Abstract
Cryo transmission x-ray microscopy in the “water window” of photon energies has recently been introduced as a method that exploits the natural contrast of biological samples. We have used cryo tomographic x-ray imaging of the intraerythrocytic malaria parasite, Plasmodium falciparum, to undertake a survey of the cellular features of this important human pathogen. We examined whole hydrated cells at different stages of growth and defined some of the structures with different x-ray density, including the parasite nucleus, cytoplasm, digestive vacuole and the hemoglobin degradation product, hemozoin. As the parasite develops from an early cup-shaped morphology to a more rounded shape, puncta of hemozoin are formed; these coalesce in the mature trophozoite into a central compartment. In some trophozoite stage parasites we observed invaginations of the parasite surface and, using a selective permeabilization process, showed that these remain connected to the RBC cytoplasm. Some of these invaginations have large openings consistent with phagocytic structures and we observed independent endocytic vesicles in the parasite cytoplasm which appear to play a role in hemoglobin uptake. In schizont stage parasites staggered mitosis was observed and x-ray-dense lipid-rich structures were evident at their apical ends of the developing daughter cells. Treatment of parasites with the antimalarial drug artemisinin appears to affect parasite development and their ability to produce the hemoglobin breakdown product, hemozoin.
Keywords: Plasmodium, digestive vacuole, hemoglobin uptake, cryo x-ray tomography, soft x-ray microscopy
INTRODUCTION
Of the different species of malaria parasite that infect humans Plasmodium falciparum is the most deadly; it is responsible for close to one million deaths per year (WHO, 2009). During its intra-erythrocytic development, the parasite grows and divides, feeding on the hemoglobin, and remodeling the host red blood cell (RBC) to promote its own survival. The blood stages are responsible for the clinical symptoms of the disease which range from uncomplicated fevers to life-threatening cerebral and placental malaria (Miller et al., 2002). The complications are due to the cytoadherence of infected RBCs to receptors on brain venule epithelial cells or (in pregnant women) to placental syncytiotrophoblasts. The adhesion process prevents phagocytic clearance in the spleen which contributes to virulence, while an inappropriate host immune response to the sequestered parasites can induce coma and death (Rogerson et al., 2004).
The intraerythrocytic parasite resides within a parasitophorous vacuole (PV) that is formed during the invasion step (Bannister et al., 2000). It develops through the ring, trophozoite and schizont stages, eventually bursting to release 16–20 daughter merozoites (Bannister et al., 2000; Garcia et al., 2008). The ring stage (0 – ~20 h) is thought to be a metabolically sluggish phase during which the parasite exports a range of proteins to the RBC cytoplasm. As it grows the parasite expands its core complement of organelles needed for metabolism (nucleus, endomembrane system, mitochondria, and apicoplast), and develops novel organelles including a modified lysosome (referred to as the digestive vacuole) and a series of apical organelles (rhoptries, dense granules and micronemes) (Bannister and Mitchell, 2009; Bannister et al., 2000; Kats et al., 2006).
As the intraerythrocytic parasite develops it needs to obtain a source of amino acid building blocks and to create sufficient space for expansion and division (Goldberg, 2005; Lew et al., 2003). To achieve this, the parasite digests its host cell from the inside, consuming ~75% of the host hemoglobin (Loria et al., 1999). Uptake of host cytoplasm is most active in the trophozoite stage and is thought to involve endocytic structures that bud from the surface of the parasite and deliver hemoglobin to the digestive vacuole (Slomianny, 1990; Yayon et al., 1984). Three recent electron microscopy (EM)-based studies have re-examined hemoglobin uptake in infected RBC leading to somewhat conflicting views of the endocytic process (Abu Bakar et al., 2010; Elliott et al., 2008; Lazarus et al., 2008).
Artemisinin, an endoperoxide-based drug, and its derivatives, are now recommended treatments for malaria in many countries (Golenser et al., 2006; Meshnick, 2002; Tilley et al., 2006). The mechanism of action of artemisinin is still debated with some studies suggesting that the primary target lies within the ER (Eckstein-Ludwig et al., 2003; Krishna et al., 2004) or the mitochondrion (Wang et al., 2010). By contrast other studies have suggested that the digestive vacuole is an important site of artemisinin action (del Pilar Crespo et al., 2008; Maeno et al., 1993; Pandey et al., 1999).
The advent of molecular transfection technology, coupled with fluorescence microscopy analyses of fluorescent protein reporters, has greatly improved our understanding of the ways in which the malaria parasite alters its host cell (Tilley et al., 2007). However the resolution of conventional fluorescence microscopy is limited and there are concerns that the labeling of cellular components may alter their morphology and function (Marks and Nolan, 2006). EM offers superb resolution however there are concerns that the harsh fixation and sample manipulation that is required can introduce artifacts (Frey et al., 2006; McIntosh et al., 2005; Perktold et al., 2007). Thus it is important to compare data from visible light and electron microscopy with alternative techniques that provide the highest possible resolution while minimizing sample manipulation.
X-ray cryo-tomography has recently been introduced as a high resolution technique that can be performed on whole hydrated cells with a lower potential for artifacts. The differential absorption of soft x-rays by organic matter and water provides natural contrast and avoids the need for exogenous stains or chromophores. Due to the short wavelengths of x-rays this technique can achieve higher resolution than conventional optical microscopy (Larabell and Le Gros, 2004; Le Gros et al., 2005; Parkinson et al., 2008; Uchida et al., 2009). In this work we have used x-ray cryo-tomography to study the development of P. falciparum in infected RBCs.
MATERIALS AND METHODS
Parasites
P. falciparum parasites were cultured in vitro in RPMI medium supplemented with 4% human serum plus 0.25% AlbuMAX (GIBCO, Invitrogen) as described previously (Jackson et al., 2007). 3D7_MAHRP1-GFP transfectants were generated as described previously (Spycher et al., 2006). RBCs and pooled sera were obtained from the Red Cross Transfusion Service (Melbourne, Australia) or from volunteers (ALS, Berkeley, USA). Trophozoite stage-infected RBCs were harvested by flotation on a Percoll/sorbitol gradient (Aley et al., 1986) or using a magnetized column (Trang et al., 2004).
Immunolabeling
Freshly harvested infected RBCs were lightly fixed with 2% paraformaldehyde in RPMI medium (10 min), permeabilized with Equinatoxin II (EqtII; (Anderluh et al., 1996; del Pilar Crespo et al., 2008)), and re-fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4. Reactive groups were blocked with 2% BSA in PBS and cells were incubated with the primary antibody for 2 h at room temperature (rabbit anti-GFP (Humphries et al., 2005); 1:20 in 3% bovine serum albumin (BSA) in PBS). After washing the cells were incubated with Protein A gold (6 nm, Aurion, NL) and fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate, pH 7.3. The gold labeling was silver enhanced using R-Gent SE-EM (Aurion, NL).
Resealing RBCs and artemisinin treatment
RBCs were lysed in 2.5 volumes of ice-cold 5 mM phosphate, pH 7.5, 1 mM Mg-ATP (Sigma) in the presence of albumin labeled with ultrasmall gold particles (Aurion BSA gold tracer, previously concentrated and exchanged into the same buffer using a Centricon Centrifugal Filter Device) and resealed as described previously (Frankland et al., 2006). After 10 minutes on ice NaCl was added from a concentrated stock to a final concentration of 0.15 M and samples were incubated at 37°C for 45 minutes to reseal the cells. Cells were washed and the amount of retained hemoglobin was estimated spectrophotometrically at 415 nm. The resealed cells were mixed with schizonts and the parasites were allowed to invade and develop under culture conditions. The infected RBCs were prepared for X-ray imaging, in some cases after permeabilization with EqtII, and gold enhancement of the gold particles. For drug treatment mid-ring stage parasites (~14 h invasion, D10 strain) were incubated in the presence or absence of 60 nM artemisinin for 16 h then purified using a magnetic column. The cells were washed and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate, pH 7.3.
X-ray tomography
Aliquots of harvested infected RBCs (3D7 wild type) or EqII-permeabilized, immuno-gold labeled infected RBCs (MAHRP1-GFP transfectants) or resealed infected RBCs were used without fixation or fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4. The infected RBCs were transferred into ~8 µm glass capillaries. Fiducial particles (100 nm) were added to the outside of the capillary (note: some enter the bottom of the tube). The samples were rapidly frozen in liquid nitrogen and mounted in a cryogenic gas stream (liquid nitrogen cooled helium gas) (Le Gros et al., 2005). Projection images were collected using a transmission soft x-ray microscope (XM-2; beamline 2.1.2 at the Advanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA). The microscope is equipped with a Fresnel zone plate condenser and objective (with 60 nm and 50 nm outer zone widths, respectively. Data were collected using x-rays with an energy of 517 eV (2.4 nm), and 16-bit images were recorded using a Peltier-cooled, back-illuminated, 2048 × 2048 soft x-ray CCD camera (Andor Technology PLC, Belfast). Projection images were collected using exposure times of 0.25 s. A full tomographic data set consisted of 90 images collected at 2° increments over 180° of rotation. In addition to the data images, 10 background images were collected (with the sample moved out of the field of view). Each data image was divided by the average of the background images, and the negative logarithm of the quotient calculated to give images whose gray values correlate directly with the x-ray absorption coefficients. The IMOD package (Kremer et al., 1996; Mastronarde, 1997) was used to align the individual images. Tomographic reconstructions were calculated using iterative reconstruction methods (Erdogan and Fessler, 1999; Stayman and Fessler, 2000). Segmentation models were generated with 3dmod (http://bio3d.colorado.edu/), Blender (www.blender.org) and Amira (Visage Imaging, USA).
RESULTS
Structural features of different stages of intraerythrocytic growth of P. falciparum
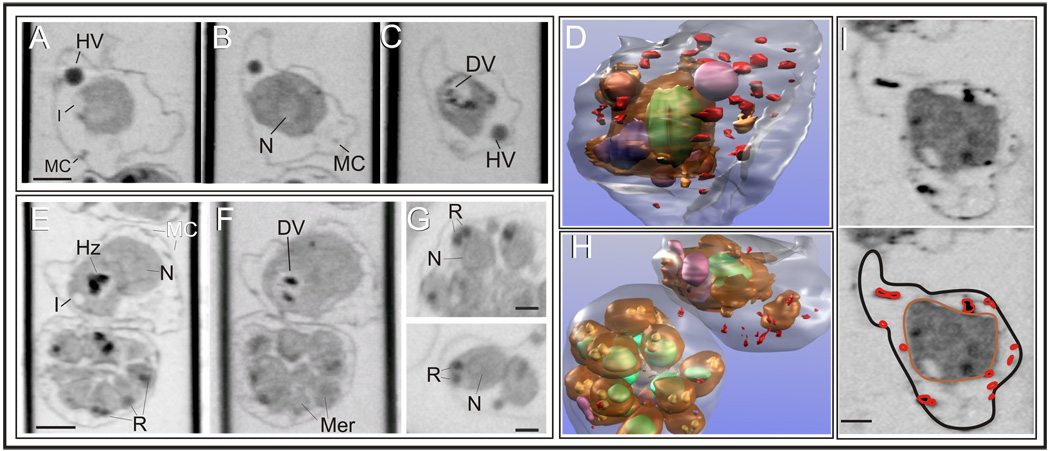
The XM-2 transmission x-ray microscope was used to examine cellular compartments of P. falciparum at different stages of development. Infected RBCs were harvested, loaded unfixed into glass capillaries, snap frozen in liquid nitrogen, and mounted in the microscope chamber. The sample was rotated through 180° collecting a series of images that were aligned to generate a tomogram. These are the first available cryo x-ray tomograms of P. falciparum-infected RBCs. In a projection image of a region of the capillary containing a trophozoite-infected RBC (Fig. 1A) the shapes of the host RBC and the intracellular parasite are evident, however the strong absorbance of hemoglobin makes it difficult to distinguish intracellular structures. By contrast in an individual virtual section (Fig. 1B), features of the intraerythrocytic parasite are revealed. The parasite cytoplasm exhibits higher x-ray absorption than the surrounding RBC and an x-ray dense feature is observed near the centre of the parasite; this is an aggregate of hemozoin crystals, a pigment that is generated during the breakdown of hemoglobin (Fig. 1B, black arrow). Regions of lower density are observed near the surface of the parasite (Fig. 1B, white arrowhead); these appear to be endocytic invaginations of the host cytoplasm into the parasite. At least some of these invaginations appear to remain open to the RBC cytoplasm.
Fig. 1. X-ray tomography of intact P. falciparum-infected RBCs.
Parasite-infected RBCs were mounted in glass capillaries then snap frozen and imaged using the XM-2 transmission x-ray microscope during rotation through 180°. (A) A projection view of a trophozoite-infected RBC in which the outline of the host cell and the parasite are visible. (B,E–G) Virtual sections through tomograms generated for parasites at different stages of development. Invaginations are observed in the surface of some of the trophozoites (B, white arrowhead). X-ray-dense hemozoin crystals are observed in more mature stage parasites (B,F,G, arrows). Membrane features are observed in the RBC cytoplasm (B, black arrowhead). (C,D) The RBC surface is rendered in translucent pink and the parasite surface in solid (C) or translucent (D) brown. Membrane features in the RBC cytoplasm are rendered in red (C) and hemozoin is depicted in purple (D). (H–I) Rendered models of an early ring (H, side and top views), a late ring (I) and a mid trophozoite (J) showing the parasite surface (translucent beige) and the hemozoin crystals (gold). The cupped parasite surface is illustrated in solid gold in H (bottom object). A translation through the virtual sections of (A) is presented in Supplementary Video S1. Scale bar, A–D 2 µm; E–J 1 µm.
Membranous features are also observed in the RBC cytoplasm (Fig. 1B, black arrowhead; best appreciated in a translation through the virtual sections, Supplementary Video S1). These are likely to represent Maurer’s cleft structures involved in the export of proteins to the RBC surface (Tilley et al., 2008). In a model of the cell (Fig. 1C) the RBC surface is rendered in translucent pink, the parasite surface in brown and the membrane-bound features in the RBC cytoplasm in red. Rendering of the parasite surface in translucent brown and hemozoin in purple (Fig. 1D) illustrates the surface invagination and shows that the hemozoin crystals are located in a central compartment (known as the digestive vacuole) in the parasite cytoplasm. We compared parasites at different stages of development. In the early ring stage (Fig. 1E,H), the parasite has a distinctly cupped profile with no evidence for intracellular hemozoin. As the parasite matures scattered hemozoin crystals are observed (Fig. 1F,I). These coalesce into a central compartment in the mid-trophozoite stage (Fig. 1G,J).
To obtain further information about the cellular features of the intraerythrocytic parasite we have used EqtII (Jackson et al., 2007) to selectively permeabilize the host cell membrane, thereby releasing the hemoglobin from the RBC cytoplasm and from connected compartments. Examples of RBCs infected with parasites of different stages (3D7 strain) are presented. The data are representative of ~90 cells that were examined.
In an early trophozoite (Fig. 2A–D) and a mid trophozoite (Fig. 2E,F,H, upper cell) the RBC membrane and the intracellular parasite are readily observed in the virtual sections (Fig. 2A–C); these have been rendered in translucent grey and brown in the models (Fig. 2D,H). The nucleus (N) is evident as a region of lower x-ray density (rendered in green). There is a single nucleus in the early trophozoite stage parasites. The digestive vacuole (rendered in dark blue) is observed as an x-ray-lucent region in the trophozoite stage parasites surrounding x-ray-dense hemozoin crystals. Hemoglobin-containing vesicles are observed in the RBC cytoplasm (Fig. 2A–C, rendered in magenta in 2D,H). Additional membranous features are observed in the RBC cytoplasm (rendered in red in the models). These are best appreciated in translations through the virtual sections (Supplementary Videos S2, S3). Rotations of the rendered models are presented in Supplementary Videos S4, S5.
Fig. 2. Tomographic analysis of EqtII-permeabilized parasitized RBCs.
(A–H) Purified mature stage-infected RBCs were permeabilized with EqtII before mounting in glass capillaries. Individual virtual slices through trophozoite (A–C, E,F, top cell) and schizont (E,F, bottom cell, G) stage parasites and rendered models of these cells (D,H). The surfaces of the trophozoites and multiple merozoites (Mer) (rendered in brown) and their nuclei (N, green) are readily observed. The digestive vacuole (blue) is resolved and the hemozoin crystals (Hz) are visible. Membrane-rich rhoptry (R) organelles are visible (rendered in gold). Hemoglobin-containing vesicles in the RBC cytoplasm are rendered in magenta and some of the membranous structures in the RBC cytoplasm are depicted in red. Translations through virtual sections and rotations of the rendered data are presented in Supplementary Videos 2–5. (I) MAHRP-GFP transfectant-infected RBCs were permeabilized and labeled with mouse anti-GFP antiserum followed by 6 nm gold-labeled protein A. The size of the gold particles was increased by silver enhancement. Dark deposits are observed in the RBC cytoplasm consistent with labeling of the Maurer’s clefts (indicated in red in the bottom panel). Scale bars: A–H, 1 µm, G,I, 500 nm.
In schizont-infected RBCs (Fig. 2E,F, lower cells, G,H) a nucleus is observed associated with each of the developing daughter cells. In some cases the nucleus is surrounded by a plasma membrane; in other cases it is not. This is consistent with previous reports that endomitotic nuclear division is initiated during the trophozoite stage before the budding of individual merozoites (Bannister et al., 2000) and indicates that development of the daughter cells can be staggered. At the outward facing end of each daughter merozoite a pair of x-ray-dense organelles is visible (Fig. 2G, rendered in gold in 2H). These are likely to be membrane-rich apical organelles known as the rhoptries. Similar morphology of trophozoite and schizont stage parasites was observed for C10 and FC27 parasite strains (data not shown).
To determine the identity of the features in the RBC cytoplasm we developed an immuno-labeling protocol and used it to examine 3D7 transfectants expressing a GFP chimera of the Membrane-Associated Histidine-Rich Protein-1 (MAHRP1) that is known to be resident in the Maurer’s clefts. EqtII-permeabilized transfectants were labeled with anti-GFP antiserum followed by 6 nm gold-labeled protein A, which was enhanced with silver. The dark deposits in the RBC cytoplasm (Fig. 2I) confirm these structures as Maurer’s clefts.
Endocytic invagination of the parasite surface
As it grows the malaria parasite takes up hemoglobin by engulfing packets of host cytoplasm. A major uptake route involves specialized structures at the parasite surface termed cytostomes (Aikawa et al., 1966). Large cytostome-independent invaginations, called phagotrophs, are also postulated to play a role in the uptake of host cytoplasm in trophozoite stage parasites (Elliott et al., 2008). These endocytic invaginations have been studied in single or serial sections of fixed, dehydrated samples prepared for EM however there are some concerns regarding the potential for artifacts with EM. Soft x-ray microscopy offers the possibility of examining these structures in whole hydrated cells.
In EqtII-permeabilized infected RBCs the invaginations of the parasite surface are clearly revealed as regions of low x-ray absorption (Fig. 3). This indicates that these compartments retain their connection to the host cell compartment, thus allowing the release of hemoglobin. The extent of the invaginations can be readily appreciated by examining different virtual sections through the tomograms (Fig. 3A–E). Similarly examining translations through the virtual sections of the reconstructions (Supplementary Video S6) show that the cell surface-connected invaginations extend deep into the parasite cytoplasm. The classical hemoglobin uptake apparatus involves cytostomal structures which remain connected to the RBC cytoplasm via a ~55 nm opening (Abu Bakar et al., 2010). The zone plates for XM-2 permit a resolution of 50 nm under optimal conditions, which may be insufficient to detect the cytostomal structures. Phagotrophic are expected to have a larger opening to the RBC cytoplasm that should be resolved. We rendered the x-ray tomograms to examine the surface opening of the invaginations. In some cases the opening is not clearly resolved; these structures likely represent cytostomal invaginations. By contrast some of the openings have diameters up to 1 µm; this is consistent with the presence of phagotrophic invaginations. Rendering of the surface of the intracellular parasite (Fig. 3F,G, grey) also highlights the convoluted extensions and invaginations of the parasite surface (see also Supplementary Video S7).
Fig. 3. X-ray tomographic analysis of surface invaginations of P. falciparum.
Purified mature stage-infected RBCs were permeabilized with EqtII. Individual virtual slices through a trophozoite in the xy (A–D) and xz (E) planes and views of a rendered model of the parasite (F,G). The convoluted surface of the parasite is rendered in brown. Surface invaginations (I) are x-ray-lucent (A–E) indicating a connection to the host cell cytoplasm; some of the openings can be resolved (G, red arrows). The digestive vacuole (DV, rendered in blue) contains hemozoin crystals (Hz). Parasite surface extensions are evident (indicted with blue arrows in the rendered model), while Maurer’s clefts (MC) are observed as independent structures in the RBC cytoplasm. A translation through the virtual sections and a rotation of the rendered data are presented in Supplementary Videos 6 and 7. Scale bar, 1 µm.
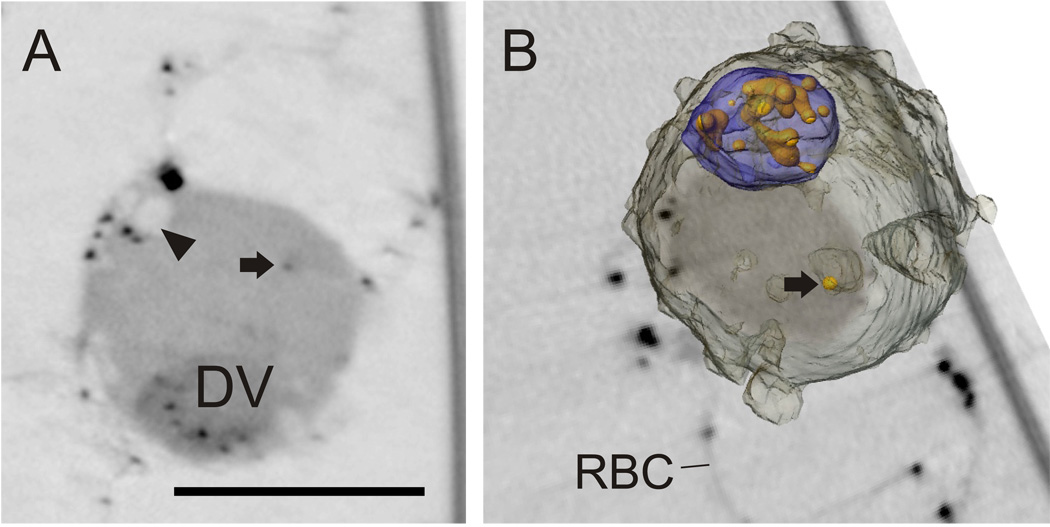
To further examine the process of hemoglobin uptake we reversibly resealed RBCs to contain ultrasmall gold particles conjugated to albumin and infected these resealed cells with P. falciparum. The resealed RBCs were invaded with ~50% efficiency ((Abu Bakar et al., 2010) and data not shown). Parasites were allowed to develop to the trophozoite stage, then magnet enriched before fixation and Eqt II permeabilization. The gold particles were enhanced by gold deposition to enable imaging by x-ray microscopy (Fig. 4). Analysis of virtual sections through a young trophozoite stage parasite shows that the gold particles are taken up into the parasite and accumulate in the digestive vacuole (Fig. 4A, DV). Gold particles are also observed associated with parasite surface invaginations (Fig. 4A, arrowhead) and in the parasite cytoplasm (Fig. 4A, arrow). Rendering of the surface of the trophozoite highlights the accumulation of gold particles in the digestive vacuole and shows that the single gold aggregate in the parasite cytoplasm is located in an additional x-ray-dense compartment (Fig. 4B). This is likely to represent an endocytic vesicle en route to the digestive vacuole (Abu Bakar et al., 2010).
Fig. 4. Uptake of gold particles from the host cytoplasm.
Parasites were allowed to invade RBCs that had been resealed to contain gold particles and were enriched at the trophozoite stage of development. (A) Individual virtual slice through a trophozoite. Gold particles are visible in the host RBC cytoplasm and in the digestive vacuole, and associated with an invagination (arrowhead) that is still linked to the RBC cytoplasm. Occasional gold particles are observed in independent structures in the parasite cytoplasm (arrow). (B) Rendered model overlaid onto a virtual section. The parasite surface is depicted in translucent grey and the digestive vacuole in blue. The model reveals the morphology of the gold particle-containing structure in the parasite cytoplasm. Scale bar, 3.5 µm.
Artemisinin treatment affects digestive vacuole development and cell growth
We examined the effect on parasite morphology of treating parasites with the endoperoxide antimalarial, artemisinin. At the start of the treatment period the parasites were at mid ring stage and no hemozoin crystals were apparent (data not shown). After incubation for 16 h in the absence of artemisinin, the parasite occupies much of the RBC volume and dark pigment granules are observed in the digestive vacuole (Fig. 5A, arrows). By contrast in the artemisinin-treated samples the parasites have failed to develop and the digestive vacuole contents are less dense (Fig. 5B,C, arrows). This indicates that hemoglobin digestion and hemozoin formation have been affected by artemisinin treatment. The data are typical of 20 different control and treated datasets.
Fig. 5. Effect of artemisinin treatment on parasite morphology and uptake of host cytoplasm.
Infected RBCs at the ring stage (~14 hours post-invasion) were cultured for 16 h in the absence (A) or presence (B,C) of 60 nM artemisinin. The cells were enriched using a magnetic column, fixed with 2.5% glutaraldehyde. The capillaries were coated with 100 nm gold (some of which enter the tube) before cryo-preservation in preparation for X-ray microscopy. Virtual slices (34 nm) from tomographic reconstructions are shown. The parasite digestive vacuole is indicated with arrows and an alignment fiducial with an arrowhead. Scale bar, 2 µm.
DISCUSSION
X-ray microscopy can provide details of the internal structure of thick (up to 10 µm), hydrated, unstained sections or whole cells (Gu et al., 2007; Le Gros et al., 2005; Uchida et al., 2009). The cryo stage that has been implemented in XM-2 limits x-ray damage during imaging and we observed no evidence for shrinkage or disruption of the sample during data collection. The tomographic capability of XM-2 and the improved optics, allow a major enhancement of image quality with correspondingly higher information content. Projection images of dehydrated samples of infected RBCs have been obtained previously (using XM-1 without a cryo-stage) (Magowan et al., 1997), however the tomograms that we have generated represent the first 3-D x-ray analysis of hydrated P. falciparum-infected RBCs.
The “water window” is the region of the x-ray spectrum between the K shell absorption edges of carbon (4.4 nm) and oxygen (2.3 nm). X-rays in this region are absorbed approximately ten times more strongly by carbon- and nitrogen-containing material than by water. The absorption adheres to the Beer-Lambert’s law and is therefore linear with thickness and concentration. Unhydrated carbon-rich materials (eg. lipids) show a particularly high absorption. Thus the absorption profile can provide quantitative information about the likely composition of different cellular features. For example the parasite surface and the membrane of the host cell are readily observed and the parasite nuclei can be distinguished from the cytoplasm due to the weaker absorbance of the nuclear material and the stronger absorbance of the nuclear double membrane. The iron-dense hemozoin is strongly x-ray absorbing and the rod-like nature of these structures is apparent. Hemozoin crystals are encased in neutral lipid nanodroplets (Pisciotta et al., 2007), which may augment the x-ray absorption.
In dividing schizonts endomitotic nuclear division is observed before cellular division, and our x-ray analysis confirmed a previous report (Bannister et al., 2000) that the development of individual daughter cells is staggered. Two x-ray-dense puncta were observed at the apical end of each of the daughter merozoites. These are likely to represent the rhoptries – secretory organelles of apicomplexan parasites that discharge during invasion of the host cell and contribute to the formation of the PV membrane (Bannister and Dluzewski, 1990). The lipid-rich nature of the rhoptries and their high protein content provide a contrast with the surrounding cell cytoplasm.
Flattened membrane features in the RBC cytoplasm, with a diameter of up to ~500 nm, were apparent in the x-ray images. We developed an immunolabeling method and used antibodies against a resident protein to confirm that these are Maurer’s clefts. We have previously reported fluorescence microscopy data showing that that Maurer’s clefts are independent organelles rather than sub-compartments or membrane extensions of the parasitophorous vacuole membrane (Spycher et al., 2008). The x-ray microscopy data provide important confirmation of this finding. Hemoglobin digestion by the parasite involves the endocytic uptake of packets of host cell cytoplasm and transfer to an acidic digestive vacuole. There is currently some discussion regarding the process of hemoglobin uptake. In single section electron micrographs hemoglobin-containing structures are observed arising from specialized structures (cytostomes) at the parasite surface (Aikawa et al., 1966). Budding from these cytostomal invaginations has been assumed to be the major feeding mechanism (Slomianny, 1990; Yayon et al., 1984). Recently, however, Lazarus et al. (2008) suggested that cytostomal invaginations remain connected to the RBC, enlarging until they abut the digestive vacuole, then bud and simultaneously fuse with the digestive vacuole. By contrast a whole cell electron tomography study provided evidence for the presence of independent hemoglobin- and hemozoin-containing structures in the parasite cytoplasm (Abu Bakar et al., 2010), suggesting that cytostomal vesicles do bud from the parasite surface. Yet another study provided evidence for the presence of phagotrophic invaginations that are not cytostome-derived (Elliott et al., 2008). Indeed a phagotrophic mechanism of hemoglobin uptake was postulated many years ago (Rudzinska and Trager, 1959). All of these studies have relied on EM analyses of fixed, dehydrated sections. The availability of x-ray imaging provides an opportunity to re-examine the hemoglobin uptake process in whole hydrated cells.
Our x-ray micrographs showed that the early ring stage parasite has a pronounced cup shape. One previous study suggested that this large invagination might be the first step in the genesis of the digestive vacuole (Elliott et al., 2008). However the x-ray data presented here support fluorescence microscopy data showing that the cup remains open to the RBC cytoplasm (Abu Bakar et al., 2010). An alternative suggestion for the role of the cupped morphology is to increase the surface area of the ring stage parasite without significantly increasing its volume (Abu Bakar et al., 2010). As the parasite develops to the late ring stage small hemozoin crystals are observed in dispersed compartments. This is consistent with previous work showing the formation of small pre-digestive vacuole compartments that coalesce into a central digestive vacuole in the mid-trophozoite stage (Abu Bakar et al., 2010; Dluzewski et al., 2008).
X-ray imaging of intact and EqtII-permeabilized trophozoite-infected RBCs revealed multiple invaginations at the parasite surface. The x-ray-lucency of these structures in the EqtII-permeabilized samples confirms that they remain connected to the host compartment thus allowing the release of hemoglobin. This is consistent with the suggestion for the existence of multiple endocytic invaginations that remain connected to the RBC cytoplasm but does not distinguish between cytostomal and phagotrophic invaginations. In some cases the opening is not clearly resolved, consistent with the ~55 nm opening of a cytostomal invagination (Abu Bakar et al., 2010). However some of the invaginations have wide openings with diameters up to 1 µm; this is consistent with the presence of phagotrophic invaginations. We considered the possibility that the EqtII treatment induces these openings however similar open invaginations are also observed in intact infected RBCs. Thus the x-ray data are consistent with the presence of two different types of endocytic invagination in trophozoite stage parasites. Whether the phagotrophic structures play a role in the uptake of hemoglobin or simply represent a previously unrecognized plasticity of the parasite surface remains to be determined.
The x-ray data also provide support for the presence of independent hemoglobin-containing structures (endocytic vesicles) in the parasite cytoplasm. Consistent with a recent EM study (Lazarus et al., 2008) we found that closed hemoglobin-containing compartments in the parasite cytoplasm are rare. To further examine this process we developed methods for infecting RBCs that had been resealed to contain gold particles. The gold particles accumulate in the digestive vacuole indicating that this is the major site for the products of hemoglobin digestion. In some cells we also observed independent compartments containing gold particles in the parasite cytoplasm. These compartments were x-ray-dense possibly indicating concentration of the hemoglobin contents or the formation of small hemozoin crystallites. The data suggest that independent hemoglobin uptake vesicles are present though they are probably short-lived. The data are also consistent with recent studies suggesting that hemoglobin digestion can be initiated in extra digestive vacuolar compartments (Abu Bakar et al., 2010; Dluzewski et al., 2008).
In the EqtII-permeabilized infected RBCs some x-ray dense spherical structures (probably containing hemoglobin) remain in the RBC cytoplasm. Indeed similar structures have been observed in sections prepared for EM, and are thought to represent extensions of the parasitophorous vacuole membrane wrapped around regions of host (del Pilar Crespo et al., 2008). The role of these structures has not been investigated. They may be involved in hemoglobin uptake; for example they could represent a store of hemoglobin that is sequestered for digestion in the final stages of parasite development. Alternatively they could represent a store of exported parasite proteins. Further work is needed to determine their function.
We cultured ring stage parasites in the presence or absence of 60 nM artemisinin for 16 h. This concentration is twice the level needed to kill 50% of parasites over a 48 h period (Taylor et al., 2004). In control cells the parasites increased in size at the expense of the host cytoplasm and generated hemozoin crystals in the digestive vacuole. We found that the artemisinin treatment slowed parasite development and inhibited the accumulation of hemozoin crystals. While it is difficult to distinguish cause and effect with respect to the alterations in digestive vacuole morphology and function under the conditions employed in this experiment, our observations are in agreement with previous reports of artemisinin-induced changes in digestive vacuole morphology observed using EM (del Pilar Crespo et al., 2008; Maeno et al., 1993). The ability of x-ray microscopy to readily measure morphology in large numbers of cells means that it could be used to examine the effects of different drugs and drug combinations.
CONCLUSIONS
In this work we have used cryo x-ray tomography to image whole P. falciparum-infected RBCs. The technique can be used to collect data from significant numbers of whole cells and has provided important new information regarding the cellular topology of P. falciparum and the structures involved in hemoglobin uptake. The spatial resolution achieved in this study is of the order of 50 nm; this is determined by the zone plates that are used to focus and image the x-rays (Gu et al., 2007; Weiss et al., 2000). With further development x-ray imaging with a resolution of ~15 nm should be achievable (Chao et al., 2005), although with a lower depth of field. Coherent x-ray diffraction imaging techniques promise even higher resolution (Miao et al., 2003; Williams et al., 2008). Correlative fluorescence and x-ray imaging will soon be implemented on XM-2 (McDermott et al., 2009); this will greatly enhance the potential applications of this new imaging modality. X-ray microscopy holds enormous promise as a technique for rapidly imaging whole hydrated cells at high resolution.
Supplementary Material
A full tomographic data set of x-ray transmission images consisted of 90 images collected at 2° increments over 180° of rotation. IMOD and Blender packages were used to align the individual images, and to generate the tomogram. Membrane features in the RBC cytoplasm, invaginations of the parasite surface and x-ray-dense hemozoin crystals are evident.
Purified trophozoite stage-infected RBCs were permeabilized with EqtII before mounting in glass capillaries. The RBC membrane, the surfaces of the trophozoites, the nuclei, the digestive vacuoles and the hemozoin crystals are visible. Hemoglobin-containing vesicles and other membranous structures are observed in the RBC cytoplasm.
Purified mature stage-infected RBCs were permeabilized with EqtII before mounting in glass capillaries. A trophozoite (top cell) and a schizont (bottom cell) were imaged. The RBC membrane, the surfaces of the trophozoites, the nuclei, the digestive vacuoles and the hemozoin crystals are visible. Some of the nuclei are enclosed in plasma membranes of daughter cells; some are less developed. Membrane-rich rhoptry organelles and nuclei are observed in each of the daughter merozoites.
The data for a trophozoite presented in Supporting Video S2 were rendered using IMOD. The RBC membrane is shown in transparent grey, the surface of the trophozoite in brown, the nucleus in green, the digestive vacuole in blue. Hemoglobin-containing vesicles in the RBC cytoplasm are rendered in magenta, while other membranous structures are rendered in red.
The data for the schizont presented in Supporting Video S3 were rendered using IMOD. The RBC membrane is shown in transparent grey, the surface of the merozoites in brown, the nuclei in green, and the paired rhoptries at the apical end of the daughter cells in gold. Hemozoin crystals in the remnant digestive vacuole are in black, hemoglobin-containing vesicles in the RBC cytoplasm in magenta, and other membranous structures in red.
Purified mature stage-infected RBCs were permeabilized with EqtII. The RBC membrane, the surface of the trophozoite with multiple x-ray-lucent invaginations and the digestive vacuole with hemozoin crystals are visible.
The data for the trophozoite presented in Supporting Video S6 were rendered using IMOD. The RBC membrane is depicted in brown and the digestive vacuole in blue. Multiple invaginations and extensions of the parasite are visible. Some of the invaginations have resolvable openings onto the parasite surface.
Parasites were allowed to invade RBCs that had been resealed to contain gold particles and were enriched at the trophozoite stage of development. The first part of the video show translations through the virtual sections of the infected RBC. Gold particles are visible in the host RBC cytoplasm and in the digestive vacuole, and associated with an invagination that is still linked to the RBC cytoplasm. Occasional gold particles are observed in independent structures in the parasite cytoplasm. The second part of the movie shows a rendered model of the data overlaid with a virtual section. The parasite surface is depicted in translucent grey and the digestive vacuole in blue. The model reveals the morphology of the gold particle-containing structure in the parasite cytoplasm.
ACKNOWLEDGEMENTS
The authors acknowledge support from the Australian Research Council and the Australian National Health and Medical Research Council, the US Department of Energy, Office of Biological and Environmental Research (DE-AC02-05CH11231), the National Center for Research Resources of the National Institutes of Health (P41RR019664) and the National Institutes of General Medicine of the National Institutes of Health (GM63948). Use of the Advanced Light Source was supported by the U. S. Department of Energy, Office of Science. We thank Sam Deed, Wei-Wei Gu, Dilworth Parkinson and Emily Wilson for technical support. We thank Professor Peter Beck, Swiss Tropical Institute for supplying transfected parasites, Assoc Prof Mike Ryan, La Trobe University, for anti-GFP antibodies.
Abbreviations
- EM
Electron Microscopy
- EqtII
Equinatoxin II
- GFP
green fluorescent protein
- MAHRP1
Membrane-Associated Histidine-Rich Protein-1
- PfEMP1
P. falciparum erythrocyte membrane protein-1
- PVM
parasitophorous vacuole membrane
- RBC
red blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abu Bakar NA, Klonis N, Hanssen E, Chan C, Tilley L. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J Cell Sci. 2010;123:441–450. doi: 10.1242/jcs.061499. [DOI] [PubMed] [Google Scholar]
- Aikawa M, Hepler PK, Huff CG, Sprinz H. The feeding mechanism of avian malarial parasites. J Cell Biol. 1966;28:355–373. doi: 10.1083/jcb.28.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley SB, Sherwood JA, Marsh K, Eidelman O, Howard RJ. Identification of isolate-specific proteins on sorbitol-enriched Plasmodium falciparum infected erythrocytes from Gambian patients. Parasitology. 1986;92:511–525. doi: 10.1017/s0031182000065410. [DOI] [PubMed] [Google Scholar]
- Anderluh G, Pungercar J, Strukelj B, Macek P, Gubensek F. Cloning, sequencing, and expression of equinatoxin II. Biochem Biophys Res Commun. 1996;220:437–442. doi: 10.1006/bbrc.1996.0391. [DOI] [PubMed] [Google Scholar]
- Bannister LH, Dluzewski AR. The ultrastructure of red cell invasion in malaria infections: a review. Blood Cells. 1990;16:257–292. discussion 293-7. [PubMed] [Google Scholar]
- Bannister LH, Mitchell GH. The malaria merozoite, forty years on. Parasitology. 2009;136:1435–1444. doi: 10.1017/S0031182009990734. [DOI] [PubMed] [Google Scholar]
- Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol Today. 2000;16:427–433. doi: 10.1016/s0169-4758(00)01755-5. [DOI] [PubMed] [Google Scholar]
- Chao W, Harteneck BD, Liddle JA, Anderson EH, Attwood DT. Soft X-ray microscopy at a spatial resolution better than 15 nm. Nature. 2005;435:1210–1213. doi: 10.1038/nature03719. [DOI] [PubMed] [Google Scholar]
- del Pilar Crespo M, Avery TD, Hanssen E, Fox E, Robinson TV, Valente P, Taylor DK, Tilley L. Artemisinin and a series of novel endoperoxide antimalarials exert early effects on digestive vacuole morphology. Antimicrob Agents Chemother. 2008;52:98–109. doi: 10.1128/AAC.00609-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzewski AR, Ling IT, Hopkins JM, Grainger M, Margos G, Mitchell GH, Holder AA, Bannister LH. Formation of the food vacuole in Plasmodium falciparum: a potential role for the 19 kDa fragment of merozoite surface protein 1 (MSP1(19)) PLoS ONE. 2008;3:e3085. doi: 10.1371/journal.pone.0003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O'Neill PM, Bray PG, Ward SA, Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- Elliott DA, McIntosh MT, Hosgood HD, 3rd, Chen S, Zhang G, Baevova P, Joiner KA. Four distinct pathways of hemoglobin uptake in the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 2008;105:2463–2468. doi: 10.1073/pnas.0711067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan H, Fessler JA. Ordered subsets algorithms for transmission tomography. Phys Med Biol. 1999;44:2835–2851. doi: 10.1088/0031-9155/44/11/311. [DOI] [PubMed] [Google Scholar]
- Frankland S, Adisa A, Horrocks P, Taraschi TF, Schneider T, Elliott SR, Rogerson SJ, Knuepfer E, Cowman AF, Newbold CI, Tilley L. Delivery of the malaria virulence protein PfEMP1 to the erythrocyte surface requires cholesterol-rich domains. Eukaryot Cell. 2006;5:849–860. doi: 10.1128/EC.5.5.849-860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey TG, Perkins GA, Ellisman MH. Electron tomography of membrane-bound cellular organelles. Annu Rev Biophys Biomol Struct. 2006;35:199–224. doi: 10.1146/annurev.biophys.35.040405.102039. [DOI] [PubMed] [Google Scholar]
- Garcia CR, de Azevedo MF, Wunderlich G, Budu A, Young JA, Bannister L. Plasmodium in the postgenomic era: new insights into the molecular cell biology of malaria parasites. Int Rev Cell Mol Biol. 2008;266:85–156. doi: 10.1016/S1937-6448(07)66003-1. [DOI] [PubMed] [Google Scholar]
- Goldberg DE. Hemoglobin degradation. Curr Top Microbiol Immunol. 2005;295:275–291. doi: 10.1007/3-540-29088-5_11. [DOI] [PubMed] [Google Scholar]
- Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol. 2006;36:1427–1441. doi: 10.1016/j.ijpara.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Gu W, Etkin LD, Le Gros MA, Larabell CA. X-ray tomography of Schizosaccharomyces pombe. Differentiation. 2007;75:529–535. doi: 10.1111/j.1432-0436.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- Humphries AD, Streimann IC, Stojanovski D, Johnston AJ, Yano M, Hoogenraad NJ, Ryan MT. Dissection of the mitochondrial import and assembly pathway for human Tom40. J Biol Chem. 2005;280:11535–11543. doi: 10.1074/jbc.M413816200. [DOI] [PubMed] [Google Scholar]
- Jackson KE, Spielmann T, Hanssen E, Adisa A, Separovic F, Dixon MW, Trenholme KR, Hawthorne PL, Gardiner DL, Gilberger T, Tilley L. Selective permeabilization of the host cell membrane of Plasmodium falciparum-infected red blood cells with streptolysin O and equinatoxin II. Biochem J. 2007;403:167–175. doi: 10.1042/BJ20061725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kats LM, Black CG, Proellocks NI, Coppel RL. Plasmodium rhoptries: how things went pear-shaped. Trends Parasitol. 2006;22:269–276. doi: 10.1016/j.pt.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Krishna S, Uhlemann AC, Haynes RK. Artemisinins: mechanisms of action and potential for resistance. Drug Resist Updat. 2004;7:233–244. doi: 10.1016/j.drup.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Larabell CA, Le Gros MA. X-ray tomography generates 3-D reconstructions of the yeast, Saccharomyces cerevisiae, at 60-nm resolution. Mol Biol Cell. 2004;15:957–962. doi: 10.1091/mbc.E03-07-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus MD, Schneider TG, Taraschi TF. A new model for hemoglobin ingestion and transport by the human malaria parasite Plasmodium falciparum. J Cell Sci. 2008;121:1937–1949. doi: 10.1242/jcs.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros MA, McDermott G, Larabell CA. X-ray tomography of whole cells. Curr Opin Struct Biol. 2005;15:593–600. doi: 10.1016/j.sbi.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lew VL, Tiffert T, Ginsburg H. Excess hemoglobin digestion and the osmotic stability of Plasmodium falciparum-infected red blood cells. Blood. 2003;101:4189–4194. doi: 10.1182/blood-2002-08-2654. [DOI] [PubMed] [Google Scholar]
- Loria P, Miller S, Foley M, Tilley L. Inhibition of the peroxidative degradation of haem as the basis of action of chloroquine and other quinoline antimalarials. Biochem J. 1999;339:363–370. [PMC free article] [PubMed] [Google Scholar]
- Maeno Y, Toyoshima T, Fujioka H, Ito Y, Meshnick SR, Benakis A, Milhous WK, Aikawa M. Morphologic effects of artemisinin in Plasmodium falciparum. Am J Trop Med Hyg. 1993;49:485–491. doi: 10.4269/ajtmh.1993.49.485. [DOI] [PubMed] [Google Scholar]
- Magowan C, Brown JT, Liang J, Heck J, Coppel RL, Mohandas N, Meyer-Ilse W. Intracellular structures of normal and aberrant Plasmodium falciparum malaria parasites imaged by soft x-ray microscopy. Proc Natl Acad Sci U S A. 1997;94:6222–6227. doi: 10.1073/pnas.94.12.6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks KM, Nolan GP. Chemical labeling strategies for cell biology. Nat Methods. 2006;3:591–596. doi: 10.1038/nmeth906. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Dual-axis tomography: an approach with alignment methods that preserve resolution. J Struct Biol. 1997;120:343–352. doi: 10.1006/jsbi.1997.3919. [DOI] [PubMed] [Google Scholar]
- McDermott G, Le Gros MA, Knoechel CG, Uchida M, Larabell CA. Soft X-ray tomography and cryogenic light microscopy: the cool combination in cellular imaging. Trends Cell Biol. 2009;19:587–595. doi: 10.1016/j.tcb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh R, Nicastro D, Mastronarde D. New views of cells in 3D: an introduction to electron tomography. Trends Cell Biol. 2005;15:43–51. doi: 10.1016/j.tcb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32:1655–1660. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- Miao J, Hodgson KO, Ishikawa T, Larabell CA, LeGros MA, Nishino Y. Imaging whole Escherichia coli bacteria by using single-particle x-ray diffraction. Proc Natl Acad Sci U S A. 2003;100:110–112. doi: 10.1073/pnas.232691299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- Pandey AV, Tekwani BL, Singh RL, Chauhan VS. Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite. J Biol Chem. 1999;274:19383–19388. doi: 10.1074/jbc.274.27.19383. [DOI] [PubMed] [Google Scholar]
- Parkinson DY, McDermott G, Etkin LD, Le Gros MA, Larabell CA. Quantitative 3-D imaging of eukaryotic cells using soft X-ray tomography. J Struct Biol. 2008;162:380–386. doi: 10.1016/j.jsb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perktold A, Zechmann B, Daum G, Zellnig G. Organelle association visualized by three-dimensional ultrastructural imaging of the yeast cell. FEMS Yeast Res. 2007;7:629–638. doi: 10.1111/j.1567-1364.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- Pisciotta JM, Coppens I, Tripathi AK, Scholl PF, Shuman J, Bajad S, Shulaev V, Sullivan DJ., Jr The role of neutral lipid nanospheres in Plasmodium falciparum haem crystallization. Biochem J. 2007;402:197–204. doi: 10.1042/BJ20060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson SJ, Grau GE, Hunt NH. The microcirculation in severe malaria. Microcirculation. 2004;11:559–576. doi: 10.1080/10739680490503311. [DOI] [PubMed] [Google Scholar]
- Rudzinska MA, Trager W. Phagotrophy and two new structures in the malaria parasite Plasmodium berghei. J Biophys Biochem Cytol. 1959;6:103–112. doi: 10.1083/jcb.6.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomianny C. Three-dimensional reconstruction of the feeding process of the malaria parasite. Blood Cells. 1990;16:369–378. [PubMed] [Google Scholar]
- Spycher C, Rug M, Klonis N, Ferguson DJP, Cowman AF, Beck H-P, Tilley L. Genesis of and trafficking to the Maurer's clefts of Plasmodium falciparum-infected erythrocytes. Mol. Cell. Biol. 2006;26:4074–4085. doi: 10.1128/MCB.00095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spycher C, Rug M, Pachlatko E, Hanssen E, Ferguson D, Cowman AF, Tilley L, Beck HP. The Maurer's cleft protein MAHRP1 is essential for trafficking of PfEMP1 to the surface of Plasmodium falciparum-infected erythrocytes. Mol Microbiol. 2008;68:1300–1314. doi: 10.1111/j.1365-2958.2008.06235.x. [DOI] [PubMed] [Google Scholar]
- Stayman JW, Fessler JA. Regularization for uniform spatial resolution properties in penalized-likelihood image reconstruction. IEEE Trans Med Imaging. 2000;19:601–615. doi: 10.1109/42.870666. [DOI] [PubMed] [Google Scholar]
- Taylor DK, Avery TD, Greatrex BW, Tiekink ER, Macreadie IG, Macreadie PI, Humphries AD, Kalkanidis M, Fox EN, Klonis N, Tilley L. Novel endoperoxide antimalarials: synthesis, heme binding, and antimalarial activity. J Med Chem. 2004;47:1833–1839. doi: 10.1021/jm0305319. [DOI] [PubMed] [Google Scholar]
- Tilley L, Davis TM, Bray PG. Prospects for the treatment of drug-resistant malaria parasites. Future Microbiol. 2006;1:127–141. doi: 10.2217/17460913.1.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley L, McFadden G, Cowman A, Klonis N. Illuminating Plasmodium falciparum-infected red blood cells. Trends Parasitol. 2007;23:268–277. doi: 10.1016/j.pt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Tilley L, Sougrat R, Lithgow T, Hanssen E. The twists and turns of Maurer's cleft trafficking in P. falciparum-infected erythrocytes. Traffic. 2008;9:187–197. doi: 10.1111/j.1600-0854.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- Trang DT, Huy NT, Kariu T, Tajima K, Kamei K. One-step concentration of malarial parasite-infected red blood cells and removal of contaminating white blood cells. Malar J. 2004;3:7. doi: 10.1186/1475-2875-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M, McDermott G, Wetzler M, Le Gros MA, Myllys M, Knoechel C, Barron AE, Larabell CA. Soft X-ray tomography of phenotypic switching and the cellular response to antifungal peptoids in Candida albicans. Proc Natl Acad Sci U S A. 2009;106:19375–19380. doi: 10.1073/pnas.0906145106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Huang L, Li J, Fan Q, Long Y, Li Y, Zhou B. Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation. PLoS ONE. 2010;5:e9582. doi: 10.1371/journal.pone.0009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D, Schneider G, Niemann B, Guttmann P, Rudolph D, Schmahl G. Computed tomography of cryogenic biological specimens based on X-ray microscopic images. Ultramicroscopy. 2000;84:185–197. doi: 10.1016/s0304-3991(00)00034-6. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2009. 2009 http://whqlibdoc.who.int/publications/2009/9789241563901_eng.pdf.
- Williams GJ, Hanssen E, Peele AG, Pfeifer MA, Clark J, Abbey B, Cadenazzi G, de Jonge MD, Vogt S, Tilley L, Nugent KA. High-resolution X-ray imaging of Plasmodium falciparum-infected red blood cells. Cytometry A. 2008;73:949–957. doi: 10.1002/cyto.a.20616. [DOI] [PubMed] [Google Scholar]
- Yayon A, Timberg R, Friedman S, Ginsburg H. Effects of chloroquine on the feeding mechanism of the intraerythrocytic human malarial parasite Plasmodium falciparum. J Protozool. 1984;31:367–372. doi: 10.1111/j.1550-7408.1984.tb02981.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A full tomographic data set of x-ray transmission images consisted of 90 images collected at 2° increments over 180° of rotation. IMOD and Blender packages were used to align the individual images, and to generate the tomogram. Membrane features in the RBC cytoplasm, invaginations of the parasite surface and x-ray-dense hemozoin crystals are evident.
Purified trophozoite stage-infected RBCs were permeabilized with EqtII before mounting in glass capillaries. The RBC membrane, the surfaces of the trophozoites, the nuclei, the digestive vacuoles and the hemozoin crystals are visible. Hemoglobin-containing vesicles and other membranous structures are observed in the RBC cytoplasm.
Purified mature stage-infected RBCs were permeabilized with EqtII before mounting in glass capillaries. A trophozoite (top cell) and a schizont (bottom cell) were imaged. The RBC membrane, the surfaces of the trophozoites, the nuclei, the digestive vacuoles and the hemozoin crystals are visible. Some of the nuclei are enclosed in plasma membranes of daughter cells; some are less developed. Membrane-rich rhoptry organelles and nuclei are observed in each of the daughter merozoites.
The data for a trophozoite presented in Supporting Video S2 were rendered using IMOD. The RBC membrane is shown in transparent grey, the surface of the trophozoite in brown, the nucleus in green, the digestive vacuole in blue. Hemoglobin-containing vesicles in the RBC cytoplasm are rendered in magenta, while other membranous structures are rendered in red.
The data for the schizont presented in Supporting Video S3 were rendered using IMOD. The RBC membrane is shown in transparent grey, the surface of the merozoites in brown, the nuclei in green, and the paired rhoptries at the apical end of the daughter cells in gold. Hemozoin crystals in the remnant digestive vacuole are in black, hemoglobin-containing vesicles in the RBC cytoplasm in magenta, and other membranous structures in red.
Purified mature stage-infected RBCs were permeabilized with EqtII. The RBC membrane, the surface of the trophozoite with multiple x-ray-lucent invaginations and the digestive vacuole with hemozoin crystals are visible.
The data for the trophozoite presented in Supporting Video S6 were rendered using IMOD. The RBC membrane is depicted in brown and the digestive vacuole in blue. Multiple invaginations and extensions of the parasite are visible. Some of the invaginations have resolvable openings onto the parasite surface.
Parasites were allowed to invade RBCs that had been resealed to contain gold particles and were enriched at the trophozoite stage of development. The first part of the video show translations through the virtual sections of the infected RBC. Gold particles are visible in the host RBC cytoplasm and in the digestive vacuole, and associated with an invagination that is still linked to the RBC cytoplasm. Occasional gold particles are observed in independent structures in the parasite cytoplasm. The second part of the movie shows a rendered model of the data overlaid with a virtual section. The parasite surface is depicted in translucent grey and the digestive vacuole in blue. The model reveals the morphology of the gold particle-containing structure in the parasite cytoplasm.