Abstract
Drosophila mitochondria contain two peroxidases, peroxiredoxin 3 (dPrx3) and peroxiredoxin 5 (dPrx5), which together constitute the sole known intra-mitochondrial mechanism for the catalytic removal of hydrogen-and organic-peroxides. dPrx3 exists exclusively within mitochondria, whereas dPrx5 is also present in some other intracellular compartments. Levels of these two peroxiredoxins were genetically manipulated, singly and together, in D. melanogaster, for the purpose of understanding their respective functions. Under-expression of dPrx3 by 90–95% had no discernable effect on life span under normal or oxidative stress conditions; the dPrx5 null flies were previously reported to exhibit a 10% shortening of mean life span and an increase in sensitivity to oxidative stress. Flies under-expressing both dPrx3 and dPrx5 showed an 80% decrease in life span, severe disruption in thiol homeostasis and a massive induction of apoptosis in the muscle and digestive system tissues. The early mortality in flies, under-expressing both peroxiredoxins, was partially offset by over-expression of thioredoxin reductase but not mitochondrion-targeted catalase. These results suggest that mitochondrial peroxiredoxins confer specific protection for thioredoxin/glutathione systems, play a critical role in the maintenance of global thiol homeostasis, prevent the age-associated apoptosis and premature death.
Keywords: peroxiredoxin, mitochondria, redox state, thiol, thioredoxin, glutathione, oxidative stress, apoptosis, aging, Drosophila
INTRODUCTION
Peroxiredoxins (Prx) comprise a family of peroxidases that reduce H2O2, organic peroxides and peroxynitrite by using thiols of the cysteine residues at the active site as suppliers of reducing equivalents. In higher eukaryotes, peroxiredoxins are classified into six subtypes, based on their structure, mode of action and location. Orthologs of all known mammalian peroxiredoxin subtypes have been identified in homologous cellular compartments in Drosophila [1–4].
Drosophila mitochondria contain two peroxiredoxin subtypes, dPrx3 and dPrx5, but unlike mammals, which have thioredoxin peroxidases (peroxiredoxins) and mitochondrial glutathione peroxidases, the orthologues of the latter are absent in Drosophila, which implies that the intra-mitochondrial elimination of peroxides is solely dependent upon the activity of these two peroxiredoxins. Other Drosophila orthologues of glutathione peroxidases, named non-selenium glutathione peroxidases or peroxiredoxin 6 (DPx-2540 and DPx-6005), are located in the cytosol [2]. dPrx3 occurs exclusively within mitochondria [5], whereas dPrx5 is distributed in several other cellular compartments [6, 7]. The respective functions of these two peroxiredoxins and advantages of their simultaneous presence within mitochondria are presently unclear.
Over-expression of Prx3 or Prx5 in mammalian cultured cells has been reported to attenuate structural damage and frequency of apoptosis under conditions of oxidative stress, whereas silencing had the opposite effects, albeit to a varying extent [8–11]. Although such results suggest that both mitochondrial peroxiredoxins provide antioxidant defenses, their specific functions, separate from each other, remain obscure due to the intra-mitochondrial presence of glutathione peroxidase, which has an overlapping function in the elimination of peroxides. Because Drosophila lack the classic glutathione peroxidases [12] and the only known peroxidase activity in the mitochondrial compartment is executed by the peroxiredoxins, it is thus feasible to determine the specific functional roles of dPrx3 and dPrx5 in this species.
Accordingly, the present study was conducted in fly lines under-expressing Drosophila peroxiredoxin 3 (dPrx3) and dPrx5 separately and together. The effects of these genetic manipulations on life span, redox state, stress resistance and apoptosis are reported herein.
MATERIALS AND METHODS
Construction of UAS-dPrx3, RNAi-dPrx3 and UAS-mtCat transgenic lines
To generate a p[UAST]-dPrx3 construct, a fragment derived from the cDNA clone SD08737 (Research Genetics, Huntsville, AL), comprising the entire coding region of the dPrx3 gene and 62 bp 5’UTR region upstream of the start codon and 100 bp 3’UTR region downstream of the stop codon, was inserted into the pUAST vector. A p[UAST]-mtCat construct was made by re-cloning of a fragment, containing mitochondrial OAT presequence and a coding region of the catalase gene, from the pCaSpeR4-OAT-Cat construct [13] into the pUAST vector. The RNAi-dPrx3 hairpin construct was produced by introducing two copies of a 649 bp dPrx3 domain, placed tail-to-tail with an intervening sequence into the P element vector, pWIZ [14], which contains an upstream activating sequence (UAS) that is recognized by the yeast transcriptional activator GAL4. The UAS-dPrx3, UAS-mtCat transgenic flies and those containing RNAi-dPrx3 constructs were then generated by P element-mediated germ-line transformation in the yellow white (yw) reference strain, either on our premises or by external services (TheBestGene company). At least three different transgenic fly lines were established for each construct.
Fly strains and procedures
The y w reference strain has been maintained in this laboratory for >15 years. The tubulin (Tub)-GAL4, actin (Act)-GAL4, daughterless (Da)-GAL4, Appl-GAL4, Elav-GAL4, D42-GAL4 and armadillo (Arm)-GAL4 driver lines were supplied by Dr. Blanka Rogina (University of Connecticut Health Science Center). The dprx5 mutant is described in previous publications [1, 4]. Flies exhibiting an approximately two-fold increase in the expression of thioredoxin reductase (formerly identified as glutathione reductase) have been previously described [15, 16]. Briefly, the transgene contains a fragment of thioredoxin reductase (dTrxR) gene, trxr-1, encoding two isoforms of the enzyme, a cytoplasmic and mitochondrial [17]. To exclude background effects on survivorship and other traits, all transgenic and GAL4 driver lines and the dprx5 (−/−) mutant were backcrossed to y w for 6–8 generations to obtain genetically homogeneous stocks.
Flies under-expressing both dPrx3 and dPrx5 were generated by expressing the RNAi-dPrx3 hairpin construct, using the ubiquitous Da-GAL4 driver in the dprx5 −/− mutant background. The genotypes of the generated flies were yw; RNAi-dPrx3/+; dprx5/Da-GAL4,dprx5 and yw; +/+; dprx5,Da-GAL4/dprx5,RNAi-dPrx3. The Da-GAL4,dprx5 and dprx5, RNAi-dPrx3 configurations were obtained by recombination.
In all experimental studies, flies were collected within 1–2 days after hatching and reared on a standard sucrose-cornmeal medium at 25°C. To induce oxidative stress, flies were fed specified concentrations of either H2O2 or paraquat in 1% sucrose. Survivorship data were obtained as described previously [4].
Isolation of mitochondria
Mitochondria were isolated from flight muscles by differential gradient centrifugation, as described previously [4, 18]. Briefly, a minimum of 50 thoraces were gently pounded in a chilled mortar, containing 500 µl of ice-cold isolation buffer (0.32M sucrose, 10 mM EDTA and 10 mM Tris/HCl, pH 7.3), supplemented with 2% (w/v) BSA (fatty acid content 0.003%). The resulting brei was filtered through Spectra/Mesh® nylon (pore size=10µm) and centrifuged for 10 min at 2200 g. The pellet was rinsed briefly in BSA-free isolation buffer and stored at −80°C. Alternatively, mitochondrial fractions were isolated using the Mitochondria Isolation Kit (Pierce), as per manufacturer’s instructions.
Immunoblotting
The isolation of antibodies raised against Drosophila Prx3 and Prx5 and procedures for analysis of dPrx3 and dPrx5 protein expression were described previously [4, 19]. Briefly, 10 µg aliquots of the protein extracts were resolved by 10% SDS-PAGE, followed by transfer to PVDF membrane (Millipore). Immunoblots were developed using anti-dPrx3 and anti-dPrx5 antibodies. Anti-actin antibodies (MP Biomedicals) were used as a control for loading. Analysis of lipid peroxidation was performed with anti-HNE antibodies (Alpha Diagnostics, San Antonio, TX).
Fluorimetric analysis of ATP and sulfhydryls
ATP content in mitochondria was determined by using the ATP Determination Kit (Invitrogen) essentially as described in the manufacturer’s protocol. Samples were prepared by boiling mitochondrial lysates for 5 min, prior to the assembly of the reaction mixture, in order to maximally extract ATP from the mitochondrial membranes and to inactivate enzymes, such as ATPase that may interfere with the detection. Glutathione and protein sulfhydryls were measured by using the fluorimetric Glutathione Assay Kit (Sigma) essentially as described in the manufacturer’s protocol. We determined that during prolonged (30 min-1h) incubation of Drosophila lysates with the reaction mixture, the thiol probe (monochlorobimane) reacts with not only GSH but also with protein sulfhydryls (unpublished observations). We thus report the obtained measurements as total sulfhydryl levels.
HPLC analysis of GSH, GSSG and reactive protein sulfhydryls
Flies were immobilized on ice for 1–2 min, weighed, and homogenized in 10 vol of freshly prepared ice-cold 5% (w/v) meta-phosphoric acid (MPA), using 1.5 ml plastic tubes and pestles, obtained from RPI (Mt. Prospect, IL). The homogenates were incubated for 30 min on ice and centrifuged at 18,000 g for 20 min at 4°C. Supernatants were filtered using 0.45 µm PTFE Acrodisc® CR 4 mm syringe filters, obtained from Gelman Laboratory (Ann Arbor, MI); filtrates were transferred to sampling vials and either analyzed immediately or stored at −80°C for up to 1 month. The procedure for the detection and quantification of GSH and GSSG used here and the precautionary measures, adopted to minimize spontaneous GSH oxidation, have been described by us previously [20]. Briefly, aminothiols were separated by HPLC, fitted with a Shimadzu Class VP solvent delivery system, using a reverse phase C18 Luna (II) column (3µ; 4.6 × 150 mm), obtained from Phenomenex (Torrance, CA). The mobile phase for isocratic elution consisted of 25 mM monobasic sodium phosphate, 0.120 mM of the ion-pairing agent 1-octane sulfonic acid, 1% (v/v) acetonitrile, pH 2.75, adjusted with 85% phosphoric acid. The flow rate was 0.7 ml/min. Under these conditions, the separation was completed in 30 min; GSSG was the last eluting peak, with a retention time of approximately 22 min. Calibration standards were prepared in 5% (w/v) MPA. Aminothiols were detected with a model 5600 CoulArray® electrochemical detector (ESA Inc., Chelmsford, MA), equipped with an eight-channel analytical cell, using potentials from +500mV in 100mV increments. GSH was monitored at +800 mV whereas GSSG at +900 mV. Each sample was injected twice, and the average of the peak areas was used for quantification.
For the measurements of reactive protein sulfhydryls, 150 µl of whole fly homogenates (15 flies homogenized in 150 µl of 50mM Na-phosphate buffer, pH7.4) were mixed with 50 µl of a solution containing 50 mM GSSG. After incubation for 2 h at 37°C, 250 µl of ice-cold 10% MPA was added. After 20 min of incubation on ice, samples were centrifuged for 20 min at 18,000 g at 4°C. Protein pellets from acid precipitation were washed 3 times in 2 ml of 5% MPA to remove the free (non-protein bound) glutathione. Protein-bound glutathione was subsequently released by incubation of washed protein pellets with 10mM Tris(2-carboxyethyl)-Phosphine (TCEP) in 50mM Phosphate buffer pH 7.6 for 60 min at 37°C. Amounts of GSH were measured by HPLC-ECD, as described previously [21, 22].
TUNEL Labeling
Tissue-specific assessment of apoptosis-induced DNA fragmentation was made in cryosections of whole flies. Slides were fixed with 2% paraformaldehyde (Sigma) for 5 min at room temperature and incubated with DNA-labeling reagents using the In Situ Cell Death Detection Kit, TMR red (Roche), following the manufacturer’s recommendations. Images were acquired by fluorescence microscopy (Nikon), using MetaMorph software.
Statistical Analysis
Differences in the levels of glutathione, protein sulfhydryls, H2O2, HNE and ATP levels, as well as enzyme activities were assessed by unpaired Student's t tests using Microsoft Excel software. Rates of oxygen consumption were compared by analysis of variance, with post hoc pairwise comparisons based on Tukey tests. The mean survivorship time and statistical significance of differences between survival curves were assessed by analysis of variance with pairwise comparisons, using Prism for Macintosh version 4.0a software (GraphPad Software, Inc., San Diego, CA). The significance of differences between survival curves was determined separately for each experimental combination and its corresponding driver-only and responder-only controls.
RESULTS
Effects of under- and over-expression of mitochondrial peroxiredoxin dPrx3 on the physiology and life span of flies
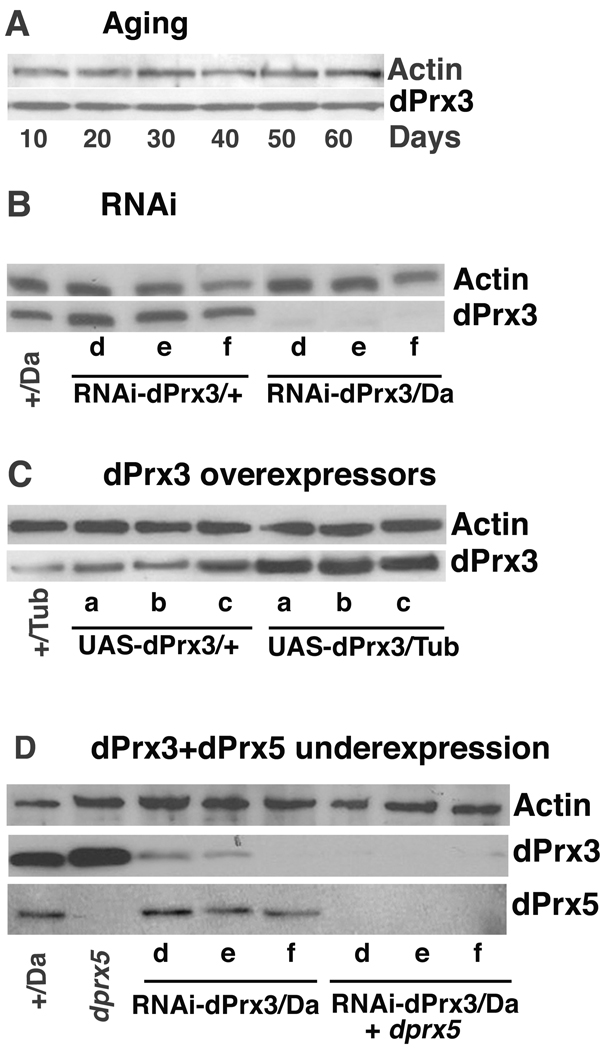
In mammals, Prx3 has been localized to the mitochondria and has been characterized as a house-keeping protein with an antioxidant function [23–25]. In Drosophila, the expression of Prx3 protein was also restricted to the mitochondria [2] and no notable variations in its level were discernable at different ages (Fig. 1 A). Similarly, gene expression, indicated by mRNA and protein levels, remained relatively unaltered in response to stressors, such as high temperature, hydrogen peroxide and paraquat (data not shown).
Fig. 1. Immunoblot analysis of expression of mitochondrial peroxiredoxins.
Immunoblots were performed using anti-dPrx3 and/or anti-dPrx5 antibodies and re-probed with anti-actin antibodies to control for loading. A, dPrx3 expression during aging. Adult yw male flies were collected at ten-day increments starting at 10 days of age. B, Underexpression of dPrx3 using RNAi constructs. Controls are Da-GAL4 driver (Da/+) and three different RNAi-dPrx3 transgenic lines (d, e and f). Experimental RNAi-dPrx3/Da lines were obtained by crossing the appropriate RNAi and Da-GAL4 driver transgenic lines. C, Over-expression of dPrx3 using UAS-dPrx3 constructs. Controls are Tub-GAL4 driver (Tub/+) and three different UAS-dPrx3 transgenic lines (a, b and c). Experimental lines UAS-dPrx3/Da were obtained by crossing the appropriate UAS-dPrx3 transgenes and Tub-GAL4 driver lines. D, Under-expression of dPrx3 and dPrx5. Da/+ designates the Da-GAL4 driver control, while dprx5 is the P element-associated null mutant. Three distinct RNAi-dPrx3/Da lines are indicated as d, e, and f, in the absence or presence of the dprx5 mutation.
A decrease in the expression of dPrx3 was induced through RNAi, by generating flies carrying both the dPrx3 hairpin constructs (RNAi-dPrx3) and global high level GAL4 drivers (Tub-GAL4, Act-GAL4 and Da-GAL4). The most effective knock-down of dPrx3 expression at the protein level was obtained using the Da-GAL4 driver (~90–95%; Fig. 1 B). This marked reduction in dPrx3 expression produced a phenotype that was indistinguishable from the controls in terms of developmental timing, fertility or physical activity levels (data not shown). Nor did the under-expressing lines exhibit any differences in life span relative to both driver and transgene controls (Fig. 2 A). However, exposure of young (10–15 day) as well as middle age (30–40 day, data not shown) flies to paraquat or hydrogen peroxide revealed small, but significant, decreases in resistance to oxidants in the experimental lines (Fig. 2 B, C and Table 1).
Fig. 2. Survivorship of flies under-expressing dPrx3 under normal (A) and oxidative stress (B, C) conditions.
A, representative data of two independent experiments with three different RNAi-dPrx3 fly lines (d, e and f). In all experiments, 200–250 flies for each fly strain were used. Controls are Da-GAL4 driver (+/Da) and three different RNAi-dPrx3 transgenic lines (d, e or f/+). Experimental flies under-expressing dPrx3 were obtained by crossing RNAi-dPrx3 transgenic lines with Da-GAL4 driver (d, e or f/Da). Similar data were obtained in an independent experiment using a second cohort. B, 100–150 young (10-day-old) flies of each group were fed 1% sucrose solution containing 5 mM paraquat and survivorship was plotted. Similar results were obtained in two independent experiments. C, 100–150 young flies from each group were fed 0.5 M hydrogen peroxide and tested for survivorship. Similar results were obtained in two independent experiments. Results of replicate experiments are provided in Table 1.
Table 1.
Mean survival time of male flies under-expressing dPrx3
| Genotype | Mean (hours) | % vs. Responder/+ | % vs. Driver/+ |
|---|---|---|---|
| Flies exposed to 5 mM paraquat | |||
| RNAi-d/+ | 45.6; 55.1 | ||
| RNAi-e/+ | 43.4; 46.8 | ||
| RNAi-f/+ | 48.4; 67.9 | ||
| +/Da | 40.9; 55.1 | ||
| RNAi-d/Da | 37.3; 42.6 | −14.0; −8.9 | −8.8; −22.6 |
| RNAi-e/Da | 39.0; 53.5 | −14.5; −2.9D | −4.7; −2.9 |
| RNAi-f/Da | 37.0; 37.4 | −23.4; −45.0 | −9.3; −32.1 |
| Flies exposed to 0.5 M H2O2 | |||
| RNAi-d/+ | 61.7; 64.5 | ||
| RNAi-e/+ | 61.4; 63.1 | ||
| RNAi-f/+ | 55.6; 68.3 | ||
| +/Da | 58.7; 59.6 | ||
| RNAi-d/Da | 53.6; 52.2 | −13.1; −19.1 | −8.7; −12.4 |
| RNAi-e/Da | 49.2; 46.3 | −19.8; −26.6 | −16.2; −22.3 |
| RNAi-f/Da | 42.0; 40.0 | −24.4; −41.4 | −28.4; −32.9 |
Values obtained for two independent cohorts are listed in column 1. In columns 2 and 3 are indicated the % differences of the experimentals vs the responder/+ and driver/+ controls. All differences are statistically significant (P < 0.05) unless indicated by a delta (D).
Global over-expression of dPrx3, using either low-level (Arm-GAL4) or high-level drivers (Da-GAL4 or Tub-GAL4, Fig. 1 C), did not have any significant effect on susceptibility to oxidative stress or longevity (Fig. 3 A). Similarly, dPrx3 over-expression by Appl-GAL4 driver in the neuronal tissues had no effect on longevity, albeit resistance to oxidative stress was increased particularly in the middle-age (30-day-old) flies (Fig. 3 B and C and Table 2). Nonetheless, another pan-neuronal driver, Elav-GAL4, as well as the motor neuronal D42-GAL4 driver, did not confer similar effects (data not shown).
Fig. 3. The effects of dPrx3 over-expression on aging (A) and resistance to oxidative stress (B, C).
A, Life spans of flies over-expressing dPrx3 globally at high levels (with the Tub-GAL4 driver), at low levels (with the Arm-GAL4 driver) and in neuronal tissues (with the Appl-GAL4 driver). Controls are Da-GAL4 drivers (+/Tub, +/Arm and +/Appl) and three different UAS-dPrx3 transgenic lines (a, b or c/+).Experimental flies over-expressing dPrx3 were obtained by crossing UAS-dPrx3 transgenic lines with Tub-, Arm- or Appl-GAL4 drivers (a, b or c/Tub, Arm or Appl). Presented data are representative of two independent experiments, with one out of three transgenic lines. Resistance of middle-age (30-day-old) flies to paraquat (B) and hydrogen peroxide (C). Approximately 100–150 flies of each group were fed 1% sucrose solution containing 2.5 mM paraquat or 0.25 M H2O2. Results of replicate experiments are provided in Table 2.
Table 2.
Mean survival time of male flies over-expressing dPrx3
| Genotype | Mean (hours) | % vs. Responder/+ | % vs. Driver/+ |
|---|---|---|---|
| Flies exposed to 2.5 mM paraquat | |||
| UAS-dPrx3-a/+ | 41.1; 39.0 | ||
| UAS-dPrx3-b/+ | 45.8; 45.1 | ||
| UAS-dPrx3-c/+ | 48.9; 46.8 | ||
| +/Appl | 41.1; 41.2 | ||
| UAS-dPrx3-a/Appl | 50.1; 42.6 | 22.1; 9.2 | 21.7; 3.1 |
| UAS-dPrx3-b/Appl | 51.6; 48.2 | 12.5; 6.8 | 25.4; 16.9 |
| UAS-dPrx3-c/Appl | 59.4; 45.3 | 21.6; −3.3D | 44.5; 9.8 |
| Flies exposed to 0.25 M H2O2 | |||
| UAS-dPrx3-a/+ | 56.6; 50.0 | ||
| UAS-dPrx3-b/+ | 61.8; 46.5 | ||
| UAS-dPrx3-c/+ | 54.3; 48.2 | ||
| +/Appl | 55.3; 43.9 | ||
| UAS-dPrx3-a/Appl | 56.4; 55.9 | 2.0D; 15.8 | 4.0; 27.4 |
| UAS-dPrx3-b/Appl | 65.0; 51.4 | 5.1; 10.4 | 17.5; 17.1 |
| UAS-dPrx3-c/Appl | 60.6; 52.9 | 7.1; 5.7 | 9.6; 20.5 |
Values obtained for two independent cohorts are listed in column 1. In columns 2 and 3 are indicated the % differences of the experimental vs the responder/+ and driver/+ controls. All differences are statistically significant (P < 0.05) unless indicated by a delta (D).
Altogether, it seems that under- or over-expression of the mitochondrial peroxiredoxin, dPrx3, has no discernible effects on survivorship under normal conditions and only a relatively minor influence in the ability to withstand oxidant stress.
Effects of Under-expression of dPrx3 and dPrx5 on fly survivorship under normal and oxidative stress conditions
Effects of the under-expression of dPrx5, using a dprx5−/− null mutant, were examined in a previous study (9). Under normal conditions, the null mutant showed an ~ 10% reduction in mean life span but showed no significant changes in median or maximum age, whereas under conditions of oxidative stress, induced by paraquat- or H2O2- intake, the length of survival was decreased by ~50%. Although it would seem that the effects of dPrx5 under-expression are relatively more severe than those observed for dPrx3, it deserves noting that the under-expression of dPrx3 was obtained through RNAi, an approach that typically causes a severe knock-down (~90–95%), but does not achieve the null condition (complete absence of activity). Thus it is plausible that the 5–10% of dPrx3 activity present in the knock-down flies is sufficient to maintain a relatively normal phenotype.
Alternatively it could be the absence of dPrx5 in other compartments, which mediate the observed effects. To test this latter hypothesis, the dPrx3 knock-down was placed in a dPrx5 null background, termed here as the "double mutant" (Fig. 1 D). It was reasoned that if mitochondrial peroxiredoxins are not involved in the modulation of resistance to oxidative stress or longevity, then the phenotype of the double mutant should be equivalent to that of the dprx5 mutant. The construction of the double mutant is described in Materials and Methods.
The knock-down of dPrx3 in a dprx5 null background had a very deleterious effect on the survival of flies under normal conditions. Mean life span was shortened from 55–70 days to 12.8 days (Fig. 4). Notably, the double mutant flies appeared relatively normal for the first 9–11 days after eclosion, but then underwent a very rapid increase in mortality rate. Thus, the depletion of both intra-mitochondrial peroxiredoxins has a severe longevity effect, suggesting that with regard to this phenotype, dPrx3 and dPrx5 appear to have at least partially redundant functions in the mitochondrial compartment. The double mutant also exhibited diminished survivability in response to either H2O2 or paraquat treatment, which was similar to that observed in the dprx5 mutant (Fig. 5).
Fig. 4. Life span of flies under-expressing dPrx3 in a dprx5 mutant background.
Control fly lines include the Driver alone (Da/+), the three RNAi transgenes with no driver (d, e or f/+) and the reference y w strain. Experimental flies include those underexpressing dPrx3 (d, e or f/Da), the dprx5 null mutant and the “double mutant” flies under-expressing dPrx3 in a dprx5 mutant background (d, e or f/Da;dprx5). Shown are representative data of two independent experiments with three different RNAi-dPrx3 fly lines (d, e and f). In all experiments, there were 100–150 flies in each group. Mean life span of flies under-expressing both peroxiredoxins together was 12.8±0.1 days; maximum life span (~10 survival) was 13.3±0.5 days. During this period, flies under-expressing dPrx3 or dPrx5 individually, > 90% remained alive.
Fig. 5. Survivorship of flies under-expressing dPrx3 in a dprx5 mutant background under conditions of oxidative stress.
The lines used here are as described in the legend to Fig. 4. 100–150 young male flies from each strain were fed a 1% sucrose solution containing 0.25 mM hydrogen peroxide. Mean survival times were reduced by ~60% in dprx5 mutant and double mutants compared to RNAi-dPrx3 fly lines and controls. Similar data were obtained in two independent experiments.
Under-expression of dPrx3 and dPrx5 disrupts thiol homeostasis
To investigate the underlying cause of the drastic reduction in the survival of the double mutant under normal conditions, we evaluated the biochemical markers of oxidative stress and mitochondrial function, just prior to the onset of rapid death (i.e. 9 to 10-day-old flies reared at SMU and 12 to 15-day-old flies reared at USC. Note: such differences in Drosophila longevity at different laboratories are relatively common. Nevertheless the nature of the observed effects at both sites was equivalent; manifestation of the observed effects was simply slightly delayed at the USC site tracking with the slightly extended life span). The most striking alterations were observed in thiol homeostasis, measured as ratios of GSH:GSSG and amounts of reactive protein sulfhydryls in mitochondria and whole body homogenates (Figs. 6 and 7). Measurements of GSH and GSSG by HPLC indicated a strong pro-oxidizing shift, demonstrated by the decrease in GSH:GSSG ratios, predominantly due to an elevation of GSSG concentration (Fig. 6 A). The decrease in GSH/GSSG ratio occurred gradually and was particularly evident in flies that were relatively near to death (Fig. 6 B). Similarly, there was a significant drop in the protein sulfhydryl levels, which also decreased gradually before the onset of rapid death (Fig. 7). In contrast to the double mutants, under-expression of dPrx3 or dPrx5 alone had no demonstrable impact on the redox state. Thus, under-expression of both mitochondrial peroxiredoxins together was found to be a prerequisite for an accelerated pro-oxidizing shift in the redox state and death.
Fig. 6. HPLC analysis of GSH and GSSG in whole body homogenates prepared from flies under-expressing mitochondrial peroxiredoxins.
Results are mean + SD of three independent preparations consisting of 25 flies in each group. A, control (yw and +/Da), dprx5 mutant (dprx5), RNAi flies under-expressing dPrx3 (d/Da) and double mutant flies (d, e or f/Da;dprx5) were collected at the age of 12–15 days, or at a time just before the onset of accelerated death. Compared to the controls, there was an ~ 10-fold decrease in the GSH/GSSG ratio in all three double mutant lines (P < 0.05). B, GSH and GSSG levels were determined in control and one experimental double mutant line at the indicated ages. Differences in GSSG and GSH:GSSG ratios between control and experimental flies were statistically significant at 15 da (P<0.032) and 18 da (P<0.0054). Differences between experimental flies at 5 da vs 15 or 18 da were also significant.
Fig. 7. HPLC analysis of reactive protein sulfhydryls in whole body homogenates prepared from flies under-expressing mitochondrial peroxiredoxins.
The lines used here are as described in the legend to Fig. 6. Results are mean ± SD of three independent preparations made from 25 flies for each group. A, control and double mutant flies were collected at a time just before the onset of accelerated death (12–15 days of age). Compared to controls, there was an ~ two-fold decrease in sulfhydryl level in all three double mutant lines (P < 0.0035). B, Protein sulfhydryl levels were determined in control and one experimental double mutant line at the indicated ages. Differences in the amounts of reactive protein thiols between the double mutant and the control flies were statistically sigificant (P<0.05) at all examined ages.
While there were striking changes in the redox state of the double mutant, other markers of oxidative stress and mitochondrial function were not affected notably. For instance, there were no significant differences between the double mutant, the single mutants and the controls in activities of mitochondrial oxidoreductases, NADH dehydrogenase (Complex I) and cytochrome c oxidase (Complex IV), mitochondrial respiration supported by alpha-glycerophosphate and mitochondrial membrane potential (Suppl. Figs. 1–3). There were also no differences in the NADP+/NADPH and NAD+/NADH redox couples (Suppl. Fig. 4). There was a statistically significant decrease in the mitochondrial ATP levels in the dprx5 mutant, compared to the control; however under-expression of dPrx3 in the dprx5 mutant background did not further enhance this phenotype (Suppl. Fig. 5). Surprisingly, a predicted increase in the rate of mitochondrial production of H2O2, measured with Amplex Red reagent (Invitrogen), was not observed in the double mutant (data not shown). There was an increase in the presence of mitochondrial protein-HNE adducts, which might have arisen through increased lipid peroxidation; however the differences were not statistically significant (Suppl. Fig. 5).
A rapid death phenotype of the double mutant is partially rescued by over-expression of thioredoxin reductase but not catalase
The dramatic accrual of GSSG in the double mutant and a drop in the protein sulfhydryl levels, compared to much more modest, if any, changes in other markers of oxidative stress, led us to hypothesize that the ablation of peroxiredoxin activity affected specific target(s) rather than causing a general shift towards a more pro-oxidizing environment. Moreover, these potential targets are likely to be components of the thioredoxin and glutathione systems that maintain the thiol: disulfide homeostasis. In Drosophila, thioredoxin reductase (dTrxR) serves the function that corresponds to glutathione reductase [26]. TrxR also supports the activity of other types of thiol oxidoreductases, thioredoxins and glutaredoxins. To test the hypothesis that peroxiredoxins protect components of thioredoxin/glutathione system from oxidation, we generated flies overexpressing thioredoxin reductase, using fly lines containing the dTrxR trangene [16], in the dPrx3/dPrx5 ‘double mutant’ background. We also generated flies expressing mitochondrion-targeted catalase with simultaneous under-expression of dPrx3 and dPrx5 using the low-level global enhancer Arm-GAL4. Ectopic expression of catalase in mitochondria has been shown previously to reduce mitochondrial production of H2O2 [13]. The level of mitochondrially-localized catalase expressed from the UAS-mtCat transgene was about 20–50% that of the endogenous cytosolic catalase. No catalase protein was detected in the mitochondria isolated from controls (data not shown). Higher levels of mtCat expression using the global Act-GAL4, Tub-GAL4 or Da-GAL4 drivers were detrimental to fly survivorship (unpublished data).
Expression of mtCat at moderate levels with Arm-GAL4 did not rescue the shortened life span phenotype in the peroxiredoxin double mutant (Fig. 8 A). It should be noted that this “double mutant” is distinct from our standard double mutant, since it uses a weaker GAL4 driver (Arm-GAL4) to knock down Prx3 expression. The result is a slightly less severe phenotype in terms of longevity (median age 16 da vs 7 da – Fig. 8 A vs Fig. 8 B). This was necessitated by the fact that strong global expression of mtCat, as indicated above, has detrimental effects on fly survivorship. It was possible, however, to use our strong double mutant in conjunction with dTrxR over-expression and we found that dTrxR substantially prolonged survivorship of flies under-expressing dPrx3/dPrx5, increasing median age by almost 60% (Fig. 8 B). Molecular analysis revealed that over-expression of dTrxR not only alleviated premature aging phenotype but also essentially restored sulfhydryl levels, which were depleted in the dPrx3/dPrx5 mutant (Fig. 8 C). Furthermore, over-expression of dTrxR resulted in reduction of GSSG levels, which were significantly elevated in the double mutant, and thus ameliorated the GSH:GSSG ratio (Fig. 8 D–F). Altogether, the data suggested that the peroxiredoxin activity in mitochondria provides protection of thioredoxin/glutathione system from oxidation and inactivation rather than serving primarily as a mechanism for the modulation of hydrogen peroxide release from mitochondria.
Fig. 8. Effects of underexpression of dPrx3 and dPrx5 were offset by over-expression of thioredoxin reductase but not catalase.
Survivorship of peroxiredoxin double mutant flies expressing mitochondion-targeted catalase (A) or thioredoxin reductase (B). Shown are representative data of two independent experiments. In all experiments, there were 100–150 flies in each group. A, control fly line (C) contained the UAS-mtCat transgene and an allele containing RNAi-dPrx3 transgene and the dprx5 mutation, resulting in a genotype UAS-mtCat/+;RNAi-dPrx3,dprx5/+. Flies under-expressing dPrx3 and dPrx5 (dprx3,dprx5) were made by using Arm-GAL4 driver and RNAi-dPrx3,dprx5 allele. The resulting genotype was Arm-GAL4/+;RNAi-dPrx3,dprx5/RNAi-dPrx3,dprx5. Flies expressing mtCat in peroxiredoxin mutant background (mtCat;dprx3,dprx5) had a genotype UAS-mtCat/Arm-GAL4; RNAi-dPrx3,dprx5/RNAi-dPrx3,dprx5. B, Control fly line (C) contained the dTrxR transgene and an allele containing RNAi-dPrx3 transgene and the dprx5 mutation, resulting in a genotype dTrxR/+;RNAi-dPrx3,dprx5/+. Flies under-expressing dPrx3 and dPrx5 (dprx3,dprx5) were those described in Fig. 4 and had a genotype RNAi-dPrx3,dprx5/Da-GAL4,dprx5. Flies over-expressing dTrxR in a peroxiredoxin mutant background (dTrxR;dprx3,dprx5) were dTrxR/+;RNAi-dPrx3,dprx5/Da-GAL4,dprx5. Median age of flies under-expressing dPrx3 and dPrx5 (dprx3,dprx5) was 7.2±0.8 days and median age of flies over-expressing dTrxR in a peroxiredoxin mutant background (dTrxR;dprx3,dprx5) was 11.4±0.6 days. C, sulfhydryl content in flies over-expressing dTrxR. Shown are flies under-expressing dPrx3 and dPrx5 (dprx3,dprx5), their heterozygous control (dprx3,dprx5/+), flies over-expressing thioredoxin reductase (dTrxR) and flies over-expressing thioredoxin reductase in peroxiredoxin mutant background (dTrxR; dprx3,dprx5). Results are mean ± SD of three independent preparations made from 25 flies for each group. Statistically significant differences (P<0.05) are indicated by asterisks. D–F, HPLC analysis of GSH (D), GSSG (E) and their ratio (F) in whole body homogenates prepared from flies under-expressing mitochondrial peroxiredoxins (dprx3,dprx5) and over-expressing thioredoxin reductase (dTrxR). Results are mean + SD of four independent preparations consisting of 25 flies in each group. Flies were collected before the onset of accelerated death. Statistically significant differences (P<0.05) are denoted by asterisks.
Under-expression of dPrx3 and dPrx5 induces tissue-specific apoptosis
We also investigated whether the reduction in peroxiredoxin activity in mitochondria and the observed changes in thiol homeostasis had an impact on the induction of apoptosis. Indeed, using TUNEL labeling to assess DNA fragmentation in cells in situ, we found a dramatic increase in the number of cells undergoing apoptosis in the thoracic muscles, digestive tract and oenocytes of double mutant flies, compared to the single RNAi-dPrx3 mutants and control flies (Fig. 9). In flies with normal levels of dPrx5, but reduced levels of dPrx3, there was a slight increase in apoptosis relative to controls (Fig. 9 B). As reported previously [4], compared to the control, the dprx5 mutant exhibited somewhat increased levels of apoptosis, localized to specific tissues (muscle, digestive tract and oenocytes). These patterns were conserved, albeit considerably more intensely, in the double mutants (Fig. 9 D), with the exception of oenocytes, where no further enhancement of apoptosis was observed. A particularly strong fluorescence was observed in the proventriculus (cardia) of the double mutants (Fig. 9 D), an effect, which was mimicked in paraquat-administered yw controls and in flies under-expressing dPrx3 (Fig. 9 B). These prominent apoptotic signatures observed in the double mutant flies have also been documented in old flies [27].
Fig. 9. TUNEL analysis of cell death in yw control (A), flies under-expressing dPrx3 (B), dprx5 mutant (C) and flies under-expressing dPrx3 in a dprx5 mutant background (D).
Representative images show DNA fragmentation in cryosections made from 10 day old flies reared under normal conditions or fed 10 mM paraquat for 6 hours (B, image on the right). Shown are selected images of the abdomen and thoracic regions, where differences in DNA fragmentation between control and mutant flies. In the double mutant, apoptosis was particularly strong in the gut epithelia, cardia (or proventriculus) and thoracic muscle. g, gut lumen; oe, oenocyte.
In summary, the double mutant flies differed from the flies with reduced levels of dPrx3 or dprx5 null flies or wild type controls by exhibiting a much earlier shift to a pro-oxidizing redox state manifested by large decreases in protein sulfhydryls and GSH/GSSG ratios. This shift was accompanied by a massive induction of apoptosis in thoracic muscles and gut tissue, previously documented biomarkers of aged flies, as well as a drastic reduction in life span.
DISCUSSION
Results of this study demonstrate that the mitochondrial peroxiredoxins, dPrx5 and dPrx3, play a key role in the maintenance of cellular redox state, prevention of apoptosis and survival beyond the first quartile of adult life. Simultaneous absence of both dPrxs results in precipitous failure of thiol homeostasis. While perhaps not the sole reason, this collapse of thiol homeostasis could certainly contribute to the functional deterioration, culminating in death. Consequently, it is possible to infer that these two peroxiredoxins display significant functional overlap in both the maintenance of redox homeostasis and achievement of potential maximum life span.
Mitochondria are a major intra-cellular site for the generation of O2− and H2O2 as well as the highly reactive oxidant, peroxynitrite, formed by a reaction between O2− and nitric oxide. Mitochondria of dipteran flies, such as Drosophila, are known to produce O2− and H2O2 at relatively high rates, which increase by 50–100% during aging. Although all three of the above reactive molecular species play a physiological role in the redox-based cell signaling, they can also be hazardous at relatively high concentrations because of their ability to impair mitochondrial function by inactivating Fe-S centers in the electron transport chain, fostering structural damage to macromolecules and triggering apoptosis [28].Because mitochondrially-generated H2O2 readily permeates into the cytosol, it can cause pan-cellular changes in redox state by reacting with thiol-containing molecules. Thus the intra-mitochondrial peroxiredoxins, by virtue of their thioredoxin peroxidase activity and the peroxynitrite reductase activity associated with Prx5, can be surmised to play a role in the maintenance of redox balance as well as to provide protection against oxidant attacks [5, 8, 29].
As described in a previous report, the absence of dPrx5 results in a small reduction in mean but not maximum life span, an increase in sensitivity to oxidative stress and an enhancement of tissue-specific apoptosis [4]. Using an RNAi strategy, a similar analysis was conducted here, in which dPrx3 has been knocked down by 90–95%. While the knock-down flies were marginally more sensitive to oxidative stress relative to controls, no differences were observed in terms of survivorship in the absence of stressors or with respect to the enhancement of apoptosis. It may thus be conjectured that the residual amounts of dPrx3 present were sufficient to maintain a normal phenotype and/or the wild type dPrx5 activity present in the mitochondria was sufficient to compensate for the reduced dPrx3 levels. The latter explanation is more plausible because the lack of Prx3 in other organisms also does not significantly affect the physiological functions. For instance, the phenotype of the Prx3 knock-out mouse has been reported to be quite benign, exhibiting a relatively normal phenotype with only some tissues or cells, such as the placenta or macrophages, displaying signs of oxidative stress [30, 31], which supports the contention that Prx5 can largely substitute for Prx3 function in mitochondria. An additional argument for the redundancy comes from the observation that over-expression of dPrx3 produces no significant increase in longevity. The longevity effects of dPrx5 are significantly greater (ca. 13–30%) indicating that some of the longevity effect may be due to extra-mitochondrial expression and/or the greater versatility of this enzyme, such as its peroxynitrite reductase activity.
The simultaneous reduction of dPrx3 via RNAi and dPrx5, using the null dprx5 mutation, resulted in flies with a severely shortened life span (mean life span = 12.8 da). These results underscore the importance of peroxiredoxin expression in the mitochondrial compartment. Somewhat surprisingly, resistance to oxidative stress, induced by H2O2 or paraquat administration, was not enhanced in the double mutant compared to dprx5 alone. Studies conducted in human cell cultures, where under-expression of both peroxiredoxin 3 and peroxiredoxin 5 were found not to further enhance susceptibility to oxidative damage and apoptosis, induced by MPP(+), are in agreement with our observations [32]. It would thus appear that the extent of the fly’s capacity to overcome H2O2 or paraquat induced stress does not fully explain the reason for the rapid death observed in these flies. This view is supported by the observation that the expression of mitochondrially-targeted catalase was found to be unable to rescue the aging phenotype of the double mutant (Fig. 8 A). Thus, it seems that the mitochondrial peroxiredoxins function to protect specific targets susceptible to H2O2 oxidation rather than the attenuation of the overall levels of H2O2 in mitochondria. The in vivo reactions of peroxiredoxins are still not fully understood. In vitro, these enzymes are active at low concentrations of hydrogen peroxide and are inactivated at higher concentrations [33]. It was therefore suggested that peroxiredoxins respond to local H2O2 fluxes in the vicinity of target groups. Present demonstrated that in the absence of peroxiredoxins the most dramatic changes occurred in thiol compounds (acuumulation of GSSG and protein mixed disulfides) and to a significantly lesser extent in other oxidation products (lipid peroxidation, H2O2 accumulation). It is plausible that peroxiredoxins utilize H2O2 to modify target thiol groups, which then form specific intermediates with varied functional outcomes.
Our provisional hypothesis is that the early death observed in the double mutant flies is a consequence of the severe disturbance in the redox state, presumably emanating from the near absence of mitochondrial peroxiredoxins. Without the presence of adequate levels of mitochondrial peroxiredoxins, GSH is likely to assume a more critical role in the maintenance of redox equilibrium in the mitochondrial compartment, hence the greater likelihood of GSH oxidation. Indeed, the increase in GSSG levels observed in the double mutant, supports this hypothesis. The relatively free movement of H2O2 through membranes may also stimulate enhanced GSH oxidation in the extra-mitochondrial milieu. However, in our opinion accumulation of GSSG occurred mainly due to the failure of mechanisms for the reduction of oxidized thiols.
In Drosophila, the reduction of GSSG to GSH is largely supported by the thioredoxin system in two consecutive reactions, where GSSG is first reduced by thioredoxin, which in turn is reduced by NADPH-dependent thioredoxin reductase activity [26]. It is conceivable that fluxes of H2O2, in the absence of mitochondrial peroxiredoxin activity, could lead to the oxidation of cysteine residues in the active sites of thioredoxins and/or thioredoxin reductase, thereby causing the broader thiol/disulfide shift (Fig. 10). Thus we presumed that thiol homeostasis is largely supported by the ability of peroxiredoxins to shield functional sulfhydryls from oxidation and consequent inactivation. Indeed, the observed decrease in the level of protein thiols (Fig. 7) and the rescue effects conferred by thioredoxin reductase (Fig. 8 B and C) support this hypothesis. The results also suggest an inter-dependence between peroxiredoxins and thioredoxin/thioredoxin reductase in their functioning. We have not yet investigated the rescue effects by thioredoxins, as no Drosophila mitochondria-localized thioredoxin(s) have as yet been identified.
Fig. 10. A model illustrating interactions among the redox maintenance system in Drosophila.
Hydrogen peroxide is reduced by the activity of peroxiredoxins (Prx), which obtain reducing equivalents from thioredoxin (Trx).Oxidized thioredoxin is reduced by thioredoxin reductase (TrxR), which, in turn, receives reducing equivalents from NADPH. GSSG is reconverted to GSH by the activity of TrxR in a reaction coupled to thioredoxin, which is involved in dithiol-disulfide exchange with GSSG and also catalyses the reduction of protein disulfide bridges. In the absence of Prx activity, concentration of peroxides and the consequent oxidation of thiols would tend to increase. Oxidation of functional thiol groups in the active centers of Trx and TrxR can halt or obstruct the recycling of GSSG and reduction of protein disulfides, thereby further aggravating the disturbance in redox homeostasis.
Decreases in redox potential and protein sulfhydryl levels may affect different signaling pathways and stimulate apoptosis in the various fly tissues. Such causal links between reduced/oxidized glutathione ratio, damage to mitochondria and the stimulation of apoptosis have been firmly established in mammalian cells [34–36]. Efforts are currently under way to identify the mechanisms, which promote apoptosis in Drosophila under these conditions. This may be particularly relevant to aging, since the phenotypes of the double mutant are strikingly similar to those observed in old flies. Indeed, the observed changes in thiol homeostasis in the double mutant with a premature aging phenotype (Fig. 6 and 7) were similar to those observed in flies undergoing normal aging, characterized by elevation in GSSG levels and an increase in protein mixed disulfides [21]. It is quite remarkable that the apoptotic patterns of the double mutant, where cardiac and muscle tissues were committed to apoptosis, were also a characteristic of old flies [27].
To our knowledge this is the first study in which the effects of decreases in the levels of both mitochondrial peroxiredoxins, Prx3 and Prx5, have been examined in the whole organism.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Genying Niu for generating UAS-dPrx3 transgenic lines. The study was supported by the grant, RO1 AG15122.from National Institutes of Health-National Institute on Aging.
ABBREVIATIONS
- Prx
peroxiredoxin
- ROS
reactive oxygen species
- UAS
upstream activating sequence
- Tub
tubulin
- Da
daughterless
- Arm
armadillo
- y w
yellow white
- TCEP
Tris(2-carboxyethyl)-Phosphine
- TrxR
thioredoxin reductase
- MPA
meta-phosphoric acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Michalak K, Orr WC, Radyuk SN. Drosophila peroxiredoxin 5 is the second gene in a dicistronic operon. Biochemical and biophysical research communications. 2008;368:273–278. doi: 10.1016/j.bbrc.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radyuk SN, Klichko VI, Spinola B, Sohal RS, Orr WC. The peroxiredoxin gene family in Drosophila melanogaster. Free radical biology & medicine. 2001;31:1090–1100. doi: 10.1016/s0891-5849(01)00692-x. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez J, Agudo M, Van Damme J, Vandekerckhove J, Santaren JF. Polypeptides differentially expressed in imaginal discs define the peroxiredoxin family of genes in Drosophila. European journal of biochemistry / FEBS. 2000;267:487–497. doi: 10.1046/j.1432-1327.2000.01022.x. [DOI] [PubMed] [Google Scholar]
- 4.Radyuk SN, Michalak K, Klichko VI, Benes J, Rebrin I, Sohal RS, Orr WC. Peroxiredoxin 5 confers protection against oxidative stress and apoptosis and also promotes longevity in Drosophila. The Biochemical journal. 2009 doi: 10.1042/BJ20082003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae HZ, Kim HJ, Kang SW, Rhee SG. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes research and clinical practice. 1999;45:101–112. doi: 10.1016/s0168-8227(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 6.Seo MS, Kang SW, Kim K, Baines IC, Lee TH, Rhee SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. The Journal of biological chemistry. 2000;275:20346–20354. doi: 10.1074/jbc.M001943200. [DOI] [PubMed] [Google Scholar]
- 7.Kropotov A, Sedova V, Ivanov V, Sazeeva N, Tomilin A, Krutilina R, Oei SL, Griesenbeck J, Buchlow G, Tomilin N. A novel human DNA-binding protein with sequence similarity to a subfamily of redox proteins which is able to repress RNA-polymerase-III-driven transcription of the Alu-family retroposons in vitro. European journal of biochemistry / FEBS. 1999;260:336–346. doi: 10.1046/j.1432-1327.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Kok KH, Chun AC, Wong CM, Wu HW, Lin MC, Fung PC, Kung H, Jin DY. Mouse peroxiredoxin V is a thioredoxin peroxidase that inhibits p53-induced apoptosis. Biochemical and biophysical research communications. 2000;268:921–927. doi: 10.1006/bbrc.2000.2231. [DOI] [PubMed] [Google Scholar]
- 9.Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. The Journal of biological chemistry. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 10.Banmeyer I, Marchand C, Clippe A, Knoops B. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS letters. 2005;579:2327–2333. doi: 10.1016/j.febslet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Kropotov A, Gogvadze V, Shupliakov O, Tomilin N, Serikov VB, Tomilin NV, Zhivotovsky B. Peroxiredoxin V is essential for protection against apoptosis in human lung carcinoma cells. Experimental cell research. 2006;312:2806–2815. doi: 10.1016/j.yexcr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Sohal RS, Arnold L, Orr WC. Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mechanisms of ageing and development. 1990;56:223–235. doi: 10.1016/0047-6374(90)90084-s. [DOI] [PubMed] [Google Scholar]
- 13.Kwong LK, Mockett RJ, Bayne AC, Orr WC, Sohal RS. Decreased mitochondrial hydrogen peroxide release in transgenic Drosophila melanogaster expressing intramitochondrial catalase. Archives of biochemistry and biophysics. 2000;383:303–308. doi: 10.1006/abbi.2000.2093. [DOI] [PubMed] [Google Scholar]
- 14.Bao S, Cagan R. Fast cloning inverted repeats for RNA interference. RNA. 2006;12:2020–2024. doi: 10.1261/rna.258406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candas M, Sohal RS, Radyuk SN, Klichko VI, Orr WC. Molecular organization of the glutathione reductase gene in Drosophila melanogaster. Archives of biochemistry and biophysics. 1997;339:323–334. doi: 10.1006/abbi.1996.9872. [DOI] [PubMed] [Google Scholar]
- 16.Mockett RJ, Sohal RS, Orr WC. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. FASEB J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 17.Missirlis F, Ulschmid JK, Hirosawa-Takamori M, Gronke S, Schafer U, Becker K, Phillips JP, Jackle H. Mitochondrial and cytoplasmic thioredoxin reductase variants encoded by a single Drosophila gene are both essential for viability. The Journal of biological chemistry. 2002;277:11521–11526. doi: 10.1074/jbc.M111692200. [DOI] [PubMed] [Google Scholar]
- 18.Toroser D, Orr WC, Sohal RS. Carbonylation of mitochondrial proteins in Drosophila melanogaster during aging. Biochemical and biophysical research communications. 2007;363:418–424. doi: 10.1016/j.bbrc.2007.08.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radyuk SN, Sohal RS, Orr WC. Thioredoxin peroxidases can foster cytoprotection or cell death in response to different stressors: over- and under-expression of thioredoxin peroxidase in Drosophila cells. The Biochemical journal. 2003;371:743–752. doi: 10.1042/BJ20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radyuk SN, Rebrin I, Luchak JM, Michalak K, Klichko VI, Sohal RS, Orr WC. The catalytic subunit of Drosophila glutamate-cysteine ligase is a nucleocytoplasmic shuttling protein. The Journal of biological chemistry. 2009;284:2266–2274. doi: 10.1074/jbc.M805913200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebrin I, Bayne AC, Mockett RJ, Orr WC, Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. The Biochemical journal. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free radical biology & medicine. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuji K, Copeland NG, Jenkins NA, Obinata M. Mammalian antioxidant protein complements alkylhydroperoxide reductase (ahpC) mutation in Escherichia coli. The Biochemical journal. 1995;307(Pt 2):377–381. doi: 10.1042/bj3070377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto T, Matsui Y, Natori S, Obinata M. Cloning of a housekeeping-type gene (MER5) preferentially expressed in murine erythroleukemia cells. Gene. 1989;80:337–343. doi: 10.1016/0378-1119(89)90297-7. [DOI] [PubMed] [Google Scholar]
- 25.Watabe S, Hiroi T, Yamamoto Y, Fujioka Y, Hasegawa H, Yago N, Takahashi SY. SP-22 is a thioredoxin-dependent peroxide reductase in mitochondria. European journal of biochemistry / FEBS. 1997;249:52–60. doi: 10.1111/j.1432-1033.1997.t01-1-00052.x. [DOI] [PubMed] [Google Scholar]
- 26.Kanzok SM, Fechner A, Bauer H, Ulschmid JK, Muller HM, Botella-Munoz J, Schneuwly S, Schirmer R, Becker K. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science (New York, N.Y. 2001;291:643–646. doi: 10.1126/science.291.5504.643. [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, Edelman SW, Tharmarajah G, Walker DW, Pletcher SD, Seroude L. Differential patterns of apoptosis in response to aging in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12083–12088. doi: 10.1073/pnas.0503374102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace DC. Mitochondrial diseases in man and mouse. Science (New York, N.Y. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 29.Dubuisson M, Vander Stricht D, Clippe A, Etienne F, Nauser T, Kissner R, Koppenol WH, Rees JF, Knoops B. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS letters. 2004;571:161–165. doi: 10.1016/j.febslet.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Shoji W, Oshima H, Obinata M, Fukumoto M, Kanno N. Crucial role of peroxiredoxin III in placental antioxidant defense of mice. FEBS letters. 2008;582:2431–2434. doi: 10.1016/j.febslet.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 31.Yang HY, Jeong DK, Kim SH, Chung KJ, Cho EJ, Jin CH, Yang U, Lee SR, Lee DS, Lee TH. Gene expression profiling related to the enhanced erythropoiesis in mouse bone marrow cells. Journal of cellular biochemistry. 2008;104:295–303. doi: 10.1002/jcb.21620. [DOI] [PubMed] [Google Scholar]
- 32.De Simoni S, Goemaere J, Knoops B. Silencing of peroxiredoxin 3 and peroxiredoxin 5 reveals the role of mitochondrial peroxiredoxins in the protection of human neuroblastoma SH-SY5Y cells toward MPP+ Neuroscience letters. 2008;433:219–224. doi: 10.1016/j.neulet.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 33.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science (New York, N.Y. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 34.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxidants & redox signaling. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 35.Martensson J, Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson CP, Tsai JM, Meek WE, Liu RM, Tang Y, Forman HJ, Reynolds CP. Depletion of glutathione by buthionine sulfoxine is cytotoxic for human neuroblastoma cell lines via apoptosis. Experimental cell research. 1999;246:183–192. doi: 10.1006/excr.1998.4303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.