Abstract
Objective
Centers for Medicare and Medicaid Services (CMS) reimbursement criteria for carotid artery stenting (CAS) require that patients be high surgical risk or enrolled in a clinical trial. This may bias comparisons of CAS and carotid endarterectomy (CEA). We evaluate mortality and stroke following CAS and CEA stratified by medical high risk criteria.
Methods
The Nationwide Inpatient Sample (2004-2007) was queried by ICD-9 code for CAS and CEA with diagnosis of carotid artery stenosis. Medical high risk criteria were identified for each patient including patients undergoing a coronary artery bypass and/or valve repair (CABG/V) during the same admission. Symptom status was defined by history of stroke, TIA, and/or amarosis fugax. The primary outcome was postoperative death, stroke (complication code 997.02), and combined stroke or death, stratified by high risk versus non-high risk status and symptom status.
Results
56,564 (10.5%) CAS and 482,394 (89.5%) CEA were identified. Half of the patients in each group were high risk. CABG/V was performed less commonly with CAS than CEA (2.8% vs. 4.0%, P<.001). Patients undergoing CAS were more likely symptomatic than those undergoing CEA (13.1% vs. 9.4%, P < .001). Mortality was higher after CAS than CEA for both high risk and non-high risk patients. Stroke was also higher after CAS for both high risk and non-high risk patients. Combined stroke or death was higher after CAS again for both high risk (asymptomatic 1.5% vs. 1.2%, P < .05, symptomatic 14.4% vs. 6.9%, P < .001) and non-high risk (asymptomatic 1.8% vs. 0.6%, P < .001, symptomatic 11.8% vs. 4.9%, P < .001). Combined stroke or death for patients undergoing CABG/V during the same admission was similar for CAS and CEA (4.8% vs. 3.2%, P = .19). Multivariate predictors of combined stroke or death adjusted for age and gender included CAS vs. CEA (OR 2.4, P < .001), symptom status (OR 6.8, P < .001), high risk (OR 1.6, P < .001), and earlier year of procedure (OR 1.1, P < .01).
Conclusions
In the United States from 2004 to 2007 CAS has a higher risk of stroke and death than CEA after adjustment for medical high risk criteria. Further analysis with prospective assessment of risk factors is needed to guide appropriate patient selection for CEA and CAS in the general population.
Introduction
Carotid artery stenting (CAS) emerged as an alternative to carotid endarterectomy (CEA) for carotid artery stenosis in 1989.1-3 Since that time, with evolving technology and experience, comparative studies have shown varying results. Randomized trials and registries have also yielded conflicting results.1-13 The Centers for Medicare and Medicaid Services (CMS) criteria for reimbursement of CAS mandates that patients be “high risk for CEA” with symptomatic >70% stenosis unless enrolled in a clinical trial. Medical high risk criteria are predominantly cardiac conditions including recent myocardial infarction, severe congestive heart failure (CHF), need for coronary revascularization or valve repair within 30 days, and unstable angina in addition to severe pulmonary and end-stage renal disease (Table I).14 Therefore comparisons of the two techniques may be biased by over representation of high risk and symptomatic patients in the CAS cohort. For this study we compare outcomes of CAS and CEA accounting for symptom status and high risk status.
Table I.
CMS High Risk Criteria.
| CMS High Risk Criteria | |
|---|---|
| Medical High Risk | ICD-9 Code |
| Age >80* | |
| Renal failure* | 585.3-585.9, 586, V42.0, V 45.1, V56.0-V56.32, V6.8, V45.11-V45.12 |
| Severe chronic lung disease* | 490-492.8, 493, 494-494.1, 495-505, 506.4 |
| Recent myocardial infarction* | 412 |
| LV ejection fraction <30% | |
| Requirement for aortocoronary bypass or cardiac valve surgery within 30 days* | 36.11- 36.14, 35 |
| Unstable angina* | 411.1 |
| Class III/IV congestive heart failure* | 428 |
Identified within the current study by ICD-9 coding
Methods
Database
The Nationwide Inpatient Sample (NIS) from 2004 to 2007 was used for this study. The NIS, maintained through the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality, currently includes over 8 million annual hospitalizations from 40 states. The sampling frame allows the NIS to represent approximately 90% of all hospitalizations, making it the largest all-payer inpatient database in the United States.15
Data Retrieval
The NIS was queried for patient selection using diagnosis and procedure codes from the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) system codes. The procedure code for CAS was introduced in October of 2004 and, at the time of study initiation, NIS data was available through 2007. Procedure codes coupled with diagnosis codes were used to identify patients undergoing either carotid endarterectomy (38.12) or carotid artery stenting (00.61, 00.63) for carotid artery stenosis (443.10, 443.11, 443.30, 443.31). Additionally, patients having coronary artery bypass (CABG) (36.11-36.16), cardiac valve repair (35), percutaneous coronary artery intervention (PCI) (00.66, 36.01, 36.02, 36.05, 36.06, 36.07), or a diagnostic cardiac catheterization (37.21, 37.22, 37.23) during the same hospital stay were identified. Cardiac procedures were recorded in a hierarchical schema (in order listed) to prevent dual counting. PCI and diagnostic cardiac catheterizations were included for descriptive purposes only and did not factor into high risk status. Patients admitted with a primary diagnosis of an acute myocardial infarction (410) were excluded from analysis as were those <18years of age. Patients with symptomatic carotid stenosis were identified by ICD-9 diagnosis codes of transient ischemic attack (TIA) (435 or 781.4), amarosis fugax (362.34 or 368.12), or stroke (433.11, 433.31, 433.91, 434.01, 434.11, 434.91).
ICD-9 diagnosis codes were also used to identify comorbid conditions. Age, gender, and race were recorded as demographic variables. General comorbid conditions were recorded as well as those that would qualify the patient for CMS medical high risk (Table I). High risk as determined by available ICD-9 coding was included as a composite variable for comparison and adjustment of outcomes. We also performed analysis of individual high risk criteria to determine relative impact. Patients without any of these factors were classified as non-high risk patients. Primary outcomes were death, stroke (997.02), and combined stroke or death occurring during the hospitalization. Secondary outcomes included complications, hospital length of stay, and hospital costs. The complications recorded were global complications (996-999), acute renal failure (584), and cardiac complications (997.1).
Statistical Analysis
Queries of the NIS data were performed with SAS (version 9.1, SAS Institute, Cary, NC) and all statistical analyses were performed using STATA statistical computing software (Stata Statistical Software: Release 8.2. College Station, TX: StataCorp LP). Population estimates were calculated based upon the weighted discharge values within the NIS. All presented information reflects weighted estimates. Comparisons between CEA and CAS as well as carotid procedures with and without CABG/valve surgery were performed using Student T-test for (parametric data) or Wilcoxon rank-sum (non-parametric data) for continuous measures and Chi-square tests for categorical variables. Univariate logistic regression was used to assess significance of predictive variables for stroke or death as well as mortality alone. Multivariate logistic regression analysis was performed by backwards selection of variables obtaining significance at the P < 0.1 level on univariate analysis with adjustment for high risk criteria. Statistical significance was determined by a P-value of < .05.
Results
There were 56,564 (10.5%) patients who underwent CAS and 482,394 (89.5%) patients who underwent CEA from 2004-2007. CAS increased from 8.5% of carotid revascularizations in 2004 (October – December) to 12.3% in 2007 with the largest percentage in 2006 at 16.1% (P < .001). CABG/V was performed more frequently during the same hospitalization with CEA (4.0%) than CAS (2.8%, P < .001) (Figure 1). Comparatively, a greater number of CAS patients had a PCI (1.0% vs. 0.4%, P < .001) or a diagnostic cardiac catheterization (3.1% vs. 1.7%, P < .001).
Figure 1.
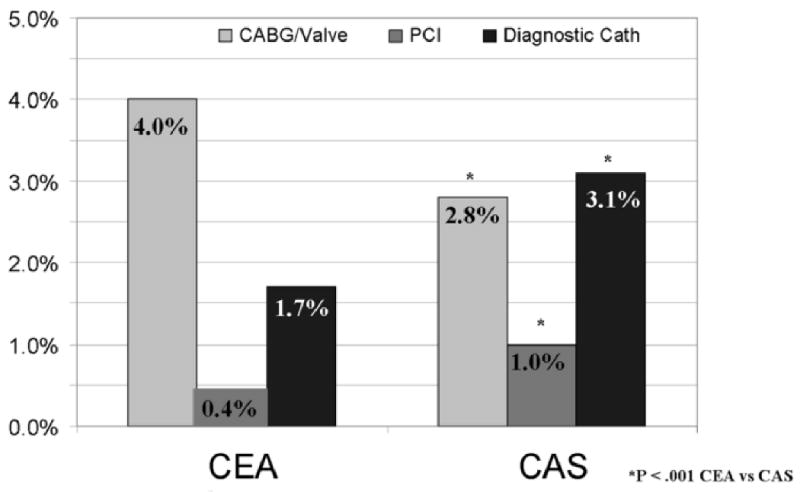
Cardiac Procedures Performed During Hospitalization for Carotid Endarterectomy or Carotid Artery Stenting from October 2004 through 2007.
Half of all patients for both methods of carotid repair were high risk (CAS 50.5% vs. CEA 50.8%, P = .58) (Table II). Patients undergoing CAS had higher rates of CHF and chronic renal failure and lower rates of COPD and urgent heart surgery (CABG/V). Age was similar although statistically significant between the two groups (CAS 69.8 +/- 11.3 years vs. CEA 71.1 +/- 9.5 years, P < .001) and the proportion of patients equal to or greater than 80 years was similar (19.7% vs. 20.1%, P = .69). There was a slightly greater proportion of males undergoing CAS (60.2%) compared to CEA overall (57.5%, P < .001), however among patients receiving concurrent CABG/Valve, there was a greater proportion of males undergoing CEA (68.6%) compared to CAS (57.9%, P < .05).
Table II.
Concurrent Cardiac Procedures, Symptom Status, and Demographics of Patients Undergoing Carotid Repair from the Nationwide Inpatient Sample 2004-2007.
| CAS N=56,564, 10.5% |
CEA N=482,394, 89.5% |
P-value | |
|---|---|---|---|
| High Risk | 28,573, 50.5% | 244,991, 50.8% | .58 |
| Age ≥ 80 Years | 11,168, 19.7% | 96,815, 20.1% | .41 |
| Renal failure | 4,218, 7.5% | 22,925, 4.8% | < .001 |
| Chronic lung disease | 10,546, 18.6% | 104,396, 21.6% | < .001 |
| Prior myocardial infarction | 5,840, 10.3% | 52,160, 10.8% | .11 |
| Congestive heart failure | 6,166, 10.9% | 35,346, 7.3% | < .001 |
| CABG/Valve | 1,592, 2.8% | 19,217, 4.0% | < .001 |
| Unstable angina | 730, 1.3% | 4,703, 1.0% | < .01 |
| Symptomatic | 7,438, 13.1% | 45,499, 9.4% | < .001 |
| Age (mean +/- SD) | 69.8 +/- 11.3 | 71.1 +/- 9.5 | < .001 |
| Male | 24,306, 60.2% | 210,805, 57.5% | < .001 |
There was a greater proportion of symptomatic patients undergoing CAS compared to CEA (13.1% vs. 9.4%, P <. 001) (Table II). This trend persisted although was not statistically significant for patients undergoing concurrent CABG/Valve (12.0% vs. 8.8%, P = .06). Symptom status for patients undergoing PCI (14.3% vs. 13.5%, P = .82) or diagnostic catheterization (15.5% vs. 16.4%) was similar for CAS and CEA.
Stroke or Death
In the overall cohort, combined stroke or death was higher following CAS (3.1%) than CEA (1.4%, P < .001) (Table III). This was true for both symptomatic (13.1% vs. 5.9%, P < .001) and asymptomatic patients (1.6% vs. 0.9%, P < .001). Combined stroke or death for patients with high risk was also higher after CAS than CEA (3.2% vs. 1.8%, P < .001). Again, this was significant for both symptomatic (14.4% vs. 7.0% P < .001) and asymptomatic patients (1.6% vs. 1.2%, P < .05). For non-high risk patients, combined stroke or death rates were also higher after CAS overall (3.1% vs. 1.0%, P < .001) and for symptomatic (11.8% vs. 4.9%, P < .001) and asymptomatic patients (1.8% vs. 0.6%, P < .001).
Table III.
Outcomes Following Carotid Repair and Concurrent Cardiac Procedures from the Nationwide Inpatient Sample 2004-2007.
| CAS N=56,564, 10.5% |
CEA N=482,394, 89.5% |
P-value | ||||
|---|---|---|---|---|---|---|
| Stroke or Death | 1,780, 3.2% | 6,670, 1.4% | < .001 | |||
| -High Risk | 906, 3.2% | 4,304, 1.8% | < .001 | |||
| -Non-High Risk | 874, 3.1% | 2,366, 1.0% | < .001 | |||
| Sx | Asx | Sx | Asx | |||
| 973, 13.1% | 807, 1.6% | 2,698, 5.9% | 3,973, 0.9% | < .001 | < .001 | |
| -High Risk | 14.4% | 1.5% | 6.9% | 1.2% | < .001 | < .05 |
| -Non-High Risk | 11.8% | 1.8% | 4.9% | 0.6% | < .001 | < .001 |
| Mortality | 846, 1.5% | 2,432, 0.5% | < .001 | |||
| -High Risk | 430, 1.5% | 1,941, 0.8% | < .001 | |||
| -Non-High Risk | 416, 1.5% | 491, 0.2% | < .001 | |||
| Sx | Asx | Sx | Asx | |||
| 448, 6.0% | 398, 0.8% | 814, 1.8% | 1,618, 0.4% | < .001 | < .001 | |
| -High Risk | 6.8% | 0.7% | 2.5% | 0.6% | < .001 | .28 |
| -Non-High Risk | 5.3% | 0.9% | 1.0% | 0.1% | < .001 | < .001 |
| Stroke | 1,093, 1.9% | 4,727, 1.0% | <.001 | |||
| -High Risk | 560, 2.0% | 2,723, 1.1% | < .001 | |||
| -Non-High Risk | 533, 1.9% | 2,004, 0.8% | < .001 | |||
| Sx | Asx | Sx | Asx | |||
| 603, 8.1% | 490, 1.0% | 2,099, 4.6% | 2,628, 0.6% | < .001 | < .001 | |
| -High Risk | 8.8% | 1.0% | 5.0% | 0.7% | < .001 | < .05 |
| -Non-High Risk | 7.5% | 1.0% | 4.2% | 0.5% | < .001 | < .001 |
| Global Complications (High Risk) | 13.6% (14.9%) | 9.6% (11.9%) | < .001 (< .001) | |||
| Cardiac Complications (High Risk) | 1.9% (2.2%) | 1.8% (2.4%) | .27 (.35) | |||
| Acute Renal Failure (High Risk) | 1.9% (2.9%) | 1.3% (2.3%) | < .001 (< .01) | |||
| LOS [median, range] | 1 [0-258] | 2 [0-120] | < .001 | |||
| Total Cost [median, range] | $12,406 (46-319,107) | $7,213 (13-459,915) | < .001 | |||
Sx Symptomatic Asx Asymptomatic
In contrast, patients undergoing concurrent CABG/Valve had similar combined stroke or death rates following CEA and CAS (4.8% vs. 3.2%, P = .19) overall. However, stroke or death was higher with CEA in asymptomatic patients (CEA 3.8% vs. CAS 1.5%, P < .05) but not symptomatic patients (CEA 15.4% vs. CAS 15.7%, P = .95).
In those with age≥80, mortality, stroke, and combined stroke or death were higher after CAS for symptomatic patients (6.6% vs. 2.7%, 9.0% vs. 4.6%, and 14.7% vs. 6.7% respectively, P < .001 all). In asymptomatic octogenarians undergoing CAS versus CEA, mortality was similar (0.6% vs. 0.6%, P = .92), stroke was higher (1.1% vs. 0.7%, P < .05) and combined stroke or death was similar (1.5% vs. 1.2%, P =.16).
Multivariate Analysis
Stroke or death
On multivariate analysis, CAS was associated with more than double the risk of stroke or death compared to CEA (OR 2.4, 95%CI 2.1-2.8) (Table IVA). Other predictors included symptomatic status (OR 6.8, 95% CI 6.1-7-6) and high risk (OR 1.6, 95% CI 1.4-1.8). Later year of procedure was associated with lower event rates (OR 0.9, 95% CI 0.8-0.97).
Table IV.
Multivariate Predictors of Stroke or Death after Carotid Repair (adjusted for age/gender). A) Composite medical high risk criteria. B) Individual high risk criteria.
| A) | |||
|---|---|---|---|
| Predictors of Combined Stroke or Death | |||
| OR | 95% CI | P-Value | |
| Carotid Stent vs. Endarterectomy | 2.4 | 2.1-2.8 | < .001 |
| Symptomatic | 6.8 | 6.1-7.6 | < .001 |
| High Risk | 1.6 | 1.4-1.8 | < .001 |
| Year of Procedure | 0.9 | 0.8-0.97 | < .01 |
| B) | |||
|---|---|---|---|
| Predictors of Combined Stroke or Death (Individual High Risk Criteria) | |||
| Carotid Stent vs. Endarterectomy | 2.5 | 2.2-2.9 | < .001 |
| Symptomatic | 7.0 | 6.3-7.9 | < .001 |
| High Risk | |||
| Age ≥ 80 Years | 1.2 | 1.03-1.3 | < .05 |
| Renal failure | 1.7 | 1.4-2.1 | < .001 |
| Chronic lung disease | 1.2 | 1.04-1.3 | < .05 |
| Prior myocardial infarction | 0.7 | 0.5-0.8 | < .001 |
| Congestive heart failure | 2.2 | 1.9-2.5 | < .001 |
| CABG/Valve | 5.4 | 4.4-6.7 | < .001 |
| Unstable angina | 1.0 | 0.7-1.4 | .95 |
| Year of Procedure | 0.9 | 0.8-0.95 | < .01 |
When individual high risk criteria were analyzed separately, CABG/Valve was the strongest predictor of stroke or death (OR 5.4, P < .001) followed by CHF (OR 2.2, P < .001), renal failure (OR 1.7, P < .001), age ≥80 (OR 1.2, P < .05), and chronic lung disease (OR 1.2, P < .05) (Table IVB) Patients with a prior myocardial infarction had a lower risk of stroke or death (OR 0.7, P < .001) and unstable angina showed no difference (OR 1.0, P = .95).
Death
Predictors of mortality were CAS (OR 3.1, 95% CI 2.5-3.8), high risk (OR 2.6, 95% CI 2.2-3.1), and symptomatic patients (OR 5.3, 95% CI 4.4-6.3) (Table VA). Analysis of individual high risk factors demonstrated similar results to those for stroke and death (Table VB)
Table V.
Multivariate Predictors of Death after Carotid Repair (adjusted for age/gender). A) Composite medical high risk criteria. B) Individual high risk criteria.
| A) | |||
|---|---|---|---|
| Predictors of Death | |||
| OR | 95% CI | P-Value | |
| Carotid Stent vs. Endarterectomy | 3.1 | 2.5-3.8 | < .001 |
| Symptomatic | 5.3 | 4.4-6.3 | < .001 |
| High Risk | 2.6 | 2.2-3.1 | < .001 |
| Year of Procedure | 0.9 | 0.8-0.99 | < .05 |
| B) | |||
|---|---|---|---|
| Predictors of Death (Individual High Risk Criteria) | |||
| Carotid Stent vs. Endarterectomy | 3.2 | 2.6-4.0 | < .001 |
| Symptomatic | 5.3 | 4.5-6.4 | < .001 |
| Female | 0.8 | 0.7-0.99 | < .05 |
| High Risk | |||
| Age ≥ 80 Years | 1.4 | 1.2-1.7 | < .001 |
| Renal failure | 2.6 | 2.0-3.4 | < .001 |
| Chronic lung disease | 1.4 | 1.2-1.8 | < .001 |
| Prior myocardial infarction | 0.5 | 0.3-0.7 | < .001 |
| Congestive heart failure | 3.5 | 2.9-4.3 | < .001 |
| CABG/Valve | 7.1 | 5.4-9.3 | < .001 |
| Unstable angina | 0.6 | 0.4-1.1 | .10 |
| Year of Procedure | 0.9 | 0.8-0.96 | < .01 |
Stroke
CAS was also predictive of stroke (OR 2.1, 95% CI 1.7-2.5) as were symptom status (OR 7.6, 95% CI 6.7-8.7) and high risk (OR 1.3, 95% CI 1.2-1.5), while later year of procedure was again protective (OR 0.9, 95% CI 0.8-0.99) (Table VIA). Analysis of individual high risk criteria showed only concomitant CABG/Valve (OR 3.7 95% CI 2.8-4.9) and CHF (OR 1.4, 95% CI 1.1-1.7) to be predictive (Table VIB).
Table VI.
Multivariate Predictors of Stroke after Carotid Repair (adjusted for age/gender). A) Composite medical high risk criteria. B) Individual high risk criteria.
| A) | |||
|---|---|---|---|
| Predictors of Stroke | |||
| OR | 95% CI | P-Value | |
| Carotid Stent vs. Endarterectomy | 2.1 | 1.7-2.5 | < .001 |
| Symptomatic | 7.6 | 6.7-8.7 | < .001 |
| High Risk | 1.3 | 1.2-1.5 | < .001 |
| Year of Procedure | 0.9 | 0.8-0.99 | < .05 |
| B) | |||
|---|---|---|---|
| Predictors of Stroke (Individual High Risk Criteria) | |||
| Carotid Stent vs. Endarterectomy | 2.1 | 1.8-2.5 | < .001 |
| Symptomatic | 7.8 | 6.8-8.9 | < .001 |
| High Risk | |||
| Age ≥ 80 Years | 1.0 | 1.0-1.3 | .65 |
| Renal failure | 1.0 | 0.7-1.3 | .84 |
| Chronic lung disease | 1.1 | 0.9-1.3 | .29 |
| Prior myocardial infarction | 0.8 | 0.7-1.1 | .14 |
| Congestive heart failure | 1.4 | 1.1-1.7 | < .01 |
| CABG/Valve | 3.7 | 2.8-4.9 | < .001 |
| Unstable angina | 1.3 | 0.9-2.0 | .20 |
| Year of Procedure | 0.9 | 0.8-0.99 | < .05 |
In a subgroup analysis of only patients undergoing concurrent CABG/Valve, carotid repair type was no longer predictive of stroke or death (CAS vs. CEA OR 1.4, 95% CI 0.6-3.0) (Table VII). In this group, only symptom status (OR 6.1, 95% CI 4.0-9.3) and the individual comorbidities of CHF (OR 3.5, 95% CI 1.8-6.7) and chronic renal failure (OR 2.0, 95 CI 1.2-3.3) were predictive of stroke or death after adjustment for age and gender. Year of procedure was not associated with outcome in this group.
Table VII.
Multivariate Predictors of Stroke or Death for Patients Undergoing Carotid Repair and CABG/Valve (adjusted for age/gender).
| OR | 95% CI | P-Value | |
|---|---|---|---|
| Carotid Stent vs. Endarterectomy | 1.4 | 0.6-3.0 | .46 |
| Symptomatic | 6.1 | 4.0-9.3 | < .001 |
| Congestive Heart Failure | 3.5 | 1.8-6.7 | < .01 |
| Chronic Renal Failure | 2.0 | 1.2-3.3 | < .01 |
| Year of Procedure | 0.9 | 0.7-1.1 | .32 |
In a separate subgroup analysis of symptomatic patients only, CAS (OR 2.6, 95% CI 2.1-3.2, P < .001) and high risk status (OR 1.5, 95% CI 1.2-1.7, P < .001) were both predictive of increased stroke or death.
Secondary Outcomes
Global complications were more common after CAS than CEA for carotid revascularization alone (13.6% vs. 9.6%, P < .001) but were less common after CAS for concurrent carotid and CABG/Valve procedures (20.8% vs. 27.2%, P < .05) (Table III). Cardiac complications were similar between repair types for the overall group (CAS 1.9% vs. CEA 1.8%) but were lower after CAS for patients undergoing CABG/Valve (4.2% vs. 8.1%, P < .05). Length of stay was longer after CAS overall (2 days vs. 1 day, P < .001) and for high risk patients (2 days vs. 1 days, P < .001) but similar if concurrent CABG/Valve (11 days vs. 12 days, P = .50). Hospital costs were higher for CAS than CEA for all subgroups.
Discussion
This study, using a large population-based database from recent years, shows that when accounting for symptom status and medical high risk criteria (including concurrent cardiac procedures), carotid stenting has a higher risk of combined stroke or death, as well as death and stroke alone compared to carotid endarterectomy in the general US population. High risk status was associated with worse outcome for CEA compared to non-high risk. Outcomes with CAS, however, were not improved in high risk patients compared to CEA.
CMS reimbursement for CAS for symptomatic high risk patients was based in part on the results of a randomized trial showing similar stroke and death rates but a lower rate of a combined endpoint of stroke, death, or MI with CAS versus CEA9 as well as on a historic control made up of patients undergoing concomitant CEA and CABG.16 Our study suggests that these results are not reflective of current national outcomes. The SAPPHIRE trial showed a stroke or death rate of 5.5% for CAS versus 8.5% for CEA (P =.36) at one year (30-day mortality 1.2% vs. 2.5%, P = .39 and 30-day stroke 3.6% vs. 3.1%, P = .77).9
Subsequent randomized trials in average risk patients have shown conflicting results. They have shown either increased stroke and death with CAS or similar results between the two repair methods but with a failure to meet non-inferiority.16,10-12,17 The recently presented CREST trial showed similar rates of stroke, death, and MI in symptomatic and asymptomatic patients with very low event rates in both the CAS and CEA groups. Stroke was lower with CEA while MI was lower with CAS. While this demonstrates the efficacy of CAS in selected patients treated by experienced physicians and supports the prior literature demonstrating a lower stroke rate with CEA, our study suggests that similar results may not be duplicated nationally.17 As an important note, there were strict criteria for interventionalists participating in CREST. They required prior performance of at least 35 carotid stent cases with a subsequent roll-in phase where 10 CAS were performed under supervision. Only physicians with adequate outcomes were invited to participate in the study.13
Wennberg et al. previously demonstrated that mortality after CEA was substantially higher in Medicare patients compared to patients enrolled in NASCET and ACAS, even if their CEA was performed in the same institutions participating in the trials.18 Our findings suggest that this increased mortality may be due to procedures performed in select high risk patients. Our national analysis suggests that mortality rates with CEA in non-high risk patients compares favorably with NASCET (1.0% vs. 0.6%) and ACAS (0.1% vs. 0.1%) suggesting these results are generalizable.19,20 CEA in high risk patients shows increased mortality and stroke/death compared to average risk, however, CAS outcomes were worse than CEA for both high and average risk patients. It is certainly possible that our high risk criteria may not detect true high risk status in some CAS patients and may also overestimate high risk in CEA patients. The lack of specificity of ICD-9 coding unfortunately means our definition of high risk is overestimated in general. However, CEA in high risk patients was associated with improved outcome compared to CAS in non-high risk patients. While these limitations hinder our ability to draw strong conclusions about the safety of CAS, the strongest conclusion to be drawn is that average risk patients undergoing CEA fare reasonably well.
These data suggest that further careful analysis should be made to be certain that the efficacy demonstrated in randomized trials with carefully selected patients being treated by highly trained physicians is translated into effectiveness with similar results in broad general practice.
Prior analyses using the NIS database as well as the SVS registry did not account for high risk status.21-24 Our analysis demonstrates the disparate outcomes in these groups for carotid revascularization and demonstrates need for this stratification for any future comparisons of these procedures. The NIS database is hampered by a questionable ability to discriminate preoperative stroke from a postoperative complication and the potential to underestimate preoperative symptomatic status. We defined symptom status by ICD-9 coding for prior stroke, TIA, or amarosis fugax, however the proportion is lower than institutional and clinical trials which likely have more accurate clinical assessments. Using the NIS, McPhee et al. found 92.1% of carotid procedures were performed for asymptomatic disease23 while Vogel et al. found a <3% symptomatic proportion using differing algorithm to define symptom status.21 Comparatively, Kang et al. reported a 43% symptomatic rate in nearly 4,000 patients from the National Surgical Quality Improvement Program from 2005-2006.25 However, results from early NSQIP data may not reflect what is occurring nationally.
The ARCHeR trial for high risk patients undergoing CAS showed a 30-day stroke or death rate of 11.6% for symptomatic patients and 5.4% for asymptomatic patients.26 This high event rate in symptomatic patients is similar to the national outcomes that we found in our analysis (14.4% in-hospital stroke or death). We found also, however, that non-high risk patients also had a high combined stroke and death rate at 11.8% whereas asymptomatic patients had significantly lower event rates (1.5% high risk, 1.8% non-high risk), showing that symptom status confers the greatest risk in patients undergoing carotid stenting. Symptomatic patients older than 80 years did particularly poorly with combined stroke and death rates of 14.7% after CAS and 6.7% after CEA. Careful patient selection should be employed when considering carotid procedures in this age group.
There was an increased likelihood of CAS patients to be symptomatic. This is likely attributable to CMS reimbursement requirements as well. Evaluation of patients pre- and post-operatively may be more accurate for CAS given that a detailed neurological evaluation is required by CMS. This may more accurately identify symptomatic patients preoperatively and may detect more minor strokes postoperatively. However, this should not impact the analysis of mortality. Adjustment for symptom status may in fact bias mortality evaluation in favor of stenting.
Our analysis showed that for patients undergoing concurrent carotid repair and CABG/Valve surgery, CAS did not have an increased risk of stroke or death compared to CEA. Concurrent CABG/Valve was performed in 3.9% of carotid revascularization procedures. It is unlikely that those patients undergoing concurrent carotid and cardiac surgery would have differing procedural risk patterns compared to those not undergoing cardiac surgery. CAS is typically performed in a separate setting prior to CABG therefore those dying after CAS would not be detected in this analysis. Additionally, patients with a major CVA after CAS are likely to have CABG deferred therefore there is inherent bias against CEA which may more commonly be performed in the same setting as CABG/Valve. The use of aspirin, plavix, and statins may be more likely in patients undergoing a CABG and blood pressure may be better optimized, however how this impacts CEA versus CAS differentially is unknown. While many publications regarding CEA and CABG exist, information specifically about combined CAS and CABG is scarce and mostly limited to single institution studies.5,16,28 Naylor et al recently systematically reviewed all published studies of staged CAS and CABG and found a 30-day stroke or death rate of 9.1% with an overall mortality of 5.5%.5 Another recent systematic review found a 30-day stroke and death rate of 12.3% and mortality of 7.6% for CAS and CABG.27 This is significantly higher than our current results of 3.2% stroke/death and 1.6% mortality for in-hospital CAS/CABG outcomes. Timaran et al. used the NIS from the years 2000-2004 (prior to CAS specific ICD-9 codes), spanning the time before the current study, to compare CAS and CABG to CEA and CABG. At that time only 3.3% (887) of combined cardiac and carotid procedures were CAS compared to 96.7% CEA. They found an in-hospital stroke and death rate after CAS and CABG of 6.9% and after CEA and CABG of 8.6% but no difference in predictive risk on multivariate analysis.28 Given the substantially higher rates of adverse events in this group of patients, it seems prudent to analyze their outcomes separately. Similarly, improved stratification of outcomes within other high risk subgroups appears warranted.
We adjusted outcomes based upon medical high risk criteria but not for anatomic high risk which is unattainable in the NIS data. Most of these have been shown to increase risk of local complications such as infection and nerve injury but not stroke or death.29,30 Failure to detect anatomic high risk as an indication for CAS is unlikely to affect these results. Contralateral occlusion has been shown to increase risk of CEA in large studies, but little data exists to evaluate the impact on CAS outcomes.31-32
Outcomes improved with time as shown in our multivariate analyses. This was true for the overall group as well as for CAS and CEA independently. With improving technology and increasing practitioner expertise, this is expected for CAS, however it was a surprising finding with CEA. This may indicate that as CAS becomes more widely used, fewer high risk patients are having CEA performed, thus improving the results of CEA over time.
We included PCI and diagnostic cardiac catheterizations in our study as coexisting cardiac procedures. There was a greater number of both performed during the same admission as CAS compared to CEA however, which may indicate that these catheter-based procedures are done prior to or with CAS while they may more likely reflect postoperative cardiac complications after CEA.
The limitations of this study are primarily due to the nature of the database as an administrative dataset some of which are noted above. Additionally, the database is limited to inpatient outcomes only, therefore we cannot identify staged carotid and cardiac procedures if a patient was discharged between procedures. We also cannot extend outcomes to 30-day results as much of the prior literature does limiting accurate comparison. Some stroke events occur after hospital discharge, so this is an important distinction.24
The dataset has no information on severity of stenosis or anatomic risk factors that may predispose a patient to adverse outcomes with either repair method. For preoperative data, we cannot determine severity of prior stroke, laterality, frequency of symptoms, or the temporal relationship of symptoms and surgical repair.
Conclusion
In the overall population, CAS is associated with a higher risk of stroke or death, death, and stroke compared to CEA for carotid stenosis even after adjustment for symptom status and medical high risk criteria. As more randomized trials define the efficacy of CAS relative to CEA, additional population based analyses with well defined high risk criteria are needed to be certain that acceptable results are obtainable in the general population. Further work is also needed to define the appropriate role of either revascularization method in those with specified high risk criteria.
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Footnotes
Author Disclosures: K.A. Giles, None; A.D. Hamdan, None; F.B. Pomposelli, None; M.C. Wyers, None; M.L. Schermerhorn, Gore Unrestricted Educational Grant; Endologix DSMB, Medtronic consultant
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery initial experience in 110 patients. J Endovasc Surg. 1996;3:42–62. doi: 10.1583/1074-6218(1996)003<0042:SITCAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Marks MP, Dake MD, Steinberg GK, Norbash AM, Lane B. Stent placement for arterial and venous cerebrovascular disease: preliminary experience. Radiology. 1994;191:441–6. doi: 10.1148/radiology.191.2.8153318. [DOI] [PubMed] [Google Scholar]
- 3.Roubin GS, New G, Iyer SS, Vitek JJ, Al-Mubarak N, Liu MW, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. Circulation. 2001;03:532–7. doi: 10.1161/01.cir.103.4.532. [DOI] [PubMed] [Google Scholar]
- 4.Wholey MH, Al-Mubarek N, Wholey MH. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv. 2003;60:259–66. doi: 10.1002/ccd.10645. [DOI] [PubMed] [Google Scholar]
- 5.Naylor AR, Mehta Z, Rothwell PM. A systematic review and meta-analysis of 30-day outcomes following staged carotid artery stenting and coronary bypass. Eur J Vasc Endovasc Surg. 2009;37:379–87. doi: 10.1016/j.ejvs.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Naylor AR, Bolia A, Abbott RJ, Pye IF, Smith J, Lennard N, et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg. 1998;28:326–34. doi: 10.1016/s0741-5214(98)70182-x. [DOI] [PubMed] [Google Scholar]
- 7.Brooks WH, McClure RR, Jones MR, Coleman TL, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol. 2001;38:1589–95. doi: 10.1016/s0735-1097(01)01595-9. [DOI] [PubMed] [Google Scholar]
- 8.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729–37. [PubMed] [Google Scholar]
- 9.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 10.Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–47. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 11.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with severe symptomatic stenosis. N Engl J Med. 2006;355:1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 12.Featherstone RL, Brown MM, Coward LJ ICSS Investigators. International carotid stenting study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis. 2004;18:69–74. doi: 10.1159/000078753. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins LN, Roubin GS, Chakhtoura EY, Gray WA, Ferguson RD, Katzen BT, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial: credentialing of interventionalists and final results of lead-in phase. J Stroke Cerebrovasc Dis. 2010;19:153–62. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pub. 100-03 Medicare National Coverage Determinations. Department of Health and Human Services, Centers for Medicare and Medicaid Services. 2006. [Google Scholar]
- 15.The Healthcare Cost and Utilization Project Nationwide Inpatient Sample (NIS) [January 12, 2010.]; http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 16.Cambria RP, Ivarsson BL, Akins CW, Moncure AC, Brewster DC, Abbott WM. Simultaneous carotid and coronary disease: safety of the combined approach. J Vasc Surg. 1989;9:56–64. [PubMed] [Google Scholar]
- 17.Forbes TL. Preliminary results of carotid revascularization endarterectomy vs stenging trial (CREST) J Vasc Surg. 2010;51:1300–1. doi: 10.1016/j.jvs.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in Carotid Endarterectomy Mortality in the Medicare Population: Trial Hospitals, Volume, and Patient Characteristics. JAMA. 1998;279:1278–81. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 19.Nascet C. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 20.Young B, Moore WS, Robertson JT, Toole JF, Ernst CB, Cohen SN, et al. An analysis of perioperative surgical mortality and morbidity in the asymptomatic carotid atherosclerosis study. ACAS Investigators. Asymptomatic Carotid Artheriosclerosis Study. Stroke. 1996;27:2216–24. doi: 10.1161/01.str.27.12.2216. [DOI] [PubMed] [Google Scholar]
- 21.Vogel TR, Dombrovskiy VY, Haser PB, Scheirer JC, Graham AM. Outcomes of carotid artery stenting and endarterectomy in the United States. J Vasc Surg. 2009;49:325–30. doi: 10.1016/j.jvs.2008.08.112. [DOI] [PubMed] [Google Scholar]
- 22.McPhee JT, Hill JS, Ciocca RG, Messina LM, Eslami MH. Carotid endarterectomy was performed with lower stroke and death rates than carotid artery stenting in the United States in 2003 and 2004. J Vasc Surg. 2007;46:1112–8. doi: 10.1016/j.jvs.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 23.McPhee JT, Schanzer A, Messina LM, Eslami MH. Carotid artery stenting has increased rates of postprocedure stroke, death, and resource utilization than does carotid endarterectomy in the United States, 2005. Journal of Vascular Surgery. 2008;48:1442–50. doi: 10.1016/j.jvs.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Sidawy AN, Zwolak RM, White RA, Siami FS, Schermerhorn ML, Sicard GA, et al. Risk-adjusted 30-day outcomes of carotid stenting and endarterectomy: Results from the SVS Vascular Registry. Journal of Vascular Surgery. 2009;49:71–9. doi: 10.1016/j.jvs.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Kang JL, Chung TK, Lancaster RT, Lamuraglia GM, Conrad MF, Cambria RP. Outcomes after carotid endarterectomy: Is there a high-risk population? A National Surgical Quality Improvement Program report. J Vasc Surg. 2009;49:331–9.e1. doi: 10.1016/j.jvs.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Gray WA, Hopkins LN, Yadav S, Davis T, Wholey M, Atkinson R, et al. Protected carotid stenting in high-surgical-risk patients: The ARCHER results. J Vasc Surg. 2006;44:258–69. doi: 10.1016/j.jvs.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 27.Guzman LA, Costa MA, Angiolillo DJ, Zenni M, Wludyka P, Silliman S, et al. A systematic review of outcomes in patients with staged carotid artery stenting and coronary artery bypass graft surgery. Stroke. 2008;39:361–5. doi: 10.1161/STROKEAHA.107.495010. [DOI] [PubMed] [Google Scholar]
- 28.Timaran CH, Rosero EB, Smith ST, Valentine RJ, Modrall JG, Clagett GP. Trends and outcomes of concurrent carotid revascularization and coronary bypass. J Vasc Surg. 2008;48:355–61. doi: 10.1016/j.jvs.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 29.Hill BB, Olcott C, Dalman RL, Harris EJ, Zarins CK. Reoperation for carotid stenosis is as safe as primary carotid endarterectomy. J Vasc Surg. 1999;30:26–35. doi: 10.1016/s0741-5214(99)70173-4. [DOI] [PubMed] [Google Scholar]
- 30.Kashyap VS, Moore WS, Quinones-Baldrich WJ. Carotid artery repair for radiation-associated atherosclerosis is a safe and durable procedure. J Vasc Surg. 1999;29:90–99. doi: 10.1016/s0741-5214(99)70351-4. [DOI] [PubMed] [Google Scholar]
- 31.Goodney PP, Likosky DS, Cronenwett JL Vascular Study Group of Northern New England. Factors associated with stroke or death after carotid endarterectomy in Northern New England. J Vasc Surg. 2008;48:1139–45. doi: 10.1016/j.jvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Rothwell PM, Slattery J, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy: systematic review. BMJ. 1997;315:1571–7. doi: 10.1136/bmj.315.7122.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]


