functional perturbation in the PKM2 molecule as shown in Table II. The figures were generated using molecular viewer tool PyMol.
Table II.
Biochemical and Biological Impacts of Dominant-Negative Heterozygous Mutations (H391Y, K422R)(37,38)
| S. no. | Features | Wild PKM2 | H391Y | K422R |
|---|---|---|---|---|
| 1 | Enzymatic activity | 100% | 80% | 25% |
| 2 | PEP affinity | Normal | Increased by sixfold | Reduced by threefold |
| 3 | Catalytic efficiency | Normal | Increased | Reduced |
| 4 | Oligomeric state | Homotetramer | Homotetramer | Homotetramer |
| 5 | Cooperativity | Allosteric | Completely lost | Increased |
| 6 | Activation by FBP | Yes | No | Yes |
| 7 | Optimum pH | 7.4 | 7.0 | 7.0 |
| 8 | Thermostability | No | Yes | No |
| 9 | α Helical content | Rich | Increased | Increased |
| 10 | Structural rigidity | Absent | Present | Absent |
| When coexpressed with PK-WT in in vitro transfection assays | ||||
| 11 | Cross-monomer interaction | Yes | Yes | Yes |
| 12 | Oligomeric state | Normal | Disturbed | Disturbed |
| 13 | Heterooligomerization | Yes | Yes | Yes |
| 14 | Heterodimerization | No | Yes | Yes |
| 15 | Enzymatic activity | Unaffected | Reduced | Reduced |
| 16 | PEP affinity | Unaffected | Reduced | Reduced |
| 17 | E. coli doubling time | Slightly reduced | Reduced | Reduced |
| 18 | Rate of cell division | Slightly increased | Promoted | Promoted |
| 19 | Polyploidy | Unaffected | Promoted | Promoted |
Figure 4.
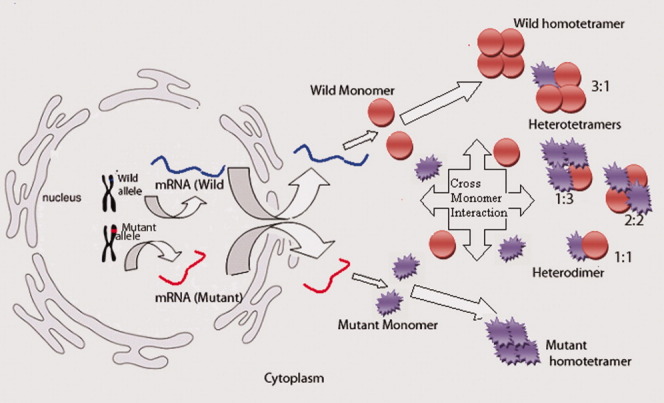
The possible hetero- and homooligomeric structures under heterozygous state of PKM2 based on in silico and experimental observations, suggesting the role of deregulated tetramer–dimer equilibrium and generation of less active tetramers affecting cell division.(38)
PKM2—cellular growth and apoptosis
The first indication of role of PKM2 in cellular growth and apoptosis came from a recent study which showed that PKM2, known to rescue cells from nutritional stress-dependent cell apoptosis, acts as a metabolic sensor, regulating cell growth, proliferation, and apoptosis.59 PKM2 senses the possible scarcity of glucose (nutritional stress) during cell division and dissociates from fully active tetramer to an inactive dimer. This channelizes the available glucose carbon in synthetic precursor formation59 while the scarcity of glucose is compensated by glutaminolysis for the energy (ATP) production. Thus, PKM2 saves the cell from nutritional stress-dependent apoptosis during cell division process (Fig. 5). Some reports also projected PK as a mediator in growth hormone signaling cascade indicating its potential role in maintaining cellular homeostasis for proliferation and apoptosis. A growth inhibiting hormone (somatostatin) caused caspase-independent cellular apoptosis by interacting and localizing the PKM2 in nucleus,13 while some cytokines were found to enhance cellular proliferation involving PKM2 in a similar way.12 In addition, growth factors such as EGFs and hormones are also known to affect the catalytic property (reduced Km) of this enzyme in freshly isolated hepatocytes in favor of cell growth and division60–63 indicating it to be an essential molecular event during cell growth.
Figure 5.
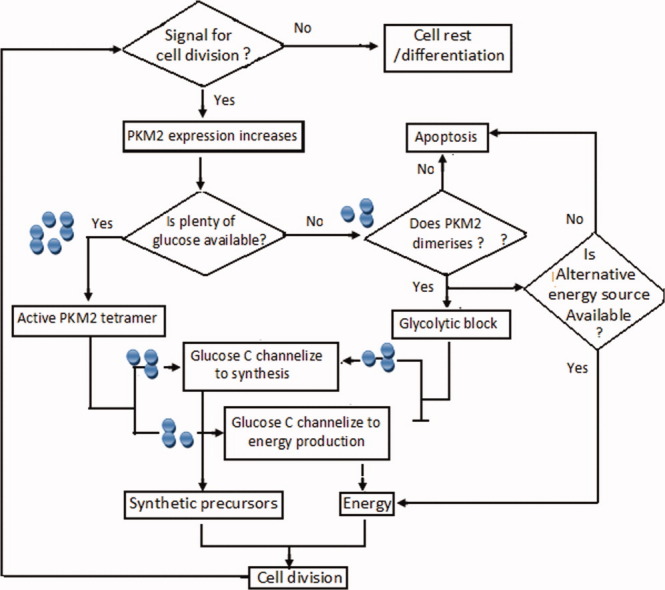
PKM2 acts as a metabolic sensor, regulating the fate of cell in division, differentiation, or apoptosis. In the presence of a growth signal, pyruvate kinase senses the presence of glucose in the medium. Availability of enough glucose distributes the glucose C into anabolic and catabolic pathways equally by maintaining its active tetrameric form and catalyzes the division. Under nutritional stress, cells are saved from apoptosis by dissociation of tetrameric PKM2 into dimer; however, in case the energy is available from an alternate source, it leads to cell division process in cancer situation where glutaminolysis acts as an extracellular energy source.
Further support for PKM2 in cell proliferation and apoptosis comes from a study of Raf kinases and their cross talk.15,52 Raf kinases are the mitogen-activated protein kinases, an important member of cell mitogenic signaling cascade, which transmit signal from receptor tyrosine kinase via Ras, Raf, MEK, and ERK, resulting in cell proliferation and differentiation.64–66 Like PKM2, Raf-A also expresses in rapidly dividing tissues, such as kidney, testis, epidymis, and ovary. Raf kinases are very well known to regulate the glycolysis by “go or stop” mechanism.15 Because PKM2 plays a central role in synthetic and energy generation processes, Raf seems to use this key molecule by physically interacting and consequently inactivating it by its dissociation (into dimer) and phosphorylation in primary fibrocytes. Only the oncogenic form a-Raf is able to reactivate PKM2 by its tetramerization as a secondary metabolic effect possibly induced by high serine level.15 It has been known that coexpression of PKM2 is required with a-Raf for cellular transformation. The dominant-negative mutants of PKM2 have been shown to block the function of a-Raf67 and hence cell growth and division.15
PKM2 and immunological responses
The role of PK in basal immunity comes from a well-known example of a woman with neutrophil deficient in PK (PK-R) activity, showing frequent staphylococcal infections. It was observed that neutrophils with defective PK activity showed defective intracellular killing,68 reflecting on the potency of genetically defective PK to affect the innate immunity and thus conferring susceptibility to infections. In a recent report, PKM2 is found to be highly immunomodulatory by interacting with SOCS3 (suppressor of cytokines signaling 3) resulting in disruption of antigen-presenting ability of dendritic cells.28 Glucose is an instant source of energy for dynamic immune cells to ensure their proper functioning, evident by the observation of T cells which not only upregulate glucose uptake (by upregulating GLUT 1 expression) but also upregulate the rate of glycolysis during interaction with APC (depending upon CD28 receptor on surface). This is to produce instant ATP to ensure a sustained immunogenic response on encountering an antigen.69,70 The defective glycolysis due to PK deficiency is likely to compromise neutrophilic immune response, hence making the parasite survival effortless in the cell.
Another evidence of involvement of PKM2 in immunological responses came from a recent report where PKM2 is shown to interact with IgE receptor on the cells, resulting in the inhibition of its activity.20 Another follow-up study has proposed that this interaction leads to mast cell degranulation, responsible for allergic reactions. It has been proposed that mast cell degranulation might require the FcvarepsilonRI-mediated inhibition of PKM2 activity, thereby showing an inverse correlation between PKM2 activity and mast cell degranulation.21 In yet another study, expression of PKM2 and annexin I was proposed to be important for contributing to granule formation containing TNFα and other mediators in mast cells, playing important role in allergic disease.71 The study showed that PKM2 plays an important role in response to allergens.
A physical interaction of pathogenic proteins like Opa (opacity associated) of Staphylococcus with PKM223 is another indicator of its role in immune modulation. Many other pathogens such as HPV19,35 and HCV17 are also known to modulate PKM2 function, promoting their pathogenesis. In Tourette syndrome, PKM1 has been identified as an antigen.72 The study has demonstrated that PK can serve as autoimmune target for staphylococcal infections. It has been reported that dendritic cells representing M1/M2 peptide can generate allergic myotitis in Balb C mice.73 It is quite likely that the release of dimeric form of PKM2 from cells74 may serve as an antigen and result in an autoimmune reaction in affected patients like BS.
Pyruvate kinase and other possible physiological effects
PKM2 has been speculated to influence other physiological pathways directly or indirectly with a significant impact. Liver PK polymorphism has been shown in association with type II diabetes,75 and the reports suggest that most of the activity of pancreatic PK is governed by M2 isoform of PK.116 The occurrence, therefore, of SNP/mutations in PKM2 becomes relevant to suggest a possible link with the susceptibility to type II diabetes, which indirectly provides opportunity for other metabolic diseases like cancer which has also been associated with diabetes.76,77 The role of PKM2 in both these diseases is explicable because both abnormalities show perturbation in glucose metabolism. The role of PK in cellular dynamics is unfolded by the report of M1 isoform of PK, which is shown to destabilize the microtubules in a PEP (substrate of PK)-dependent manner by physically interacting with it.78,79 Some high dynamic specialized cells, e.g., sperms produce some special cytoskeleton-bound isoform of PK called sperm-specific PK (PK-S),80,81 also indicated its role in cellular dynamics. Physical interaction between PKM2 and c-PML leading to its nuclear localization indicates the role of PKM2 in genomic instability, adding another dimension to PKM2 mutifunctionality.14 Glycolytic enzymes are also known to play important role in vital processes like aging.82 In erythrocytes, the activity of PK is reported to be reduced with age,83,84 and the regulatory power of M2 isozyme in dividing cells (dimer:tetramer equilibrium) has been shown to go down by 90%.85 As the older animals rely maximally on glycolysis, while the use of glycolysis as the only source of energy results in plenty of wasteful synthesis in body, ultimately leading to aging.85 In vitro stimulation with AMP has been shown to induce senescence in human fibroblast,86 a condition expected to be created by mutant (and/or less active) PKM2. Whether aging is a consequence of dysregulated glycolytic enzymes or vice versa is as yet equivocal.
PKM2 Possibly Shares the Burden of Multiphenotypic BS
BS, an autosomal recessive disorder, is prevalent in Ashkenazi Jewish population87–89 or in others.58,90–92 Affected individuals express some unique combination of other phenotypes such as predisposition to cancer,93 type II diabetes,94,95 early aging, growth retardation,96 male-specific infertility,97 hypersensitivity, immunodeficiency,98 and the characteristic feature of genomic instability (high frequency of sister chromatid exchanges, SCEs)93,99–101 and mitotic recombination.102,103 The primary reason of the syndrome is identified as the genetically defective BLM protein (159 kDa).104–107 However, the number of clinical abnormalities in BS patients has remained unexplained by defective BLM protein except for genomic instability. This has left enough scope to look for another probable candidate(s), which could explain the pleiotropic features of BS. In our independent observations, many BS cell lines have shown differentially downregulated PKM2 activity (unpublished work),38 which correlated in some cell lines with the presence of either missense or frame shift mutation in a heterozygous state. It was, therefore, pertinent to consider PKM2 as an active candidate to explain BS features. Further, another missense mutation correlated with the downregulated activity of PKM2, detected in an Indian BS patient.39,58 These reassuring observations suggested to characterize both these missense mutations in heterozygous state to establish the role of malfunctioning PKM2 and extrapolate in developing BS phenotypes.37 Our study has prompted us to suggest for the first time the role of PKM2 toward the inherent susceptibility of BS patients to cancer, although the possibility of any other defective multifunctional protein(s), apart from PKM2, giving rise to multiple phenotypes is not ruled out. (Fig. 6)
Figure 6.
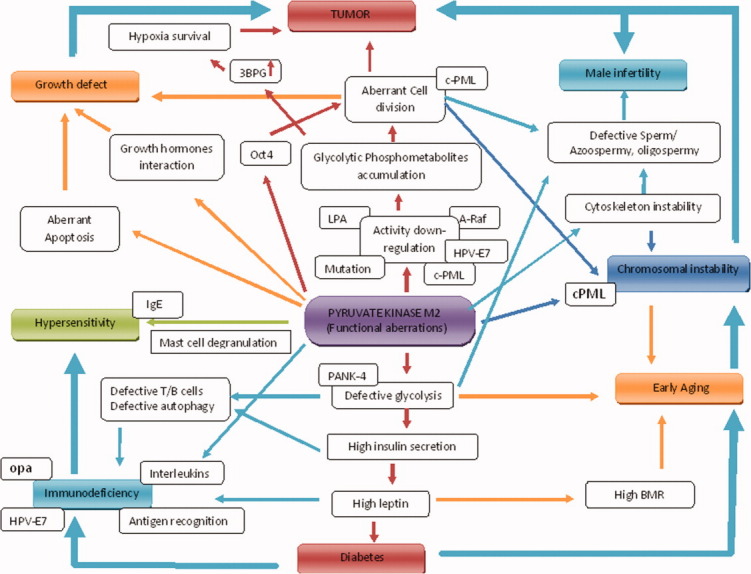
Pyruvate kinase M2, a multifunctional protein with possible implications. Malfunctions of PKM2 may lead to multiple phenotypes as in syndromes like Bloom syndrome. PML, promyelocytic leukemia protein; BMR, basal metabolic rate; Opa, opacity-associated protein; HPV, human papillomavirus; LPA, lysophosphatidic acid; 3BPG, 2,3-bisphosphoglycerate; PANK4, pantothenate kinase 4.
Arguments against “BLM involvement in immunodeficiency”108 and the role of PKM2 deficiency in neutrophils reported for the cause of immunodeficiency68 provide an interesting alternative explanation for the feature of immunodeficiency in the syndrome. In addition, reduced PKM2 activity associated with mast cell degranulation21 could make one susceptible to develop allergic reactions, very frequently observed in BS. Similarly, the early development of type II diabetes in BS may essentially be associated with affected glucose metabolism due to malfunctioning PKM2. Incidentally, the Ashkenazi Jews with a high incidence of BS are genetically susceptible to type II diabetes.109,110 The incredible insulin resistance in BS patients111 and the absence of reports on defective insulin receptor suggest the possibility of an involvement of aberrant key glycolytic enzyme like PKM2. Another typical phenotype of infertility in BS males112 could be explained on the ground of exclusive importance of glycolysis for sperm motility.113–115 For this sperms produce special membrane-bound isoform of PK called sperm-specific PK (PK-S),81 and a genetic-defective PK would render the sperm incapable of fertilizing an ovum. The defective sperm motility, however, could also be defined on the ground of instability of microtubules due to M1 isoform of PK. Interestingly, one of our mutant H391Y of M2 isoform behaved like M1.37 This also causes the abnormal chromosomal movement during cell division leading to genomic instability, a typical feature of BS. It is to be noted that although BLM protein is important to maintain genome stability, there are ∼7% cases of BS with high SCE in normal BLM gene background (James German, personal communication), indicating a probable contribution of some other factors to the occurrence of SCEs, mitotic recombination, and chromosome instability, as observed in BS. A probable role of defective PKM2 in BS condition responsible for such genomic instability features is supported by our observations of increased rate of cellular growth and polyploidy after the overexpression of PKM2 mutants in mammalian cell line.38 Similarly, the role of PK in cellular growth and development (hence the process of aging) could explain the phenomena of growth malfunction including early aging in BS patients. The surmised correlations between PKM2 and a variety of unexplainable clinical features of BS are necessitated because of two major reasons, the support one finds from literature and our own work and inability to explain through the BLM gene implicated in the syndrome. We are confident that future research on the lines suggested directly in Bloom patients and their cells would suggest the importance of the observations made in this review.
Conclusion
In evolution, majority of biomolecules and pathways that evolved to counterstress throughout the ontogeny and were designed to play a role in multiple pathways have remained conserved from bacteria to human, fulfilling the Darwinian paradigm of survival and selection. PKM2 provides one such example of a multifunctional protein, involved in many nonglycolytic pathways, influencing the cellular physiology. Any defect in this “commandant molecule” is expected to lead to a severe multiphenotypic genetic defect. It is only in recent past that a role is being assigned in cellular biology to this molecule; the role, however, of PKM2 is understudied and its significance underestimated, except for its recent role in cancer development. The review widens the scope of research on this multifunctional molecule to look at the possible effects of inhibiting or maneuvering it before being considered as a therapeutic target for cancer.
Glossary
Abbreviations
- BS
Bloom syndrome
- PK
pyruvate kinase
- PKM2
pyruvate kinase M2 isozyme
References
- 1.Kayne FJ, Price NC. Amino acid effector binding to rabbit muscle pyruvate kinase. Arch Biochem Biophys. 1973;159:292–296. doi: 10.1016/0003-9861(73)90455-4. [DOI] [PubMed] [Google Scholar]
- 2.Valentini G, Chiarelli L, Fortin R, Speranza ML, Galizzi A, Mattevi A. The allosteric regulation of pyruvate kinase. J Biol Chem. 2000;275:18145–18152. doi: 10.1074/jbc.M001870200. [DOI] [PubMed] [Google Scholar]
- 3.Yamada K, Noguchi T, Matsuda T, Takenaka M, Monaci P, Nicosia A, Tanaka T. Identification and characterization of hepatocyte-specific regulatory regions of the rat pyruvate kinase L gene. The synergistic effect of multiple elements. J Biol Chem. 1990;265:19885–19891. [PubMed] [Google Scholar]
- 4.Yamada K, Noguchi T. Regulation of pyruvate kinase M gene expression. Biochem Biophys Res Commun. 1999;256:257–262. doi: 10.1006/bbrc.1999.0228. [DOI] [PubMed] [Google Scholar]
- 5.Muirhead H, Clayden DA, Barford D, Lorimer CG, Fothergill-Gilmore LA, Schiltz E, Schmitt W. The structure of cat muscle pyruvate kinase. EMBO J. 1986;5:475–481. doi: 10.1002/j.1460-2075.1986.tb04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boles E, Schulte F, Miosga T, Freidel K, Schluter E, Zimmermann FK, Hollenberg CP, Heinisch JJ. Characterization of a glucose-repressed pyruvate kinase (Pyk2p) in Saccharomyces cerevisiae that is catalytically insensitive to fructose-1,6-bisphosphate. J Bacteriol. 1997;179:2987–2993. doi: 10.1128/jb.179.9.2987-2993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imamura K, Tanaka T. Pyruvate kinase isozymes from rat. Methods Enzymol. 1982;90(Part E):150–165. doi: 10.1016/s0076-6879(82)90121-5. [DOI] [PubMed] [Google Scholar]
- 8.Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261:13807–13812. [PubMed] [Google Scholar]
- 9.Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44:9417–9429. doi: 10.1021/bi0474923. [DOI] [PubMed] [Google Scholar]
- 10.Muirhead H. Isoenzymes of pyruvate kinase. Biochem Soc Trans. 1990;18:193–196. doi: 10.1042/bst0180193. [DOI] [PubMed] [Google Scholar]
- 11.Takenaka M, Noguchi T, Sadahiro S, Hirai H, Yamada K, Matsuda T, Imai E, Tanaka T. Isolation and characterization of the human pyruvate kinase M gene. Eur J Biochem. 1991;198:101–106. doi: 10.1111/j.1432-1033.1991.tb15991.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino A, Hirst JA, Fujii H. Regulation of cell proliferation by interleukin-3-induced nuclear translocation of pyruvate kinase. J Biol Chem. 2007;282:17706–17711. doi: 10.1074/jbc.M700094200. [DOI] [PubMed] [Google Scholar]
- 13.Stetak A, Veress R, Ovadi J, Csermely P, Keri G, Ullrich A. Nuclear translocation of the tumor marker pyruvate kinase M2 induces programmed cell death. Cancer Res. 2007;67:1602–1608. doi: 10.1158/0008-5472.CAN-06-2870. [DOI] [PubMed] [Google Scholar]
- 14.Shimada N, Shinagawa T, Ishii S. Modulation of M2-type pyruvate kinase activity by the cytoplasmic PML tumor suppressor protein. Genes Cells. 2008;13:245–254. doi: 10.1111/j.1365-2443.2008.01165.x. [DOI] [PubMed] [Google Scholar]
- 15.Mazurek S, Drexler HC, Troppmair J, Eigenbrodt E, Rapp UR. Regulation of pyruvate kinase type M2 by A-Raf: a possible glycolytic stop or go mechanism. Anticancer Res. 2007;27:3963–3971. [PubMed] [Google Scholar]
- 16.Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Zhou Y, Zhang K, Liu Q, Guo D. Isoform-specific interaction of pyruvate kinase with hepatitis C virus NS5B. FEBS Lett. 2008;582:2155–2160. doi: 10.1016/j.febslet.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Gonzalo FR, Cruz C, Munoz P, Mazurek S, Eigenbrodt E, Ventura F, Bartrons R, Rosa JL. Interaction between HERC1 and M2-type pyruvate kinase. FEBS Lett. 2003;539:78–84. doi: 10.1016/s0014-5793(03)00205-9. [DOI] [PubMed] [Google Scholar]
- 19.Zwerschke W, Mazurek S, Massimi P, Banks L, Eigenbrodt E, Jansen-Durr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci USA. 1999;96:1291–1296. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oak MH, Cheong H, Kim KM. Activation of Fc epsilon RI inhibits the pyruvate kinase through direct interaction with the gamma-chain. Int Arch Allergy Immunol. 1999;119:95–100. doi: 10.1159/000024183. [DOI] [PubMed] [Google Scholar]
- 21.Ryu H, Walker JK, Kim S, Koo N, Barak LS, Noguchi T, Kang BY, Kim KM. Regulation of M2-type pyruvate kinase mediated by the high-affinity IgE receptors is required for mast cell degranulation. Br J Pharmacol. 2008;154:1035–1046. doi: 10.1038/bjp.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Kim HK, Han YM, Kim J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol. 2008;40:1043–1054. doi: 10.1016/j.biocel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Williams JM, Chen GC, Zhu L, Rest RF. Using the yeast two-hybrid system to identify human epithelial cell proteins that bind gonococcal Opa proteins: intracellular gonococci bind pyruvate kinase via their Opa proteins and require host pyruvate for growth. Mol Microbiol. 1998;27:171–186. doi: 10.1046/j.1365-2958.1998.00670.x. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Chang Y, Zhang L, Feng Q, Liu Z, Zhang Y, Zuo J, Meng Y, Fang F. High glucose upregulates pantothenate kinase 4 (PanK4) and thus affects M2-type pyruvate kinase (Pkm2) Mol Cell Biochem. 2005;277:117–125. doi: 10.1007/s11010-005-5535-1. [DOI] [PubMed] [Google Scholar]
- 25.Siwko S, Mochly-Rosen D. Use of a novel method to find substrates of protein kinase C delta identifies M2 pyruvate kinase. Int J Biochem Cell Biol. 2007;39:978–987. doi: 10.1016/j.biocel.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glossmann H, Presek P, Eigenbrodt E. Association of the src-gene product of Rous sarcoma virus with a pyruvate-kinase inactivation factor. Mol Cell Endocrinol. 1981;23:49–63. doi: 10.1016/0303-7207(81)90116-7. [DOI] [PubMed] [Google Scholar]
- 27.Presek P, Glossmann H, Eigenbrodt E, Schoner W, Rubsamen H, Friis RR, Bauer H. Similarities between a phosphoprotein (pp60src)-associated protein kinase of Rous sarcoma virus and a cyclic adenosine 3′:5′-monophosphate-independent protein kinase that phosphorylates pyruvate kinase type M2. Cancer Res. 1980;40:1733–1741. [PubMed] [Google Scholar]
- 28.Zhang Z, Liu Q, Che Y, Yuan X, Dai L, Zeng B, Jiao G, Zhang Y, Wu X, Yu Y, Yang R. Antigen presentation by dendritic cells in tumors is disrupted by altered metabolism that involves pyruvate kinase M2 and its interaction with SOCS3. Cancer Res. 2010;70:89–98. doi: 10.1158/0008-5472.CAN-09-2970. [DOI] [PubMed] [Google Scholar]
- 29.Spoden GA, Morandell D, Ehehalt D, Fiedler M, Jansen-Durr P, Hermann M, Zwerschke W. The SUMO-E3 ligase PIAS3 targets pyruvate kinase M2. J Cell Biochem. 2009;107:293–302. doi: 10.1002/jcb.22125. [DOI] [PubMed] [Google Scholar]
- 30.Duan HF, Hu XW, Chen JL, Gao LH, Xi YY, Lu Y, Li JF, Zhao SR, Xu JJ, Chen HP, Chen W, Wu CT. Antitumor activities of TEM8-Fc: an engineered antibody-like molecule targeting tumor endothelial marker 8. J Natl Cancer Inst. 2007;99:1551–1555. doi: 10.1093/jnci/djm132. [DOI] [PubMed] [Google Scholar]
- 31.Desmaret S, Qian L, Vanloo B, Meerschaert K, Van Damme J, Grooten J, Vandekerckhove J, Prestwich GD, Gettemans J. Lysophosphatidic acid affinity chromatography reveals pyruvate kinase as a specific LPA-binding protein. Biol Chem. 2005;386:1137–1147. doi: 10.1515/BC.2005.130. [DOI] [PubMed] [Google Scholar]
- 32.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 33.Kato H, Fukuda T, Parkison C, McPhie P, Cheng SY. Cytosolic thyroid hormone-binding protein is a monomer of pyruvate kinase. Proc Natl Acad Sci USA. 1989;86:7861–7865. doi: 10.1073/pnas.86.20.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkison C, Ashizawa K, McPhie P, Lin KH, Cheng SY. The monomer of pyruvate kinase, subtype M1, is both a kinase and a cytosolic thyroid hormone binding protein. Biochem Biophys Res Commun. 1991;179:668–674. doi: 10.1016/0006-291x(91)91424-b. [DOI] [PubMed] [Google Scholar]
- 35.Mazurek S, Zwerschke W, Jansen-Durr P, Eigenbrodt E. Effects of the human papilloma virus HPV-16 E7 oncoprotein on glycolysis and glutaminolysis: role of pyruvate kinase type M2 and the glycolytic-enzyme complex. Biochem J. 2001;356:247–256. doi: 10.1042/0264-6021:3560247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 37.Akhtar K, Gupta V, Koul A, Alam N, Bhat R, Bamezai RN. Differential behavior of missense mutations in the intersubunit contact domain of the human pyruvate kinase M2 isozyme. J Biol Chem. 2009;284:11971–11981. doi: 10.1074/jbc.M808761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta V, Kalaiarasan P, Faheem M, Singh N, Iqbal MA, Bamezai RN. Dominant negative mutations affect oligomerization of human pyruvate kinase M2 isozyme and promote cellular growth and polyploidy. J Biol Chem. 2010;285:16864–16873. doi: 10.1074/jbc.M109.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anitha M, Kaur G, Baquer NZ, Bamezai R. Dominant negative effect of novel mutations in pyruvate kinase-M2. DNA Cell Biol. 2004;23:442–449. doi: 10.1089/1044549041474797. [DOI] [PubMed] [Google Scholar]
- 40.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson EC, Rathmell JC. New roles for pyruvate kinase M2: working out the Warburg effect. Trends Biochem Sci. 2008;33:359–362. doi: 10.1016/j.tibs.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazurek S, Boschek CB, Eigenbrodt E. The role of phosphometabolites in cell proliferation, energy metabolism, and tumor therapy. J Bioenerg Biomembr. 1997;29:315–330. doi: 10.1023/a:1022490512705. [DOI] [PubMed] [Google Scholar]
- 43.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Eigenbrodt E, Reinacher M, Scheefers-Borchel U, Scheefers H, Friis R. Double role for pyruvate kinase type M2 in the expansion of phosphometabolite pools found in tumor cells. Crit Rev Oncog. 1992;3:91–115. [PubMed] [Google Scholar]
- 45.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase—development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Okoshi R, Ozaki T, Yamamoto H, Ando K, Koida N, Ono S, Koda T, Kamijo T, Nakagawara A, Kizaki H. Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J Biol Chem. 2008;283:3979–3987. doi: 10.1074/jbc.M705232200. [DOI] [PubMed] [Google Scholar]
- 48.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 49.Eigenbrodt E, Kallinowski F, Ott M, Mazurek S, Vaupel P. Pyruvate kinase and the interaction of amino acid and carbohydrate metabolism in solid tumors. Anticancer Res. 1998;18:3267–3274. [PubMed] [Google Scholar]
- 50.Mazurek S, Grimm H, Boschek CB, Vaupel P, Eigenbrodt E. Pyruvate kinase type M2: a crossroad in the tumor metabolome. Br J Nutr. 2002;87(Suppl 1):S23–S29. [PubMed] [Google Scholar]
- 51.Presek P, Reinacher M, Eigenbrodt E. Pyruvate kinase type M2 is phosphorylated at tyrosine residues in cells transformed by Rous sarcoma virus. FEBS Lett. 1988;242:194–198. doi: 10.1016/0014-5793(88)81014-7. [DOI] [PubMed] [Google Scholar]
- 52.Mazurek S. Pyruvate kinase type M2: a key regulator within the tumour metabolome and a tool for metabolic profiling of tumours. Ernst Schering Found Symp Proc. 2007;4:99–124. doi: 10.1007/2789_2008_091. [DOI] [PubMed] [Google Scholar]
- 53.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 54.Benesch R, Benesch RE. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967;26:162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- 55.Gupta RK, Benovic JL, Rose ZB. Location of the allosteric site for 2,3-bisphosphoglycerate on human oxy- and deoxyhemoglobin as observed by magnetic resonance spectroscopy. J Biol Chem. 1979;254:8250–8255. [PubMed] [Google Scholar]
- 56.Torrance JD, Lenfant C, Cruz J, Marticorena E. Oxygen transport mechanisms in residents at high altitude. Respir Physiol. 1970;11:1–15. doi: 10.1016/0034-5687(70)90098-8. [DOI] [PubMed] [Google Scholar]
- 57.Samaja M, Di Prampero PE, Cerretelli P. The role of 2,3-DPG in the oxygen transport at altitude. Respir Physiol. 1986;64:191–202. doi: 10.1016/0034-5687(86)90041-1. [DOI] [PubMed] [Google Scholar]
- 58.Reddy BS, Kochhar AM, Anitha M, Bamezai R. Bloom's syndrome—a first report from India. Int J Dermatol. 2000;39:760–763. doi: 10.1046/j.1365-4362.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- 59.Spoden GA, Rostek U, Lechner S, Mitterberger M, Mazurek S, Zwerschke W. Pyruvate kinase isoenzyme M2 is a glycolytic sensor differentially regulating cell proliferation, cell size and apoptotic cell death dependent on glucose supply. Exp Cell Res. 2009;315:2765–2774. doi: 10.1016/j.yexcr.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 60.Taunton OD, Stifel FB, Greene HL, Herman RH. Rapid reciprocal changes of hepatic glycolytic enzymes and fructose-1,6-diphosphatase following glucagon and insulin injection in vivo. Biochem Biophys Res Commun. 1972;48:1663–1670. doi: 10.1016/0006-291x(72)90906-0. [DOI] [PubMed] [Google Scholar]
- 61.Blair JB, Cimbala MA, Foster JL, Morgan RA. Hepatic pyruvate kinase. Regulation by glucagon, cyclic adenosine 3′-5′-monophosphate, and insulin in the perfused rat liver. J Biol Chem. 1976;251:3756–3762. [PubMed] [Google Scholar]
- 62.Feliu JE, Hue L, Hers HG. Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proc Natl Acad Sci USA. 1976;73:2762–2766. doi: 10.1073/pnas.73.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Berkel TJ, Kruijt JK, Koster JF, Hulsmann WC. Cyclic nucleotide-, pyruvate- and hormone-induced changes in pyruvate kinase activity in isolated rat hepatocytes. Biochem Biophys Res Commun. 1976;72:917–925. doi: 10.1016/s0006-291x(76)80219-7. [DOI] [PubMed] [Google Scholar]
- 64.Naumann U, Eisenmann-Tappe I, Rapp UR. The role of Raf kinases in development and growth of tumors. Recent Results Cancer Res. 1997;143:237–244. doi: 10.1007/978-3-642-60393-8_16. [DOI] [PubMed] [Google Scholar]
- 65.Mahon ES, Hawrysh AD, Chagpar RB, Johnson LM, Anderson DH. A-Raf associates with and regulates platelet-derived growth factor receptor signalling. Cell Signal. 2005;17:857–868. doi: 10.1016/j.cellsig.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, Tzivion G. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196–1212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Mellay V, Houben R, Troppmair J, Hagemann C, Mazurek S, Frey U, Beigel J, Weber C, Benz R, Eigenbrodt E, Rapp UR. Regulation of glycolysis by Raf protein serine/threonine kinases. Adv Enzyme Regul. 2002;42:317–332. doi: 10.1016/s0065-2571(01)00036-x. [DOI] [PubMed] [Google Scholar]
- 68.Burge PS, Johnson WS, Hayward AR. Neutrophil pyruvate kinase deficiency with recurrent staphylococcal infections: first reported case. Br Med J. 1976;1:742–745. doi: 10.1136/bmj.1.6012.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 70.Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol. 2004;25:193–200. doi: 10.1016/j.it.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Kim JY, Kim DY, Ro JY. Granule formation in NGF-cultured mast cells is associated with expressions of pyruvate kinase type M2 and annexin I proteins. Int Arch Allergy Immunol. 2008;146:287–297. doi: 10.1159/000121463. [DOI] [PubMed] [Google Scholar]
- 72.Kansy JW, Katsovich L, McIver KS, Pick J, Zabriskie JB, Lombroso PJ, Leckman JF, Bibb JA. Identification of pyruvate kinase as an antigen associated with Tourette syndrome. J Neuroimmunol. 2006;181:165–176. doi: 10.1016/j.jneuroim.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawachi I, Tanaka K, Tanaka M, Tsuji S. Dendritic cells presenting pyruvate kinase M1/M2 isozyme peptide can induce experimental allergic myositis in BALB/c mice. J Neuroimmunol. 2001;117:108–115. doi: 10.1016/s0165-5728(01)00327-7. [DOI] [PubMed] [Google Scholar]
- 74.Ewald N, Schaller M, Bayer M, Akinci A, Bretzel RG, Kloer HU, Hardt PD. Fecal pyruvate kinase-M2 (tumor M2-PK) measurement: a new screening concept for colorectal cancer. Anticancer Res. 2007;27:1949–1952. [PubMed] [Google Scholar]
- 75.Wang H, Chu W, Das SK, Ren Q, Hasstedt SJ, Elbein SC. Liver pyruvate kinase polymorphisms are associated with type 2 diabetes in northern European Caucasians. Diabetes. 2002;51:2861–2865. doi: 10.2337/diabetes.51.9.2861. [DOI] [PubMed] [Google Scholar]
- 76.Adami HO, McLaughlin J, Ekbom A, Berne C, Silverman D, Hacker D, Persson I. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991;2:307–314. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 77.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 78.Vertessy BG, Bankfalvi D, Kovacs J, Low P, Lehotzky A, Ovadi J. Pyruvate kinase as a microtubule destabilizing factor in vitro. Biochem Biophys Res Commun. 1999;254:430–435. doi: 10.1006/bbrc.1998.9957. [DOI] [PubMed] [Google Scholar]
- 79.Kovacs J, Low P, Pacz A, Horvath I, Olah J, Ovadi J. Phosphoenolpyruvate-dependent tubulin-pyruvate kinase interaction at different organizational levels. J Biol Chem. 2003;278:7126–7130. doi: 10.1074/jbc.M210244200. [DOI] [PubMed] [Google Scholar]
- 80.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feiden S, Stypa H, Wolfrum U, Wegener G, Kamp G. A novel pyruvate kinase (PK-S) from boar spermatozoa is localized at the fibrous sheath and the acrosome. Reproduction. 2007;134:81–95. doi: 10.1530/REP-06-0250. [DOI] [PubMed] [Google Scholar]
- 82.Chapman RG, Schaumburg L. Glycolysis and glycolytic enzyme activity of aging red cells in man. Changes in hexokinase, aldolase, glyceraldehyde-3-phosphate dehydrogenase, pyruvate kinase and glutamic-oxalacetic transaminase. Br J Haematol. 1967;13:665–678. doi: 10.1111/j.1365-2141.1967.tb08832.x. [DOI] [PubMed] [Google Scholar]
- 83.Luque J, Delgado MD, Rodriguez-Horche P, Company MT, Pinilla M. Bisphosphoglycerate mutase and pyruvate kinase activities during maturation of reticulocytes and ageing of erythrocytes. Biosci Rep. 1987;7:113–119. doi: 10.1007/BF01121874. [DOI] [PubMed] [Google Scholar]
- 84.Jimeno P, Garcia-Perez AI, Luque J, Pinilla M. Changes in glycolytic enzyme activities in aging erythrocytes fractionated by counter-current distribution in aqueous polymer two-phase systems. Biochem J. 1991;279(Part 1):237–243. doi: 10.1042/bj2790237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arkin R. The biology of aging, observation and principle. 3rd Ed. Oxford University Press, USA: 2006. Chapter 6; p. 208. [Google Scholar]
- 86.Zwerschke W, Mazurek S, Stockl P, Hutter E, Eigenbrodt E, Jansen-Durr P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem J. 2003;376:403–411. doi: 10.1042/BJ20030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shahrabani-Gargir L, Shomrat R, Yaron Y, Orr-Urtreger A, Groden J, Legum C. High frequency of a common Bloom syndrome Ashkenazi mutation among Jews of Polish origin. Genet Test. 1998;2:293–296. doi: 10.1089/gte.1998.2.293. [DOI] [PubMed] [Google Scholar]
- 88.Oddoux C, Clayton CM, Nelson HR, Ostrer H. Prevalence of Bloom syndrome heterozygotes among Ashkenazi Jews. Am J Hum Genet. 1999;64:1241–1243. doi: 10.1086/302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roa BB, Savino CV, Richards CS. Ashkenazi Jewish population frequency of the Bloom syndrome gene 2281 delta 6ins7 mutation. Genet Test. 1999;3:219–221. doi: 10.1089/gte.1999.3.219. [DOI] [PubMed] [Google Scholar]
- 90.Ellis NA, Ciocci S, Proytcheva M, Lennon D, Groden J, German J. The Ashkenazic Jewish Bloom syndrome mutation blmAsh is present in non-Jewish Americans of Spanish ancestry. Am J Hum Genet. 1998;63:1685–1693. doi: 10.1086/302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peleg L, Pesso R, Goldman B, Dotan K, Omer M, Friedman E, Berkenstadt M, Reznik-Wolf H, Barkai G. Bloom syndrome and Fanconi's anemia: rate and ethnic origin of mutation carriers in Israel. Isr Med Assoc J. 2002;4:95–97. [PubMed] [Google Scholar]
- 92.Kaneko H, Isogai K, Fukao T, Matsui E, Kasahara K, Yachie A, Seki H, Koizumi S, Arai M, Utunomiya J, Miki Y, Kondo N. Relatively common mutations of the Bloom syndrome gene in the Japanese population. Int J Mol Med. 2004;14:439–442. [PubMed] [Google Scholar]
- 93.Amor-Gueret M. Bloom syndrome, genomic instability and cancer: the SOS-like hypothesis. Cancer Lett. 2006;236:1–12. doi: 10.1016/j.canlet.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 94.Mori S, Kondo N, Motoyoshi F, Yamaguchi S, Kaneko H, Orii T. Diabetes mellitus in a young man with Bloom's syndrome. Clin Genet. 1990;38:387–390. doi: 10.1111/j.1399-0004.1990.tb03601.x. [DOI] [PubMed] [Google Scholar]
- 95.Kondo N, Asano J, Kimura S, Asano T, Orii T. Insulin-dependent diabetes developed in a young man with Bloom's syndrome. Clin Genet. 1991;40:251–252. doi: 10.1111/j.1399-0004.1991.tb03088.x. [DOI] [PubMed] [Google Scholar]
- 96.Keller C, Keller KR, Shew SB, Plon SE. Growth deficiency and malnutrition in Bloom syndrome. J Pediatr. 1999;134:472–479. doi: 10.1016/s0022-3476(99)70206-4. [DOI] [PubMed] [Google Scholar]
- 97.Moens PB, Freire R, Tarsounas M, Spyropoulos B, Jackson SP. Expression and nuclear localization of BLM, a chromosome stability protein mutated in Bloom's syndrome, suggest a role in recombination during meiotic prophase. J Cell Sci. 2000;113(Part 4):663–672. doi: 10.1242/jcs.113.4.663. [DOI] [PubMed] [Google Scholar]
- 98.Hutteroth TH, Litwin SD, German J. Abnormal immune responses of Bloom's syndrome lymphocytes in vitro. J Clin Invest. 1975;56:1–7. doi: 10.1172/JCI108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pedrazzi G, Perrera C, Blaser H, Kuster P, Marra G, Davies SL, Ryu GH, Freire R, Hickson ID, Jiricny J, Stagljar I. Direct association of Bloom's syndrome gene product with the human mismatch repair protein MLH1. Nucleic Acids Res. 2001;29:4378–4386. doi: 10.1093/nar/29.21.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Honma M, Tadokoro S, Sakamoto H, Tanabe H, Sugimoto M, Furuichi Y, Satoh T, Sofuni T, Goto M, Hayashi M. Chromosomal instability in B-lymphoblasotoid cell lines from Werner and Bloom syndrome patients. Mutat Res. 2002;520:15–24. doi: 10.1016/s1383-5718(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 101.Wu L, Hickson ID. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 102.Chaganti RS, Schonberg S, German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci USA. 1974;71:4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bamezai R, Shiraishi Y. Three-way differentiation of sister chromatids in endoreduplicated (M3) chromosomes of Bloom syndrome B-lymphoid cell line. Hum Genet. 1987;75:239–243. doi: 10.1007/BF00281066. [DOI] [PubMed] [Google Scholar]
- 104.Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, German J. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–666. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 105.Karow JK, Chakraverty RK, Hickson ID. The Bloom's syndrome gene product is a 3′-5′ DNA helicase. J Biol Chem. 1997;272:30611–30614. doi: 10.1074/jbc.272.49.30611. [DOI] [PubMed] [Google Scholar]
- 106.Karow JK, Constantinou A, Li JL, West SC, Hickson ID. The Bloom's syndrome gene product promotes branch migration of holliday junctions. Proc Natl Acad Sci USA. 2000;97:6504–6508. doi: 10.1073/pnas.100448097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.German J, Sanz MM, Ciocci S, Ye TZ, Ellis NA. Syndrome-causing mutations of the BLM gene in persons in the Bloom's Syndrome Registry. Hum Mutat. 2007;28:743–753. doi: 10.1002/humu.20501. [DOI] [PubMed] [Google Scholar]
- 108.Sack SZ, Liu Y, German J, Green NS. Somatic hypermutation of immunoglobulin genes is independent of the Bloom's syndrome DNA helicase. Clin Exp Immunol. 1998;112:248–254. doi: 10.1046/j.1365-2249.1998.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Permutt MA, Wasson JC, Suarez BK, Lin J, Thomas J, Meyer J, Lewitzky S, Rennich JS, Parker A, DuPrat L, Maruti S, Chayen S, Glaser B. A genome scan for type 2 diabetes susceptibility loci in a genetically isolated population. Diabetes. 2001;50:681–685. doi: 10.2337/diabetes.50.3.681. [DOI] [PubMed] [Google Scholar]
- 110.Bronstein M, Pisante A, Yakir B, Darvasi A. Type 2 diabetes susceptibility loci in the Ashkenazi Jewish population. Hum Genet. 2008;124:101–104. doi: 10.1007/s00439-008-0520-x. [DOI] [PubMed] [Google Scholar]
- 111.Diaz A, Vogiatzi MG, Sanz MM, German J. Evaluation of short stature, carbohydrate metabolism and other endocrinopathies in Bloom's syndrome. Horm Res. 2006;66:111–117. doi: 10.1159/000093826. [DOI] [PubMed] [Google Scholar]
- 112.German J, Bloom D, Passarge E, Fried K, Goodman RM, Katzenellenbogen I, Laron Z, Legum C, Levin S, Wahrman Bloom's syndrome. VI. The disorder in Israel and an estimation of the gene frequency in the Ashkenazim. Am J Hum Genet. 1977;29:553–562. [PMC free article] [PubMed] [Google Scholar]
- 113.Ford WC. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update. 2006;12:269–274. doi: 10.1093/humupd/dmi053. [DOI] [PubMed] [Google Scholar]
- 114.Miki K. Energy metabolism and sperm function. Soc Reprod Fertil Suppl. 2007;65:309–325. [PubMed] [Google Scholar]
- 115.Nascimento JM, Shi LZ, Tam J, Chandsawangbhuwana C, Durrant B, Botvinick EL, Berns MW. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J Cell Physiol. 2008;217:745–751. doi: 10.1002/jcp.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shih DQ, Screenan S, Munoz KN, Philipson L, Pontoglio M, Yaniv M, Polonsky KS, Stoffe M. Loss of HNF-1 function in mice leads to abnormal expression of genes involved in pancreatic islet development and metabolism. Diabetes. 2001;50:2472–2480. doi: 10.2337/diabetes.50.11.2472. [DOI] [PubMed] [Google Scholar]


